1. Introduction
Carotenoids have gained significant attention for
its potential therapeutic and preventive benefits against various
types of non-communicable diseases including diabetes, obesity,
cardiovascular disease, cancers, respiratory disorders, as well as
impaired neurodevelopment outcomes (1-6).
In preventive medicine, diet and nutrition, play a crucial role as
they are important risk factors for non-communicable diseases
(7-10).
Therefore, they should be prioritized in public health policies and
programs. Moreover, promoting healthier diets could lead to
substantial savings in health care costs and gains in
quality-adjusted life years (11,12).
Astaxanthin (AXT) is derived from various sources,
including marine organisms such as shrimp and krill, as well as
freshwater microalgae, particularly Haematococcus pluvialis
(13,14). It exhibits strong antioxidant
properties, which contribute to its anti-inflammatory,
anti-proliferative and possibly anticancer effects which are
discussed in the present review. These sources are critical not
only for their high AXT content but also for their role in
sustainable production practices, which are crucial in the current
global push for environmentally friendly and ethically sourced
supplements. In a search for more sustainable and healthy food
systems, microalga is one of the most auspicious sustainable
sources of food ingredients with a great potential in promoting
healthy diet and nutrition, thanks to the numerous pigments and
nutrients that contain, which can be used as dietary supplements
and functional foods (15-18).
In addition, microalgae are rich in bioactive
compounds with different chemical structures that can be extracted
from numerous species and can be used in the pharmaceutical field
for therapeutic purposes or to formulate drug delivery systems
(19). Nevertheless, 95% of AXT
available in the market is produced synthetically using
petrochemicals, due to cost-efficiency for mass production
(20) and the natural production is
facing challenges of a limited yield and high cost of cultivation
and extraction, both with an increasing demand for a more and more
sustainable economy, promoting biological production processes;
which until now, have been associated with very high costs
(21).
The present review, gathered and summarized the main
findings on the application of AXT in preventive and therapeutic
oncology, focusing on the hypothesized mechanisms of action
underlying its cytotoxic effects on cancer cells. It highlighted
the anti-proliferative features of AXT, particularly its ability to
induce cell death, inhibit metastasis and modulate key molecular
pathways involved in cancer progression. The present review
emphasized cancers with the most substantial body of research,
providing a clearer understanding of how AXT can be effectively
leveraged in combating these prevalent and impactful
malignancies.
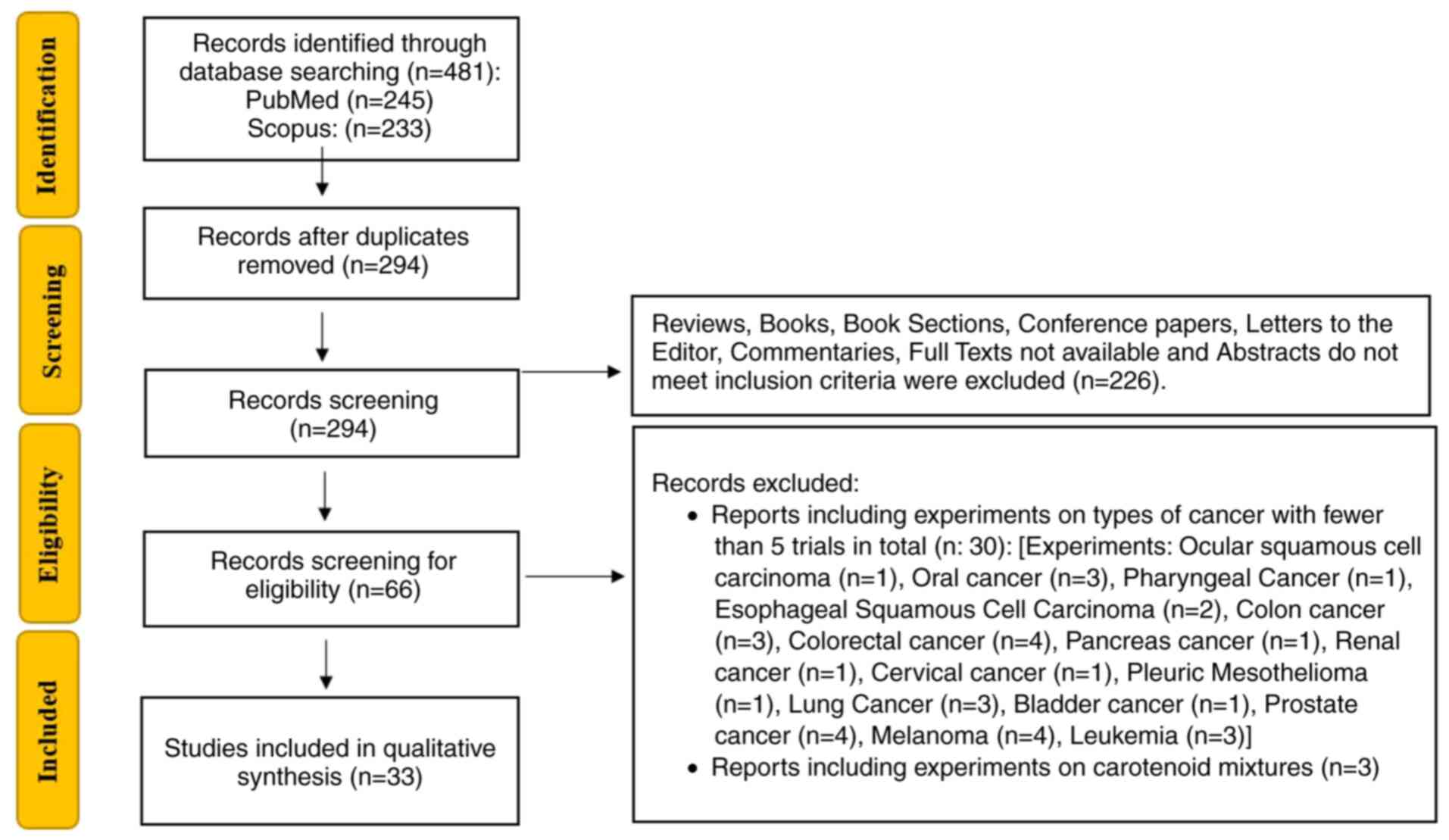
2. Protocol
A comprehensive literature search was conducted
using the PubMed (https://pubmed.ncbi.nlm.nih.gov) and Scopus
(https://www.scopus.com) databases, following a
clearly defined retrieval strategy. The keywords ‘Astaxanthin’,
‘Cancer’ and ‘Tumor’ were used for searches within the Title and
Abstract fields. The exact search string applied was: [Astaxanthin
(Title/Abstract)] AND [cancer (Title/Abstract)] OR [cancers
(Title/Abstract)] OR [tumor (Title/Abstract)] OR [tumors
(Title/Abstract)]. Filters were set to include only peer-reviewed
articles published from 2014 onwards. The inclusion criteria
encompassed studies published in English, conducted in vitro
or in vivo, with a minimum of five experimental articles for
each type of cancer and peer-reviewed articles providing detailed
insights into the cytotoxic mechanisms of AXT and its applications
in cancer treatment or prevention. Exclusion criteria included
articles not written in English and studies on types of cancer with
fewer than five published experiments, with the exception of
matters concerning central nervous system tumors. Additionally,
studies conducted on carotenoid mixtures containing astaxanthin
were excluded to maintain a sole focus on the specific effects of
AXT. Initial screening of titles and abstracts was performed to
assess relevance, followed by full-text reviews to confirm the
eligibility of the selected studies. Data extraction was
independently conducted by two reviewers to ensure accuracy and
consistency. The synthesized data emphasized the role of
astaxanthin in promoting cell death, inhibiting metastasis and
modulating key molecular pathways involved in cancer progression.
The flowchart summarizing the process of the present review is in
Fig. 1.
3. Astaxanthin and cancer
Nervous system tumors
AXT has been investigated for its potential in
treating some nervous system tumors, including glioblastoma
multiforme (GBM) and neuroblastoma. Furthermore, phytochemicals,
secondary metabolites in food, have been reported to protect
neuronal cells from death in neurodegenerative disorder models
through anti-oxidant and anti-inflammatory activities, opening
potential multifaceted perspectives for their application (22).
GBM is one of the most aggressive forms of primary
brain cancer, with very low survival rates and a significant impact
on the patient's quality of life. As the most prevalent primary
malignant brain tumor, glioblastoma accounts for 57% of all gliomas
and 48% of all primary malignant central nervous system tumors
(23). Patients diagnosed with
glioblastoma often experience a rapid deterioration in neurological
function, suffering from significant symptoms caused not only by
the tumor but also by the side effects of various treatments
(24).
When human GBM cell lines U251-MG and T98-MG, but
not CRT-MG and U87-MG, are pretreated with AXT their sensitivity to
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is
significantly enhanced; thereby increasing the apoptotic response
(25). Furthermore, the combined
treatment shows improved effectiveness in inducing apoptosis
compared with AXT or TRAIL alone, especially in the more responsive
cell lines such as U251-MG and U87-MG, indicating that AXT may
sensitize tumor cells to apoptosis-inducing agents. The differing
sensitivity of these cell lines likely stems from distinct
mechanisms, such as antioxidant expression and mitochondrial
dynamics. U251-MG cells have lower Superoxide dismutase 2 (SOD2)
expression and enzymatic activity compared with CRT-MG.
Overexpression of SOD2 in U251-MG eliminates the AXT-induced
sensitization to TRAIL treatment, indicating that SOD2 plays a
critical role in the differential response between the two cell
lines (25). These findings
underscore that the ability of AXT to enhance cancer treatment may
depend on the genetic and metabolic profile. Additionally, AXT
exhibits hormetic effects on U251-MG, T98G and CRT-MG cell lines,
where low doses stimulate cell proliferation by upregulating
markers such as cyclin-dependent kinases, while higher doses induce
apoptosis by triggering a dose-dependent oxidative stress response,
significantly increasing reactive oxygen species (ROS) levels and
promoting apoptosis (26).
Targeting cyclin-dependent kinases with specific inhibitors could
potentially improve treatment efficacy by reducing chemoresistance
and enhancing the response to chemotherapy, as indicated also by
Patel et al (27) for
colorectal and oral cancers.
Further investigations into the effects of AXT on
glioblastoma were conducting both in vitro in U251MG and
GL261 cell lines and in vivo in C57BL/6J mice model. The
in vitro results indicate that AXT could suppress cell
viability and proliferation in a concentration-dependent manner,
reducing cell migration and influencing key signaling molecules
(ERK1/2, Akt and p38 MAPK) involved in tumor progression (28). The combined treatment of AXT with
temozolomide, a standard chemotherapy drug for glioblastoma,
enhanced the antitumor effects compared with AXT alone. In
vivo, AXT significantly suppressed glioblastoma tumor growth,
demonstrating its capacity to accumulate in brain tissue and
effectively inhibit tumor progression (28).
Neuroblastoma is a common and aggressive pediatric
cancer that originates in nerve tissues, responsible for a
significant number of childhood cancer mortalities and presenting
challenges for effective treatment due to its diverse genetic,
morphological and clinical presentations; it most often begins in
the adrenal glands but can also develop in other areas such as the
neck, chest, abdomen, or spine (29). The effects of AXT were explored on
SK-N-SH neuroblastoma cell line both in vitro and in
vivo. In vitro AXT revealed strong anti-tumor activity by
inhibiting proliferation, migration and invasion, while also
promoting apoptosis (30). When AXT
was combined with small interfering (si)RNA targeting the STAT3
signaling pathway, a synergistic effect was observed, further
enhancing the reduction of tumor cell viability and aggressive
behavior in vivo (30) The
STAT3 pathway is implicated in resistance to chemotherapy and the
ability of AXT to inhibit STAT3 could theoretically help counteract
this resistance, making it a promising candidate for further
investigation in chemoresistant cancer models.
AXT is known for being able to modulate cell
survival, redox biology and bioenergetics status by influencing key
signaling pathways (31). García
et al (32) demonstrate that
AXT reduces mitochondrial superoxide (O2-•) production in SH-SY5Y
cells exposed to N-methyl-D-aspartate. Zhang et al (33) found that AXT activates the
PI3K/Akt/GSK3β pathway, leading to the upregulation of nuclear
factor erythroid 2-related factor 2 (Nrf2), a transcription factor,
in SH-SY5Y cells under oxygen and glucose deprivation conditions.
More recently AXT was shown being able to reduces mitophagy through
the Akt/mTOR pathway, suggesting its potential in preventing
neurotoxicity in neurodegenerative diseases (34). As a matter of fact, the regulation
of mitochondrial membrane pore formation has been previously
proposed as a neuroprotective mechanism of AXT in aging and
neurodegenerative disorders and thus also explains their dual
functions in neuronal and cancer cells (22). As an antioxidant, AXT has the
potential to act as both an anticancer drug and a neuroprotectant.
In cancer, AXT limits tumor growth by modulating ROS produced under
stressful conditions in rapidly proliferating cells. Conversely,
AXT protects against oxidative stress, which causes mitochondrial
dysfunction and apoptosis, thereby reducing the detrimental effects
associated with neurodegenerative diseases such as Alzheimer's,
Parkinson's and amyotrophic lateral sclerosis (35).
Breast cancer
Breast cancer is the most frequently diagnosed type
of cancer in women worldwide, with higher incidence rates in high
High Development Index (HDI) countries and markedly lower in medium
and low HDI countries. Survival rates also vary markedly between
developed and less developed countries, highlighting disparities in
healthcare quality and access to healthcare (36). Research findings reveal that AXT
treatment of human breast cancer cell lines effectively reduces
cancer progression in a dose-dependent manner by inhibiting cell
growth and inducing apoptosis without affecting non-tumorigenic
cell lines. AXT alleviates inflammation and the immunosuppressive
environment within tumors, creating less favorable conditions for
cancer growth and its spread, reducing cancer cell migration and
metastasis formation (37-43).
AXT may modulate various pathways involved in the activation of
apoptosis, targeting key signaling pathways critical in breast
cancer progression, including angiogenesis, cellular stress
response, lipid metabolism and the regulation of inflammation
(40,41,44).
In vitro, AXT significantly reduces cell
proliferation and migration in both the MCF7 (ER-positive) and
MDA-MB-231 (triple-negative) breast cancer cell lines in a
dose-dependent manner. However, the MDA-MB-231 cell line was more
sensitive to the anti-migratory effects of AXT due to its higher
metastatic potential and apoptotic gene expression was also more
prominently observed in these cells (45). In SKBR3 cells, AXT decreased the
expression of human epidermal growth factor receptor 2 (HER2) and
other related oncogenic proteins that are typically overexpressed
in SKBR3 cells (40). This
downregulation of HER2 and associated signaling pathways indicates
that AXT may interfere with survival and proliferation signals in
HER2-positive breast cancer cells. Astaxanthin treatment also
significantly reduces the expression of pontin in T47D and BT20
cell lines (37) and mutant p53
(mutp53) in T47D, BT20 and SKBR3 cell lines (37,40).
Notably, the knockdown of pontin through siRNA mirrors the effect
of AXT on gene expression and markedly reduces the proliferation,
migration and invasion of cancer cells. Therefore, by lowering both
pontin levels and ROS, AXT also reduces mutp53. Sowmya et al
(46) studied the effects of AXT
derived from shrimp, alone and combined with β-carotene and lutein
from greens, on MCF-7 breast cancer cells. This combination
exhibits enhanced cytotoxicity and oxidative stress compared with
individual treatments or saponified shrimp carotenoid extract. This
combination selectively killed MCF-7 cancer cells by modulating key
proteins involved in cell cycle regulation and apoptosis leading to
cell cycle arrest in the G0/G1 phase, with no
adverse effects on normal breast epithelial cells (MCF-10A).
In vivo, Yuri et al (47) examined dietary AXT effects on
mammary carcinogenesis in female Sprague-Dawley rats treated with
N-methyl-N-nitrosourea; higher concentrations of AXT significantly
reduces the incidence of palpable mammary tumors compared with the
control group. AXT's anticarcinogenic effects are also associated
with decreased adiponectin expression in mammary adipose tissues,
which is linked with anti-inflammatory and anti-tumor properties
and its upregulation may help reduce tumor progression (47). Furthermore, a protective effect on
tissue is shown by histological analysis, indicating that AXT
preserves tissue integrity in mammary glands, exerting a protective
role against cellular damage caused by carcinogens (47).
Combined treatments were tested to enhance the
efficacy of cell death induction in breast cancer cells. The
synergistic cytotoxic effect of AXT with melatonin showed enhanced
efficacy in the T47D cell line compared with the MDA-MB-231 line
(39). The increased effectiveness
in T47D cells is attributed to its triple-positive receptor status
(expressing estrogen, progesterone and HER2 receptors), which may
increase the sensitivity of these cells to apoptosis-inducing
effects. By contrast, MDA-MB-231, being a triple-negative breast
cancer line, exhibited lower sensitivity to the combined treatment.
This variability suggested that receptor status and genetic
differences between cell lines play a significant role in the
differential response to astaxanthin and melatonin (39). Fouad et al (48) investigated the combined effects of
eugenol (EUG) and AXT with doxorubicin (DOX) on MCF7 cells, a
hormone receptor-positive breast cancer cell line. Key findings
showed that EUG and AXT markedly increased the cytotoxic effect of
DOX on MCF7 cells through epigenetic modifications and immune
modulation. Notably the EUG and AXT combination increased histone
acetylation and histone acetyltransferase expression and decreased
aromatase and EGFR expression which is critical in breast cancer
cell proliferation and survival. Vijay et al (44) studied the combined effects of AXT
and other carotenoids with low doses of DOX on MCF-7 and MDA-MB-231
breast cancer cells; combining carotenoids with a minimal cytotoxic
dose of DOX resulted in greater cytotoxic effects, more
significantly in MCF-7 cells, than either higher doses of DOX alone
or carotenoids alone through the modulation of redox balance. This
combination not only promoted apoptosis and arrested the cell cycle
in the G0/G1 phase but also exhibited minimal cytotoxicity in
normal breast epithelial cells (MCF 10A). Atalay et al
(49) focused on the effects of AXT
combined with carbendazim on MCF-7 breast cancer cells; this
combination significantly increased the anti-proliferative effects
compared with carbendazim and AXT alone and increased cell cycle
arrest in the G2/M phase. Furthermore, co-treatment with
AXT reduced the elevated ROS levels induced by carbendazim,
potentially lessening oxidative stress-related side effects while
maintaining the anti-cancer efficacy, suggesting that combining ATX
with carbendazim could enhance anti-cancer effects while mitigating
some of the oxidative stress typically induced by chemotherapy
agents (49). Malhão et al
(45) investigated the effects of
AXT on breast cancer cells, both as a standalone treatment and in
combination with conventional chemotherapy drugs (cisplatin and
DOX). AXT alone did not show significant cytotoxic effects on the
cancer cell lines tested, including luminal MCF7 SKBR3 (HER-2
positive) and MDA-MB-231 (triple-negative), as well as a
non-tumoral breast cell line, MCF12A. However, in combination with
cisplatin or DOX, the effect of AXT is varied. In some instances,
it reduces the cytotoxicity of cisplatin, particularly with
cisplatin on the SKBR3 breast cancer cell line, indicating a
potential protective effect. In certain cases, AXT enhances the
cytotoxic effect of the chemotherapy drugs, specifically when
combined with DOX on the MCF7 cell line. The results suggest that
while AXT itself does not directly induce strong cytotoxic effects
against breast cancer cells, its interactions with chemotherapy
drugs can influence treatment outcomes, though not always in a
favorable way. This highlights the need for further research to
improve understanding of the role of AXT in combination
therapies.
Research also shows promising potential for using
functionalized nanoparticles in breast cancer therapy. A
nanoparticle formulation combining DOX with low-molecular-weight
heparin (LMWH) and AXT, known as LMWH-AST/DOX nanoparticle (LA/DOX
NP), has been studied. The findings highlight that LA/DOX NPs
effectively suppressed liver metastasis by inhibiting the formation
of neutrophil extracellular traps (NETs) (41). Furthermore, ATX-reduced gold
nanoparticles (ATX-AuNPs) were studied in MDA-MB-231 human breast
cancer cells, showing dose-dependent cytotoxic effects and
significant antiproliferative activity, indicating potent efficacy
in inhibiting cell growth. The results illustrate that ATX-AuNPs
exhibit enhanced cytotoxic effects compared with free ATX alone,
indicating that these nanoparticles can induce programmed cell
death more effectively than free AXT and improved cellular uptake
(50). ATX-loaded chitosan
oligosaccharide/alginate nanoparticles (ATX-COANPs) also offer a
promising solution to the challenges of low solubility, bio
accessibility and bioavailability of ATX. By encapsulating ATX
within COANPs, the bioactive properties of astaxanthin are
enhanced, with improved in vitro anti-inflammatory activity
and significant cytotoxic effects on MCF-7 breast cancer cells.
This nanostructure, optimized using Box-Behnken design and response
surface methodology, presents an effective delivery mechanism for
ATX in nutraceutical and functional food application (51).
Gastrointestinal cancers
Gastrointestinal cancers encompass a diverse group
of malignancies affecting the digestive system, including the
esophagus, stomach, liver, pancreas, gallbladder and intestines.
The incidence of gastrointestinal cancer is rising, with
age-standardized diagnosis rates showing significant global
variations, with the highest rates observed in South America,
Eastern Asia and Central and Eastern Europe (52). Due to delayed detection and the lack
of effective treatments, GC has a poor prognosis, with a number of
patients diagnosed at advanced stages and mortality often occurring
within the first year of diagnosis (53). Understanding the specific
characteristics and risk factors of each type is crucial for early
detection, effective treatment and improving patient outcomes. The
chemo preventive and cytotoxic potential of AXT has been
extensively studied in relation to oral, liver and gastric cancers,
demonstrating promising results and showcasing AXT's ability to
inhibit tumor growth and induce apoptosis in cancer cells.
Liver cancer
Liver cancer, or hepatocellular carcinoma (HCC), is
a major cause of cancer-related mortality worldwide and its
increasing incidence, particularly in patients with cirrhosis,
poses significant treatment challenges (54). The complexity of HCC treatment
arises from the variability in tumor size, number of nodules and
patient liver function. Liver transplantation is considered the
most effective long-term treatment, but is limited by strict
eligibility criteria and organ availability (55). AXT has been extensively studied for
its potential therapeutic and preventive benefits primarily
focusing on various HCC cell lines and animal models. In
vitro and in vivo studies are mainly based on the HepG2
hepatoma cell line. By using Kunming mice, Shao et al
(56) demonstrate that AXT inhibits
the proliferation of H22 hepatoma cells in vitro and in
vivo and induces both apoptosis and necrosis as indicated by
nuclear condensation and fragmentation analysis in treated cells.
Although the apoptosis rate did not vary significantly between low-
and high-dose groups, cell necrosis increased markedly, with
high-dose AXT performing similarly to cisplatin. Additionally, AXT
effectively reduced tumor size and the number of cancer cells in
mice, supporting its potential anti-tumor activity. These findings
highlight AXT's role in inhibiting tumor growth and inducing cell
death. Zhang et al (57)
encapsulated AXT in biodegradable calcium alginate microspheres to
enhance its bioavailability and stability, protecting it from
degradation in harsh environmental conditions such as gastric
acids. This study demonstrates that AXT treatment reduces ROS
levels specifically in HepG2 cells, leading to significant
inhibition of cell growth. Notably, this cytotoxicity was
selective, sparing normal hepatocytes (THLE-2 cells), thereby
highlighting AXT's potential for targeted cancer therapy without
harming healthy cells (57). Thus,
encapsulated AXT in calcium alginate microspheres enhances
stability, enables controlled release and improves selective
cytotoxicity toward cancer cells, minimizing effects on healthy
cells. This approach reduces oxidative stress in normal cells,
improves cellular uptake in cancer cells and offers a safer, more
effective delivery method, making encapsulated AXT a promising
option for targeted cancer therapy.
Another approach involves using natural sources of
AXT, as in the study by Messina et al (58), which assesses the bioactive
properties of AXT extracted from shrimp by-products on hepatoma
cells (Hep-G2). This research shows dose-dependent
anti-proliferative effects, where increasing concentrations of AXT
decreased cell vitality and induced apoptosis, marked by the
increase in p53 protein levels and activation of caspase-3
Considering that 53 is among the most well-known tumor progression
inhibitors. it is reasonable that AXT activates apoptosis
modulating p53 expression/activity.
These markers are critical in the cellular apoptotic
pathway, reinforcing AXT's potential to induce programmed cell
death in cancer cells (58).
Further extending the scope of the effect of AXT on liver cancer,
Li et al (59) examined both
natural and synthetic forms of AXT on different human hepatoma cell
lines, LM3 and SMMC-7721. Their findings highlight that AXT
inhibits growth by downregulating critical signaling pathways such
as NF-κB p65 and Wnt/β-catenin. These pathways are instrumental in
the regulation of gene expression linked to cell proliferation and
survival. AXT stabilizes IκB-α, preventing the nuclear
translocation of NF-κB p65 and thus inhibiting this pathway and the
consequent survival of cancer cells. Additionally, AXT influences
the Wnt/β-catenin pathway, further strengthening its role in
reducing proliferation and inducing apoptosis in a dose-dependent
manner, with significant effects observed at higher concentrations
(59). The study observed that AXT
was comparably effective in both LM3 and SMMC-7721 HCC cell lines,
demonstrating dose- and time-dependent inhibition of cell
proliferation and induction of apoptosis in both of the lines
(59). The anti-proliferative
effects of carotenoid mixture extracted from microalgae
(Monoraphidium sp. and Scenedesmus obliquus),
includes AXT as a major component, was also explored using the HCC
cell line HUH7(60). The study
highlights that Monoraphidium sp., which had the highest
amounts of AXT, exhibits stronger antioxidant and
anti-proliferation activities compared with Scenedesmus
obliquus. This underscores the significance of exploring
freshwater microalgae as a source of high-yielding AXT for
pharmaceutical applications (60).
AXT's potential in combination with chemotherapy
drugs is confirmed in studies from animal studies. Ren et al
(61), in an in vivo mouse
mode, shows that combining AXT with sorafenib enhances anti-tumor
immune responses in hepatocellular carcinoma. AXT increases CD8+ T
cell infiltration, polarizes macrophages towards the M1 phenotype,
boosts anti-tumor cytokines and positively alters gut microbiota,
improving overall immune function and treatment efficacy, thus
reducing some of the systemic and gut-related side effects of
sorafenib (61). By enhancing
immune responses, AXT may overcome some of the mechanisms that
allow chemo-resistant tumors to evade treatment. Subsequently, the
same authors demonstrated in vivo that AXT combined with
sorafenib enhances tumor suppression more significantly than in
vitro, indicating that the efficacy of AXT may depend on
mechanisms beyond direct cytotoxicity observed in cell culture,
such as its role in modifying the tumor microenvironment or
impacting pathways relevant to in vivo models (62). Furthermore, the combination
treatment appeared to reduce symptoms linked to cachexia; such as
muscle wasting, which are typically observed with sorafenib alone
(62). By enhancing the
effectiveness of a lower, subclinical dose of sorafenib, AXT
potentially allows for a reduction in the standard sorafenib dose,
thereby decreasing the likelihood of its associated toxicities.
Gastric cancer
Gastric cancer is a significant global health issue,
with >1 million new cases estimated annually, making it the
fifth most diagnosed cancer worldwide. Due to often being diagnosed
at an advanced stage, gastric cancer has a high mortality rate,
ranking as the third leading cause of cancer-related deaths with
784,000 deaths reported globally in 2018(63). Helicobacter pylori is the
primary risk factor for gastric cancer, being the main cause of
chronic gastritis and peptic ulcer disease. Despite decreasing
infection rates in a number of countries due to improved living
standards, its prevalence remains high, particularly in the Far
East and is linked to socioeconomic status and hygiene levels
(64).
The effects of AXT on gastric cancer were studied
using the AGS adenocarcinoma cell line to investigate how H.
pylori infection markedly alters gene expression, upregulating
genes associated with inflammation, cell proliferation and cancer
pathways (65-67).
Kim et al (65) found that
AXT administration results in altered expression of several genes
involved in cell motility and cytoskeleton remodeling, notably
c-MET, PI3KC2, PLCγ1, Cdc42 and ROCK1, which are critical for these
processes. The role of AXT in mitigating the effects of H.
pylori infection was further studied by Lee et al
(66) on human gastric epithelial
cell line AGS. AXT effectively inhibits H. pylori-induced
apoptosis in AGS cells as demonstrated by the reduction in key cell
death markers such as DNA fragmentation, caspase-3 activity and the
cytochrome c release. Furthermore, the study indicates that,
in H. pylori-stimulated AGS cells, AXT enhances autophagy,
through increasing the phosphorylation of AMP-activated protein
kinase (AMPK) and consequently downregulating mTOR. It was also
found that pretreatment of AGS cells with AXT counteracted H.
pylori-induced changes by reducing the expression of key genes
involved in the Wnt/β-catenin pathway (67). This includes the downregulation of
porcupine, Fos-like 1 and c-myc, which are associated with cancer
cell proliferation and survival (67). In addition, AXT reversed H.
pylori-induced repression of tumor suppressor genes such as
SMAD4 and BAMBI, which are involved in the regulation of cell
proliferation and survival, and reduced expression of spermine
oxidase (SMOX) involved in oxidative stress (67).
AXT effectively inhibits the expression of MMP-7 and
MMP-10 in H. pylori-infected gastric epithelial cells. These
enzymes are crucial for degrading the extracellular matrix, which
typically facilitates tumor invasion and metastasis. By reducing
the invasive and migratory capabilities of these cells, AXT limits
their ability to spread following H. pylori infection
(68). Furthermore, H.
pylori infection increases the expression of integrin α5 in AGS
cells, which in turn enhances cell adhesion and migration, key
processes in cancer metastasis. This increase is linked to elevated
ROS levels and activation of the JAK1/STAT3 signaling pathway
(69). AXT effectively lowers ROS
levels in AGS cells and suppressed the activation of JAK1/STAT3.
Consequently, AXT also decreases the expression of integrin α5,
leading to reduced cell adhesion and migration in the H.
pylori-stimulated AGS cells (69).
A dose-dependent inhibition of cell proliferation
was also documented for a number of other adenocarcinoma cell lines
including AGS, KATO-III, MKN-45 and SNU-1. When administered to
KATO-III and SNU-1 cells, AXT has a more pronounced effect,
inhibiting cell proliferation in a dose-dependent manner and
decreasing the percentage of cells in the S phase. Accordingly
p27kip-1 expression, a cyclin-dependent kinase inhibitor that
regulates the cell cycle, is increased, thereby contributing to the
observed G0/G1 arrest (70). By contrast, AXT shows minimal
effects on the viability of AGS and MKN-45 cells and did not
significantly alter cell cycle progression in these cell lines
(70). Others mechanisms explaining
the antiproliferative features of AXT in AGS cancer lines have been
reported, including the involvement of ERK and the activation of
necroptosis-related proteins such as NADPH oxidase,
receptor-interacting protein kinase (RIP) 1, RIP3 and mixed lineage
kinase domain-like protein (71).
In vivo, on male C57BL/6 mice used as animal
model, AXT was observed to reduce oxidative stress, as indicated by
decreased lipid peroxide levels and myeloperoxidase activity and
suppression of inflammatory cytokine IFN-γ, as well as oncogene
expression, such as c-myc and cyclin D1, which are associated with
cancer progression (72). The study
suggests that AXT can help to protect against oxidative damage,
inflammation and oncogene activation in gastric tissues,
potentially offering a preventive strategy against H.
pylori-associated gastric carcinogenesis.
4. Conclusions
In conclusion, AXT holds considerable promise as a
bioactive compound with therapeutic and preventive potential in
cancer management. Extensive in vitro and in vivo
research underscores its efficacy across multiple cancer types,
including nervous system, breast and gastrointestinal cancers,
largely due to its antioxidant, anti-inflammatory and
anti-proliferative properties. AXT exerts significant effects by
modulating various key molecular pathways involved in cancer
progression, cellular stress responses and neuroprotection. It has
been shown to activate the PI3K/Akt/GSK3β pathway, leading to the
upregulation of Nrf2, a transcription factor crucial for oxidative
stress regulation. It also modulates the Akt/mTOR pathway,
influencing mitophagy and preventing excessive apoptosis, thus
exhibiting potential in reducing neurotoxicity and affecting cancer
cell behavior. It was also found to inhibit the JAK1/STAT3 pathway,
which is associated with cell adhesion and migration, particularly
in the context of cancer metastasis. Additionally, it downregulates
the Wnt/β-catenin pathway, thereby reducing cancer cell
proliferation and survival. The NF-κB pathway is also affected by
AXT, as it stabilizes IκB-α to prevent the nuclear translocation of
NF-κB p65, thereby inhibiting survival signals in cancer cells.
Likewise, p53, the most well-known tumor progression inhibitor may
be influenced by AXT in several cancers preventing cancer growth
and invasiveness. By targeting cyclin-dependent kinase pathways,
AXT promotes cell cycle arrest, contributing to reduced cancer cell
proliferation.
Furthermore, AXT enhances autophagy through the
AMPK/mTOR pathway, reducing cell proliferation in cancer models.
Last, AXT affects the ERK1/2 and p38 MAPK signaling pathways, which
are involved in tumor suppression, as demonstrated in studies on
glioblastoma. These regulatory effects underscore AXT's potential
as a multifaceted agent in cancer therapy and neuroprotection,
warranting further clinical research to fully validate its
therapeutic role. The synergy observed when AXT is combined with
chemotherapeutic agents highlights its role in enhancing treatment
efficacy and minimizing adverse effects, especially in breast and
liver cancers. Moreover, innovations in AXT delivery, such as
encapsulation in nanoparticles, have further improved its
bioavailability and targeted action, enabling more effective cancer
cell targeting and potentially reducing systemic side effects.
However, despite promising laboratory results, further in
vivo studies and clinical trials are essential to validate
AXT's therapeutic effects in human populations. These studies would
allow the establishment of standardized dosing regimens, confirm
safety profiles and AXT's interactions with conventional therapies
across diverse cancer populations. It is important to note that
research on certain types of cancer remains limited. Therefore, the
conclusions drawn for these specific cancers should be interpreted
with caution, as they may not be as robust or comprehensive as
those for more extensively studied malignancies.
As a future direction, it would be valuable to
conduct comparative studies between natural and synthetic AXT to
improved understand their respective efficacies and potential
differences in biological activity. Additionally, further research
combining AXT with other bioactive compounds from natural sources
would be beneficial, as these combinations can exhibit synergistic
effects that enhance its overall therapeutic potential. This
synergy can result in superior antioxidant, anti-inflammatory and
anticancer effects compared with synthetic AXT alone. Investigating
these synergistic interactions and exploring the potential of
combining AXT with other naturally occurring compounds could pave
the way for more effective, multi-targeted cancer treatments and
preventive strategies.
Continued research into the bioavailability,
delivery mechanisms and role of AXT in combination therapies could
facilitate its incorporation into clinical oncology, potentially
offering a natural, complementary approach to current cancer
treatments.
Acknowledgements
Authors are grateful to Professor R. Villagonzalo
Rountree for his assistance with English editing.
Funding
Funding: The present study was supported by the Ministry of
University and Research (MUR) as part of the FSE REACT-EU-PON
2014-2020 Research and Innovation resources-Green/Innovation
Action-DM MUR 1062/2021-GrEEnoncoprev. The present study was also
supported by the Department of Medical, Surgical and Advanced
Technologies ‘G.F. Ingrassia’, University of Catania, Italy.
Availability of data and materials
Not applicable.
Authors' contributions
CC and MF contributed to the study design; CF and CS
developed the methodology; CC and MFT were responsible for data
curation and original draft preparation; AG, HGD and GOC reviewed
and edited the manuscript; MF supervised the project and secured
funding for the study. Data authentication is not applicable. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed S, Hasan MM, Heydari M, Rauf A,
Bawazeer S, Abu-Izneid T, Rebezov M, Shariati MA, Daglia M and
Rengasamy KR: Therapeutic potentials of crocin in medication of
neurological disorders. Food Chem Toxicol.
145(111739)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bakac ER, Percin E, Gunes-Bayir A and
Dadak A: A narrative review: The effect and importance of
carotenoids on aging and aging-related diseases. Int J Mol Sci.
24(15199)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferreira YAM, Jamar G, Estadella D and
Pisani LP: Role of carotenoids in adipose tissue through the
AMPK-mediated pathway. Food Funct. 14:3454–3462. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Melo van Lent D, Leermakers ETM, Darweesh
SKL, Moreira EM, Tielemans MJ, Muka T, Vitezova A, Chowdhury R,
Bramer WM, Brusselle GG, et al: The effects of lutein on
respiratory health across the life course: A systematic review.
Clin Nutr ESPEN. 13:e1–e7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sani A, Tajik A, Seiiedi SS, Khadem R,
Tootooni H, Taherynejad M, Sabet Eqlidi N, Alavi Dana SMM and
Deravi N: A review of the anti-diabetic potential of saffron. Nutr
Metab Insights. 15(11786388221095223)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sui J, Guo J, Pan D, Wang Y, Xu Y, Sun G
and Xia H: The efficacy of dietary intake, supplementation, and
blood concentrations of carotenoids in cancer prevention: Insights
from an umbrella meta-analysis. Foods. 13(1321)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ferrante M, Fiore M, Conti GO, Fiore V,
Grasso A, Copat C and Signorelli SS: Transition and heavy metals
compared to oxidative parameter balance in patients with deep vein
thrombosis: A case-control study. Mol Med Rep. 15:3438–3444.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fiore M, Cristaldi A, Okatyeva V, Lo
Bianco S, Oliveri Conti G, Zuccarello P, Copat C, Caltabiano R,
Cannizzaro M and Ferrante M: Dietary habits and thyroid cancer
risk: A hospital-based case-control study in Sicily (South Italy).
Food Chem Toxicol. 146(111778)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fiore M, Barone R, Copat C, Grasso A,
Cristaldi A, Rizzo R and Ferrante M: Metal and essential element
levels in hair and association with autism severity. J Trace Elem
Med Biol. 57(126409)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Phillips CM, Chen LW, Heude B, Bernard JY,
Harvey NC, Duijts L, Mensink-Bout SM, Polanska K, Mancano G,
Suderman M, et al: Dietary inflammatory index and non-communicable
disease risk: A narrative review. Nutrients.
11(1873)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang Y, Kypridemos C, Liu J, Lee Y,
Pearson-Stuttard J, Collins B, Bandosz P, Capewell S, Whitsel L,
Wilde P, et al: Cost-effectiveness of the US food and drug
administration added sugar labeling policy for improving diet and
health. Circulation. 139:2613–2624. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee Y, Mozaffarian D, Sy S, Huang Y, Liu
J, Wilde PE, Abrahams-Gessel S, Jardim TSV, Gaziano TA and Micha R:
Cost-effectiveness of financial incentives for improving diet and
health through medicare and medicaid: A microsimulation study. PLoS
Med. 16(e1002761)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chekanov K: Diversity and distribution of
carotenogenic Algae in Europe: A review. Mar Drugs.
21(108)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elbahnaswy S and Elshopakey GE: Recent
progress in practical applications of a potential carotenoid
astaxanthin in aquaculture industry: A review. Fish Physiol
Biochem. 50:97–126. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Azam MS, Choi J, Lee MS and Kim HR:
Hypopigmenting effects of brown algae-derived phytochemicals: A
review on molecular mechanisms. Mar Drugs. 15(297)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hentati F, Tounsi L, Djomdi D, Pierre G,
Delattre C, Ursu AV, Fendri I, Abdelkafi S and Michaud P: Bioactive
polysaccharides from seaweeds. Molecules. 25(3152)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kiran BR and Venkata Mohan S: Microalgal
cell biofactory-therapeutic, nutraceutical and functional food
applications. Plants (Basel). 10(836)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ren Y, Deng J, Huang J, Wu Z, Yi L, Bi Y
and Chen F: Using green alga Haematococcus pluvialis for
astaxanthin and lipid co-production: Advances and outlook.
Bioresour Technol. 340(125736)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mularczyk M, Michalak I and Marycz K:
Astaxanthin and other nutrients from Haematococcus
pluvialis-multifunctional applications. Mar Drugs.
18(459)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Davinelli S, Nielsen ME and Scapagnini G:
Astaxanthin in skin health, repair, and disease: A comprehensive
review. Nutrients. 10(522)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Basiony M, Ouyang L, Wang D, Yu J, Zhou L,
Zhu M, Wang X, Feng J, Dai J, Shen Y, et al: Optimization of
microbial cell factories for astaxanthin production: Biosynthesis
and regulations, engineering strategies and fermentation
optimization strategies. Synth Syst Biotechnol. 7:689–704.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu Y, Shamoto-Nagai M, Maruyama W, Osawa T
and Naoi M: Phytochemicals prevent mitochondrial membrane
permeabilization and protect SH-SY5Y cells against apoptosis
induced by PK11195, a ligand for outer membrane translocator
protein. J Neural Transm (Vienna). 124:89–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ostrom QT, Patil N, Cioffi G, Waite K,
Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2013-2017. Neuro Oncol. 22 (Suppl 2):iv1–iv96.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shin J, Nile A, Saini RK and Oh JW:
Astaxanthin sensitizes low SOD2-expressing GBM cell lines to TRAIL
treatment via pathway involving mitochondrial membrane
depolarization. Antioxidants (Basel). 11(375)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shin J, Saini RK and Oh JW: Low dose
astaxanthin treatments trigger the hormesis of human astroglioma
cells by up-regulating the cyclin-dependent kinase and
down-regulated the tumor suppressor protein P53. Biomedicines.
8(434)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patel M, Singh P, Gandupalli L and Gupta
R: Identification and evaluation of survival-associated common
chemoresistant genes in cancer. Biomed Biotechnol Res J. 8:320–327.
2024.
|
|
28
|
Tsuji S, Nakamura S, Maoka T, Yamada T,
Imai T, Ohba T, Yako T, Hayashi M, Endo K, Saio M, et al:
Antitumour effects of astaxanthin and adonixanthin on glioblastoma.
Mar Drugs. 18(474)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zheng M, Kumar A, Sharma V, Behl T, Sehgal
A, Wal P, Shinde NV, Kawaduji BS, Kapoor A, Anwer MK, et al:
Revolutionizing pediatric neuroblastoma treatment: Unraveling new
molecular targets for precision interventions. Front Cell Dev Biol.
12(1353860)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun SQ, Du FX, Zhang LH, Hao-Shi Gu FY,
Deng YL and Ji YZ: Prevention of STAT3-related pathway in SK-N-SH
cells by natural product astaxanthin. BMC Complement Med Ther.
23(430)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang
J, Yang S and Wang Y: Astaxanthin as a potential neuroprotective
agent for neurological diseases. Mar Drugs. 13:5750–5766.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
García F, Lobos P, Ponce A, Cataldo K,
Meza D, Farías P, Estay C, Oyarzun-Ampuero F, Herrera-Molina R,
Paula-Lima A, et al: Astaxanthin counteracts excitotoxicity and
reduces the ensuing increases in calcium levels and mitochondrial
reactive oxygen species generation. Mar Drugs.
18(335)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang J, Ding C, Zhang S and Xu Y:
Neuroprotective effects of astaxanthin against oxygen and glucose
deprivation damage via the PI3K/Akt/GSK3β/Nrf2 signalling pathway
in vitro. J Cell Mol Med. 24:8977–8985. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yan T, Ding F, Zhang Y, Wang Y, Wang Y,
Zhang Y, Zhu F, Zhang G, Zheng X, Jia G, et al: Astaxanthin
inhibits H2O2-induced excessive mitophagy and
apoptosis in SH-SY5Y cells by regulation of Akt/mTOR activation.
Mar Drugs. 22(57)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Korczowska-Łącka I, Słowikowski B, Piekut
T, Hurła M, Banaszek N, Szymanowicz O, Jagodziński PP, Kozubski W,
Permoda-Pachuta A and Dorszewska J: Disorders of endogenous and
exogenous antioxidants in neurological diseases. Antioxidants
(Basel). 12(1811)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Łukasiewicz S, Czeczelewski M, Forma A,
Baj J, Sitarz R and Stanisławek A: Breast cancer-epidemiology, risk
factors, classification, prognostic markers, and current treatment
strategies-an updated review. Cancers (Basel).
13(4287)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ahn YT, Kim MS, Kim YS and An WG:
Astaxanthin reduces stemness markers in BT20 and T47D breast cancer
stem cells by inhibiting expression of pontin and mutant p53. Mar
Drugs. 18(577)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Badak B, Aykanat NEB, Kacar S, Sahinturk
V, Arik D and Canaz F: Effects of astaxanthin on metastasis
suppressors in ductal carcinoma. A preliminary study. Ann Ital
Chir. 92:565–574. 2021.PubMed/NCBI
|
|
39
|
Karimian A, Mir Mohammadrezaei F,
Hajizadeh Moghadam A, Bahadori MH, Ghorbani-Anarkooli M, Asadi A
and Abdolmaleki A: Effect of astaxanthin and melatonin on cell
viability and DNA damage in human breast cancer cell lines. Acta
Histochem. 124(151832)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim MS, Ahn YT, Lee CW, Kim H and An WG:
Astaxanthin modulates apoptotic molecules to induce death of SKBR3
breast cancer cells. Mar Drugs. 18(266)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu Z, Long Y, Li J, Li J, Ren K, Zhao W,
Wang X, Xia C, Wang Y, Li M, et al: Simultaneous inhibition of
breast cancer and its liver and lung metastasis by blocking
inflammatory feed-forward loops. J Control Release. 338:662–679.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
McCall B, McPartland CK, Moore R,
Frank-Kamenetskii A and Booth BW: Effects of astaxanthin on the
proliferation and migration of breast cancer cells in vitro.
Antioxidants (Basel). 7(135)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shokrian Zeini M, Pakravesh SM, Jalili
Kolour SM, Soghala S, Dabbagh Ohadi MA, Ghanbar Ali Akhavan H,
Sayyahi Z, Mahya L, Jahani S, Shojaei Baghini S, et al: Astaxanthin
as an anticancer agent against breast cancer: An in vivo and in
vitro investigation. Curr Med Chem: Apr 17, 2024 (Epub ahead of
print).
|
|
44
|
Vijay K, Sowmya PRR, Arathi BP, Shilpa S,
Shwetha HJ, Raju M, Baskaran V and Lakshminarayana R: Low-dose
doxorubicin with carotenoids selectively alters redox status and
upregulates oxidative stress-mediated apoptosis in breast cancer
cells. Food Chem Toxicol. 118:675–690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Malhão F, Ramos AA, Macedo AC and Rocha E:
Cytotoxicity of seaweed compounds, alone or combined to reference
drugs, against breast cell lines cultured in 2D and 3D. Toxics.
9(24)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sowmya PRR, Arathi BP, Vijay K, Baskaran V
and Lakshminarayana R: Astaxanthin from shrimp efficiently
modulates oxidative stress and allied cell death progression in
MCF-7 cells treated synergistically with β-carotene and lutein from
greens. Food Chem Toxicol. 106:58–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yuri T, Yoshizawa K, Emoto Y, Kinoshita Y,
Yuki M and Tsubura A: Effects of dietary xanthophylls,
canthaxanthin and astaxanthin on N-Methyl-N-nitrosourea-induced rat
mammary carcinogenesis. In Vivo. 30:795–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fouad MA, Sayed-Ahmed MM, Huwait EA, Hafez
HF and Osman AMM: Epigenetic immunomodulatory effect of eugenol and
astaxanthin on doxorubicin cytotoxicity in hormonal positive breast
Cancer cells. BMC Pharmacol Toxicol. 22(8)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Atalay PB, Kuku G and Tuna BG: Effects of
carbendazim and astaxanthin co-treatment on the proliferation of
MCF-7 breast cancer cells. In Vitro Cell Dev Biol Anim. 55:113–119.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bharathiraja S, Manivasagan P, Quang Bui
N, Oh YO, Lim IG, Park S and Oh J: Cytotoxic induction and
photoacoustic imaging of breast cancer cells using
astaxanthin-reduced gold nanoparticles. Nanomaterials (Basel).
6(78)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kulpreechanan N and Sorasitthiyanukarn FN:
Astaxanthin-loaded chitosan oligosaccharide/alginate nanoparticles:
Exploring the anti-inflammatory and anticancer potential as a
therapeutic nutraceutical: An in vitro study. Res J Pharm Technol.
16:5378–5383. 2023.
|
|
52
|
Burz C, Pop V, Silaghi C, Lupan I and
Samasca G: Prognosis and treatment of gastric cancer: A 2024
update. Cancers (Basel). 16(1708)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li H, Shen M and Wang S: Current therapies
and progress in the treatment of advanced gastric cancer. Front
Oncol. 14(1327055)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Global Burden of Disease Liver Cancer
Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu
MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The
burden of primary liver cancer and underlying etiologies from 1990
to 2015 at the global, regional, and national level: Results from
the global burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mazzaferro V, Citterio D, Bhoori S,
Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M,
Romagnoli R, Antonelli B, et al: Liver transplantation in
hepatocellular carcinoma after tumour downstaging (XXL): A
randomised, controlled, phase 2b/3 trial. Lancet Oncol. 21:947–956.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shao Y, Ni Y, Yang J, Lin X, Li J and
Zhang L: Astaxanthin inhibits proliferation and induces apoptosis
and cell cycle arrest of mice H22 hepatoma cells. Med Sci Monit.
22:2152–2160. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang X, Li W, Dou X, Nan D and He G:
Astaxanthin encapsulated in biodegradable calcium alginate
microspheres for the treatment of hepatocellular carcinoma in
vitro. Appl Biochem Biotechnol. 191:511–527. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Messina CM, Manuguerra S, Renda G and
Santulli A: Biotechnological applications for the sustainable use
of marine by-products: In vitro antioxidant and pro-apoptotic
effects of astaxanthin extracted with supercritical CO2
from parapeneus longirostris. Mar Biotechnol (NY). 21:565–576.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li J, Dai W, Xia Y, Chen K, Li S, Liu T,
Zhang R, Wang J, Lu W, Zhou Y, et al: Astaxanthin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells via inhibition of Nf-Κb P65 and Wnt/Β-catenin in
vitro. Mar Drugs. 13:6064–6081. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yadav K, Saxena A, Gupta M, Saha B, Sarwat
M and Rai MP: Comparing pharmacological potential of freshwater
microalgae carotenoids towards antioxidant and anti-proliferative
activity on liver cancer (HUH7) cell line. Appl Biochem Biotechnol.
196:2053–2066. 2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ren P, Yu X, Yue H, Tang Q, Wang Y and Xue
C: Dietary supplementation with astaxanthin enhances anti-tumor
immune response and aids the enhancement of molecularly targeted
therapy for hepatocellular carcinoma. Food Funct. 14:8309–8320.
2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ren P, Tang Q, He X, Xu J, Wang Y and Xue
C: Astaxanthin augmented the anti-hepatocellular carcinoma efficacy
of sorafenib through the inhibition of the JAK2/STAT3 signaling
pathway and mitigation of hypoxia within the tumor
microenvironment. Mol Nutr Food Res. 68(e2300569)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JCY, et al: Global prevalence of Helicobacter pylori
infection: Systematic review and meta-analysis. Gastroenterology.
153:420–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim SH and Kim H: Transcriptome analysis
of the inhibitory effect of astaxanthin on Helicobacter
pylori-induced gastric carcinoma cell motility. Mar Drugs.
18(365)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lee H, Lim JW and Kim H: Effect of
astaxanthin on activation of autophagy and inhibition of apoptosis
in Helicobacter pylori-infected gastric epithelial cell line
AGS. Nutrients. 12(1750)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kim SH and Kim H: Inhibitory effect of
astaxanthin on gene expression changes in Helicobacter
pylori-infected human gastric epithelial cells. Nutrients.
13(4281)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lee J, Lim JW and Kim H: Astaxanthin
inhibits matrix metalloproteinase expression by suppressing
PI3K/AKT/mTOR activation in Helicobacter pylori-infected
gastric epithelial cells. Nutrients. 14(3427)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Woo J, Lim JW and Kim H: Astaxanthin
inhibits integrin α5 expression by suppressing activation of
JAK1/STAT3 in Helicobacter pylori-stimulated gastric
epithelial cells. Mol Med Rep. 27(127)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kim JH, Park JJ, Lee BJ, Joo MK, Chun HJ,
Lee SW and Bak YT: Astaxanthin inhibits proliferation of human
gastric cancer cell lines by interrupting cell cycle progression.
Gut Liver. 10:369–374. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kim S, Lee H, Lim JW and Kim H:
Astaxanthin induces NADPH oxidase activation and
receptor-interacting protein kinase 1-mediated necroptosis in
gastric cancer AGS cells. Mol Med Rep. 24(837)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Han H, Lim JW and Kim H: Astaxanthin
inhibits Helicobacter pylori-induced inflammatory and
oncogenic responses in gastric mucosal tissues of mice. J Cancer
Prev. 25:244–251. 2020.PubMed/NCBI View Article : Google Scholar
|