Introduction
Cervical cancer is a public health problem and is
the fourth most prevalent cancer in women worldwide (1). Most women diagnosed in middle- and
low-income countries are in advanced stages. The treatment and
outcome of cervical cancer are determined by the clinical stage of
the patient, which is classified according to the criteria of the
International Federation of Gynecology and Obstetrics (FIGO)
(2). Early stages are commonly
treated with surgery, while for locally advanced cancer the
treatment is concurrent chemoradiation (3). Patients with locally advanced cervical
cancer that respond to concurrent chemoradiation therapy have
increased numbers of tumor-infiltrating lymphocytes and those with
no response present increased immune reactivity to programmed cell
death receptor-ligand 1 (PD-L1) (4).
For patients with cervical cancer with metastasis
and recurrence, the alternative treatment is immunotherapy.
Anti-programmed cell death receptor 1 (PD-1) is the most used
treatment and the clinical response to anti-PD-1 varies among
different monoclonal anti-PD-1 antibodies. Other options have been
evaluated as combination therapies and improved the treatment
response (5). Patients who received
anti-PD1 and anti-cytotoxic T-lymphocyte associated protein 4
bispecific antibodies and expressed PD-L1 in tumors had an improved
objective response rate compared with those who were negative for
PD-L1 (5,6).
PD-1 and PD-L1 are transmembrane proteins: PD-1 is a
receptor, and its ligand is PD-L1. PD-1 is a negative regulator of
T cells and is involved in the maintenance of immune tolerance
through interaction with its ligand PD-L1(7). Tumor cells overexpress PD-L1 to escape
immune surveillance (8), therefore
the binding of PD-L1 to PD-1 inhibits T-cell activation, playing a
negative role in the anticancer immune response (9). It has been reported that genetic
aberration modifies PD-L1 expression in cancer cells. PD-L1 maps to
chromosome 9p24.1 and amplification of this locus has been reported
in nodular sclerosing Hodgkin lymphoma. In primary mediastinal
large B-cell lymphomas, renal cell carcinoma, breast cancer and
non-small cell lung cancer, among others, this amplification has
been associated with increased PD-L1 expression (10-14).
PD-1 and PD-L1 were initially identified as membrane
proteins but also found in blood circulation. Soluble immune
checkpoints (sICs) are relevant in cancer. Soluble (s)PD-1 and
sPD-L1 can be released into the circulation because of alternative
splicing, which generates protein variants that lack the
transmembrane domain, or by proteolytic cleavage in the cell
membrane (15-17).
PD-1 is primarily expressed by T-cells, but also by
B cells, natural killer cells, dendritic cells and monocytes. On
the other hand, PD-L1 can be expressed by tumor cells,
cancer-associated fibroblasts and less frequently by epithelial and
endothelial cells, immune cells such as antigen-presenting cells,
activated T and B cells, dendritic cells, monocytes and macrophage
lineage cells (16,18-21).
Therefore, the origin of these soluble proteins in circulation
could be of different cell types and the changes in the levels of
these soluble proteins could be a consequence of changes in their
expression or their release from the cells that express them. sPD-1
has been related to higher expression of the splice variant that
encodes the soluble form (17). For
PD-L1, it has been proposed that increased expression of the
metalloproteases that mediate its proteolytic cleavage of the
membrane could increase the levels of the sPD-L1. In relation to
this, increased levels of sPD-L1 have been associated with higher
expression of these metalloproteases (15,22).
But the exact origin of the soluble forms is not enough
documented.
sPD-1 and sPD-L1 have been evaluated in some cancer
types, and the results revealed that, in most cancers, high sPD-1
and sPD-L1 levels are related to poor prognosis. In Hodgkin
lymphoma, lower levels of sPD-L1 are associated with decreased
progression-free survival (23). In
patients with bladder cancer, higher levels of sPDL-1 were
associated with poorer prognosis, independent of the treatment
method, whether chemotherapy or immune checkpoint inhibitors
(24). In pancreatic adenocarcinoma
and gastrointestinal stromal tumors, higher levels of both sPD-L1
and sPD-L1 correlate with poor survival (25,26).
For patients with hepatocellular carcinoma, sPD-L1 and sPD-1 are,
respectively, a negative and a positive prognostic factor, showing
different behaviors, as has been reported in other cancer types
(27).
In patients with locally advanced cervical cancer,
the effect of concurrent chemoradiotherapy on the serum levels of
sPD-1 and sPD-1 was evaluated, and the results revealed that
treatment increased the levels of sPD-1 and sPD-L1, but there is no
information on the possible role of these changes in the immune
response (28). At present, the
relationships between the serum levels of sPD-1 and sPD-L1 and
clinicopathological characteristics and the clinical outcomes of
cancer remain unclear. Therefore, the current study provides
information about the potential use of sPD-1 and sPD-L1 as
prognostic biomarkers of cervical cancer and their relationships
with clinical outcomes.
Materials and methods
Patients
The present investigation was a prospective and
observational study performed on a cohort of 194 patients diagnosed
with cervical cancer at the Radiotherapy Service of the National
Health Centre, Manuel Avila Camacho, from the Mexican Institute of
Social Security (IMSS; Puebla, Mexico) from November 2017 to
October 2023. Patients included in the study were diagnosed with
cervical cancer by a pathologist and were staged according to the
FIGO system (Stage I, II, III and IV) (2). Patients had not received any previous
treatment. The exclusion criteria included previous or concurrent
cancer, pregnancy, autoimmune sickness or the presence of acute
infection.
The tumor histological types included in the study
were squamous cell carcinoma (SCC), adenocarcinoma (AC) and
adenosquamous cell carcinoma (ASC). The treatment received by the
patients during the study was chemo-radiotherapy, which was
determined according to their clinical characteristics. No
alternative treatment, such as anti-PD-L1, was applied to the
patients.
The control group included 40 women whose cytology
reports were negative for intraepithelial lesions or malignancy.
The exclusion criteria were pregnancy, autoimmune sickness or the
presence of acute infection.
The mean age of patients with cervical cancer was
53.24 years (range, 26-87 years). The mean age of the control group
was 31.7 years (range, 21-60 years).
The present study was conducted in accordance with
the Declaration of Helsinki and the protocol was approved (approval
no. R-2023-2106-004) by the Local Ethics Committee of the IMSS
(Puebla, Mexico). All the women included in the study signed a
consent form before sample collection.
Sample collection
Serum was obtained through phlebotomy, centrifuged
at 4,000 x g for 10 min at 4˚C and stored in aliquots at -20˚C
until use. Demographic data, pathological diagnosis, histological
type, tumor differentiation grade, clinical stage and clinical
outcome (total remission of disease or death) were collected from
the clinical records.
Serum sPD-1 and sPD-L1 ELISA
To determine the sPD-1 serum concentration, the
Quantikine ELISA Human PD1 Kit (cat. no. DPD10; R&D Systems,
Inc.) was used. Serum PD-L1 levels were determined via a
PD-L1/B7-H1 Quantikine ELISA kit (cat. no. DB7H10; R&D Systems,
Inc.). No serum dilution was performed and each sample was analyzed
in duplicate. The absorbance of the plates was read in a BioTek
Synergy-4 plate reader (Agilent Technologies, Inc.) at 450 nm and
the absorbance at 570 nm was subtracted. The average of the
duplicates of each sample was calculated and the sPD-L1 and sPD-L1
concentrations were calculated via a standard curve provided with
the kit.
Statistical analysis
The Mann-Whitney test was performed to compare the
serum levels of sPD-1 and sPD-L1 in the control and cervical cancer
groups, between the keratinizing types and between the clinical
outcomes. The Kruskal-Wallis test followed by Dunn's test was
performed to compare the serum levels of sPD-1 and sPD-L1 between
the different clinicopathological characteristics, such as
histological type, differentiation grade and clinical stage. Data
are presented as the medians and ranges. P≤0.05 was considered to
indicate a statistically significant difference.
Additionally, Spearman's correlation analysis was
used to analyze the correlation between sPD-1 and sPD-L1
concentrations. Statistical analyses were performed using GraphPad
Prism version 10.2.3 (Dotmatics) for Windows.
Receiver operating characteristic (ROC) curve
analysis was performed to determine the diagnostic value of sPD-1
and sPD-L1. In this ROC curve, the point with the maximum
sensitivity and specificity was selected as the cut-off value. ROC
curve analysis was performed with the method of DeLong et al
(29) using MedCalc software
version 22.030 (MedCalc software, Ltd.).
Results
Patient characteristics
The clinicopathological characteristics considered
in the present study were histological type, keratinizing,
differentiation grade, clinical stage and clinical outcome (total
remission and death). The numbers of patients included in the
different clinicopathological groups are shown in Table I.
 | Table IClinicopathological characteristics of
patients with cervical cancer. |
Table I
Clinicopathological characteristics of
patients with cervical cancer.
| Characteristics | Number |
|---|
| Histological
type | SCC | AC | ASC |
| | 156 | 28 | 10 |
| Keratinizing
type | Keratinizing |
Non-Keratinizing |
| | 77 | 30 |
| Differentiation
grade | G1 | G2 | G3 |
| | 11 | 101 | 47 |
| Clinical stage | I | II | III | IV |
| | 17 | 68 | 68 | 24 |
| Clinical
outcome | Total
remission | Deceased |
| | 15 | 7 |
sPD-1 and sPD-L1 concentrations are
increased in patients with cervical cancer
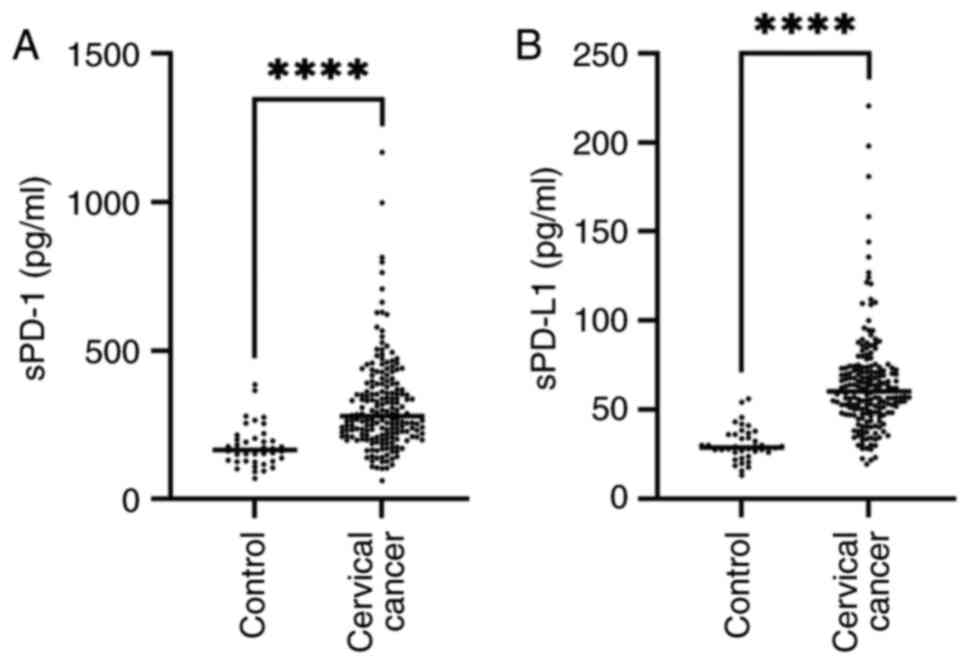
The sPD-1 and sPD-L1 concentrations increased
significantly in the patients with cervical cancer (Fig. 1A and B). The median concentration of sPD-1 in
the control group was 165.3 pg/ml (range, 69.96-385 pg/ml) while it
was 279.4 pg/ml (range, 62.48-1,168.35 pg/ml) in the cervical
cancer group. The median concentration of sPD-L1 in the control
group was 28.88 pg/ml (range, 13.25-56.07 pg/ml), while it was
60.38 pg/ml (range, 19.39-220.4 pg/ml) in the cervical cancer
group. The results showed a significantly increased concentration
of sPD-1 and sPD-L1 in the cervical cancer group P<0.0001.
ROC curve analysis
To determine the diagnostic value of sPD-1 and
sPD-L1, ROC curve analysis was performed. The specificity,
sensitivity, area under the curve (AUC) and cut-off values are
shown in Fig. 2A and B. sPD-1 was a favorable discriminator for
patients with cervical cancer with a sensitivity of 0.79 and a
specificity of 0.775; however, sPD-L1 proved superior,
demonstrating a higher sensitivity of 0.80 and a higher specificity
of 0.95. These results support the fact that important changes in
sPD-1 and sPD-L1 levels occur during the development of cervical
cancer, which allows them to be distinguished from healthy
women.
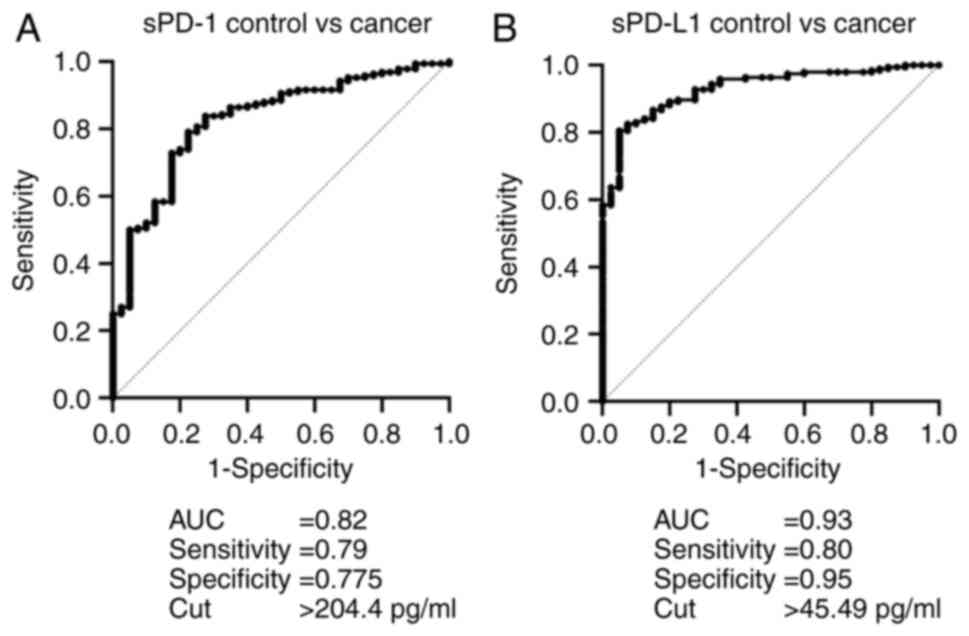 | Figure 2ROC analysis of sPD-1 and sPD-L1 in
the control vs. cervical cancer groups. (A) ROC curve of sPD1 of
the control group (n=40) compared with the cervical cancer group
(n=192); the AUC, sensitivity, specificity, the Youden index and
cut-off value are shown. (B) ROC curve of sPD-L1 of the control
group (n=40) compared with the cervical cancer group (n=194); the
AUC, sensitivity, specificity, the Youden index and cut-off value
are shown. ROC, receiver operating characteristic; AUC, area under
the curve; s, soluble; PD-L1, programmed cell death receptor-ligand
1; PD-1, programmed cell death protein 1. |
Relationships of sPD-1 and sPD-L1
concentrations with clinicopathological parameters
sPD-1 and sPD-L1 concentrations were not associated
with histological type, keratinizing type or differentiation grade.
The median concentration of sPD-1 was 269 pg/ml for patients with
SCC, 281.2 pg/ml for patients with AC and 289.83 pg/ml for patients
with ASC (P=0.9143). The median concentration of sPD-L1 was 59.82
pg/ml for patients with SCC, 62.5 pg/ml for patients with AC and
59.22 pg/ml for patients with ASC (P=0.84497). For the keratinizing
type, the median sPD-1 concentration was 284.2 pg/ml while 278.4
pg/ml for the non-keratinizing type (P=0.9533). For sPD-L1, the
median for the keratinizing type was 56.96 pg/ml while for the
non-keratinizing type was 61.23 pg/ml (P=0.4919). Concerning
differentiation grade, a slight increase was observed in the
concentration of both soluble proteins as tumor differentiation
decreased, although it did not reach significance. The median sPD-1
level was 264.4 pg/ml in the well differentiated (G1) group, 267.9
pg/ml in the moderately differentiated (G2) group and 326.4 pg/ml
in the poorly differentiated and undifferentiated (G3) group
(P=0.3749). The median sPD-L1 concentration was 57 pg/ml in the G1
subgroup, 58.92 pg/ml in the G2 subgroup and 63.79 pg/ml in the G3
subgroup (P=0.5576).
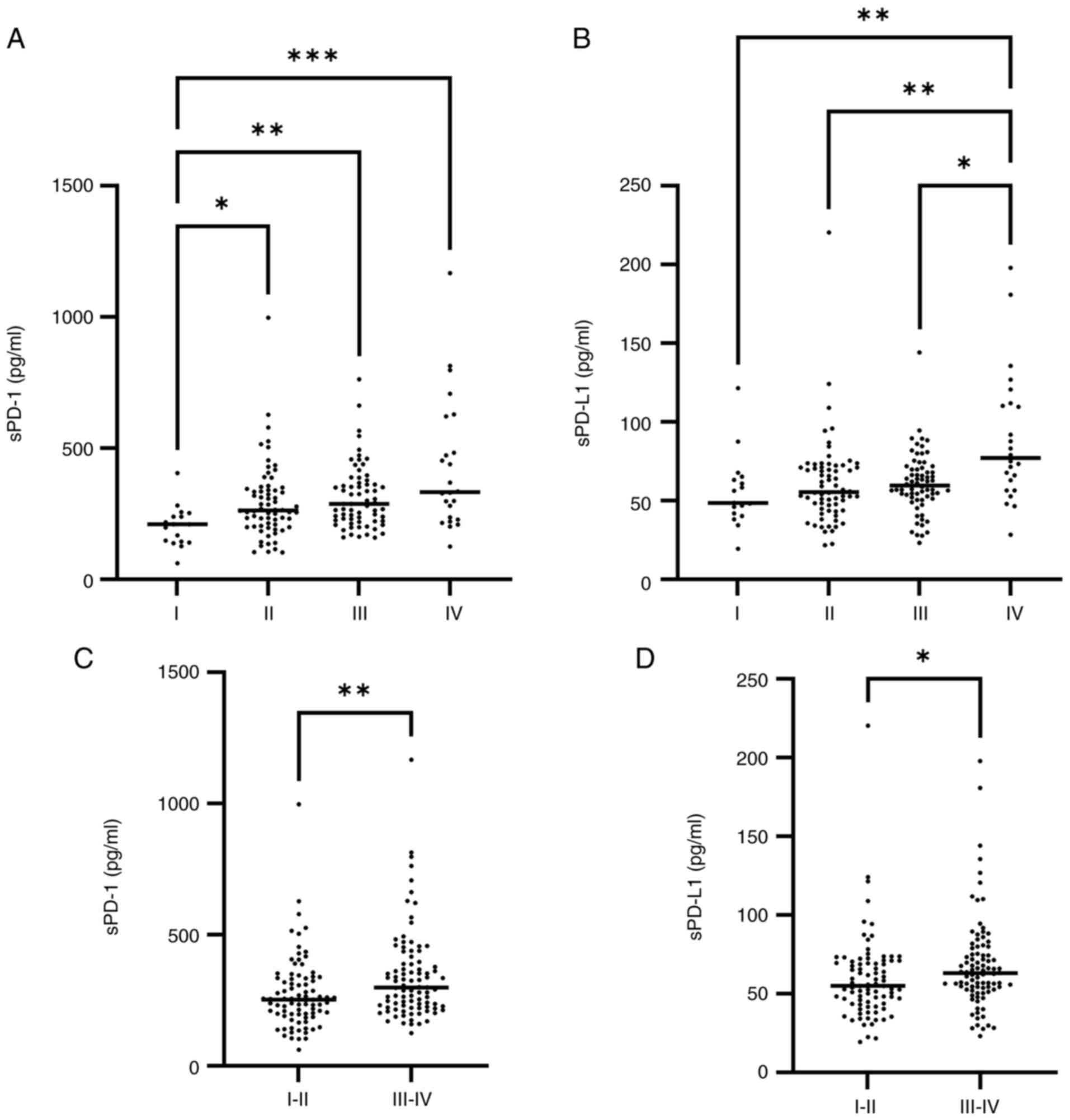
The analysis of sPD-1 concentrations for clinical
stage revealed significantly higher concentrations in patients with
clinical stage II, III and IV than in those with clinical stage I
(Fig. 3A) (P=0.0317, P=0.0025 and
P=0.0001, respectively; Kruskal-Wallis test and Dunn's multiple
comparisons test). The sPD-L1 concentration was also significantly
higher in patients with clinical stage IV disease than in those
with stages I, II and III (Fig. 3B)
(P=0.0026, P=0.0021 and P=0.0135, respectively). Analysis of sPD-1
and sPD-L1 in advanced clinical stages (III and IV) vs. early
clinical stages (I and II) indicated significantly higher levels in
advanced clinical stages (Fig. 3C
and D) (P=0.0025 and P=0.0146,
respectively). It is important to highlight that a gradual increase
was observed as the clinical stage progressed, although there were
no significant differences between all groups.
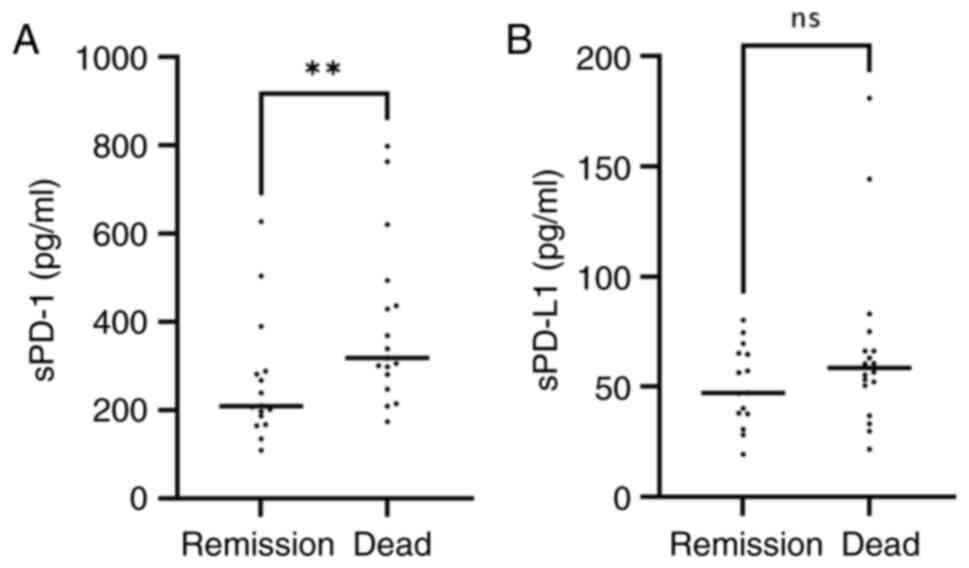
sPD-1 levels are higher in the group
of deceased patients
The clinical outcome (total remission or deceased)
was analyzed in relation to the serum levels of sPD-1 or sPD-L1.
The results revealed an increased concentration of both proteins in
the group of deceased patients. The median sPD-1 level was 209.3
pg/ml (range, 109.5-627.52 pg/ml) for the total remission group,
while it was 318.9 pg/ml (range, 174.19-798.72 pg/ml) for the
deceased patients. For sPD-L1, the median concentration in the
total remission group was 47.27 pg/ml (range, 19.39-80.2 pg/ml),
while, in the deceased group, it was 58.51 pg/ml (range,
21.8-180.87 pg/ml); however, the statistical analysis only showed
significant difference for sPD-1 (P=0.0077) (Fig. 4A and B).
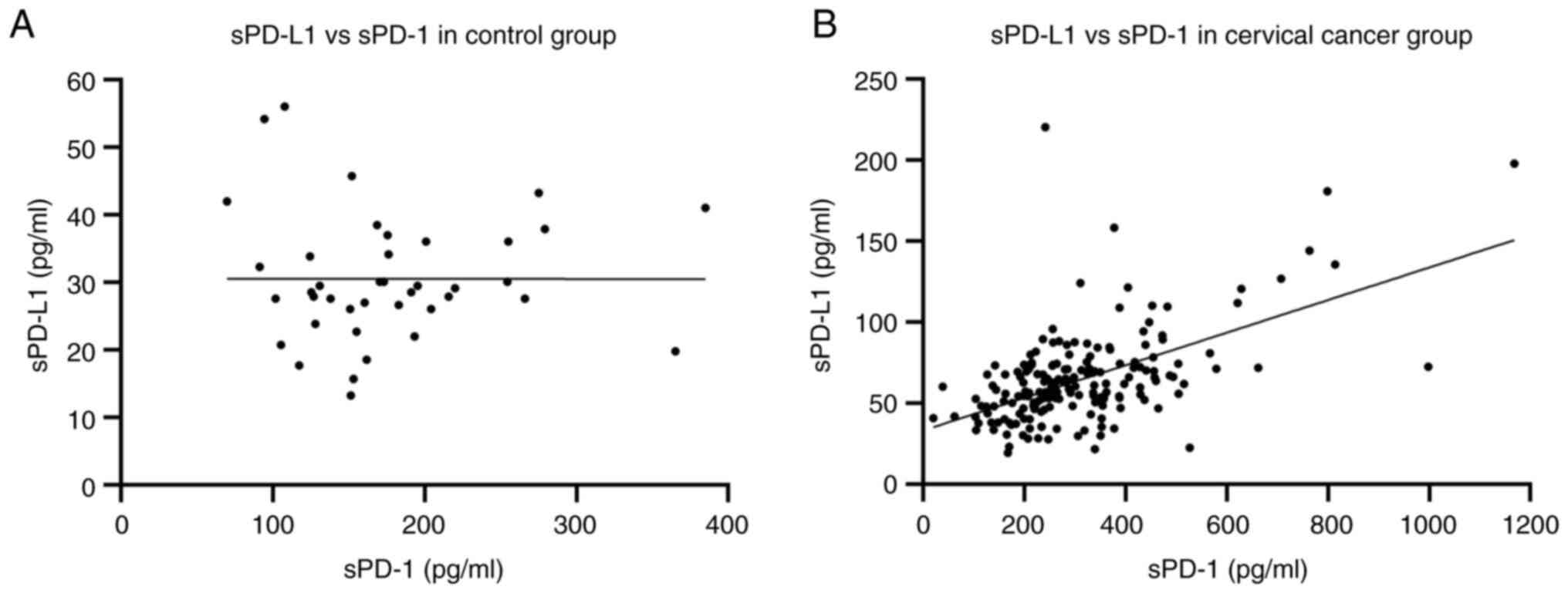
Correlations between sPD-1 and sPD-L1
in the control and cervical cancer groups
To determine whether there was a correlation between
levels of sPD-1 and sPD-L1 in the control and cervical cancer
groups, a Spearman's correlation analysis was performed. The
results revealed no correlation in the control group, with r=0.066
and P=0.6875 (Fig. 5A). In the
cervical cancer group, the results indicated a strong positive
correlation, with r=0.4660 and P<0.0001 (Fig. 5B). The correlation between these
soluble proteins in the cancer group could be related to the role
of immunosuppression which is not present in the control group.
Spearman's correlation of sPD-1 and
sPD-L1 by clinical stage
Additionally, a Spearman's correlation by clinical
stage was performed. The clinical stage I group showed a positive
association (r=0.3676), but this was found non-significant
(P=01471) (Fig. 6A). For patients
at clinical stage II, a moderately positive correlation (r=0.3665)
with P=0.0023 was calculated (Fig.
6B). For patients at clinical stage III, a moderately positive
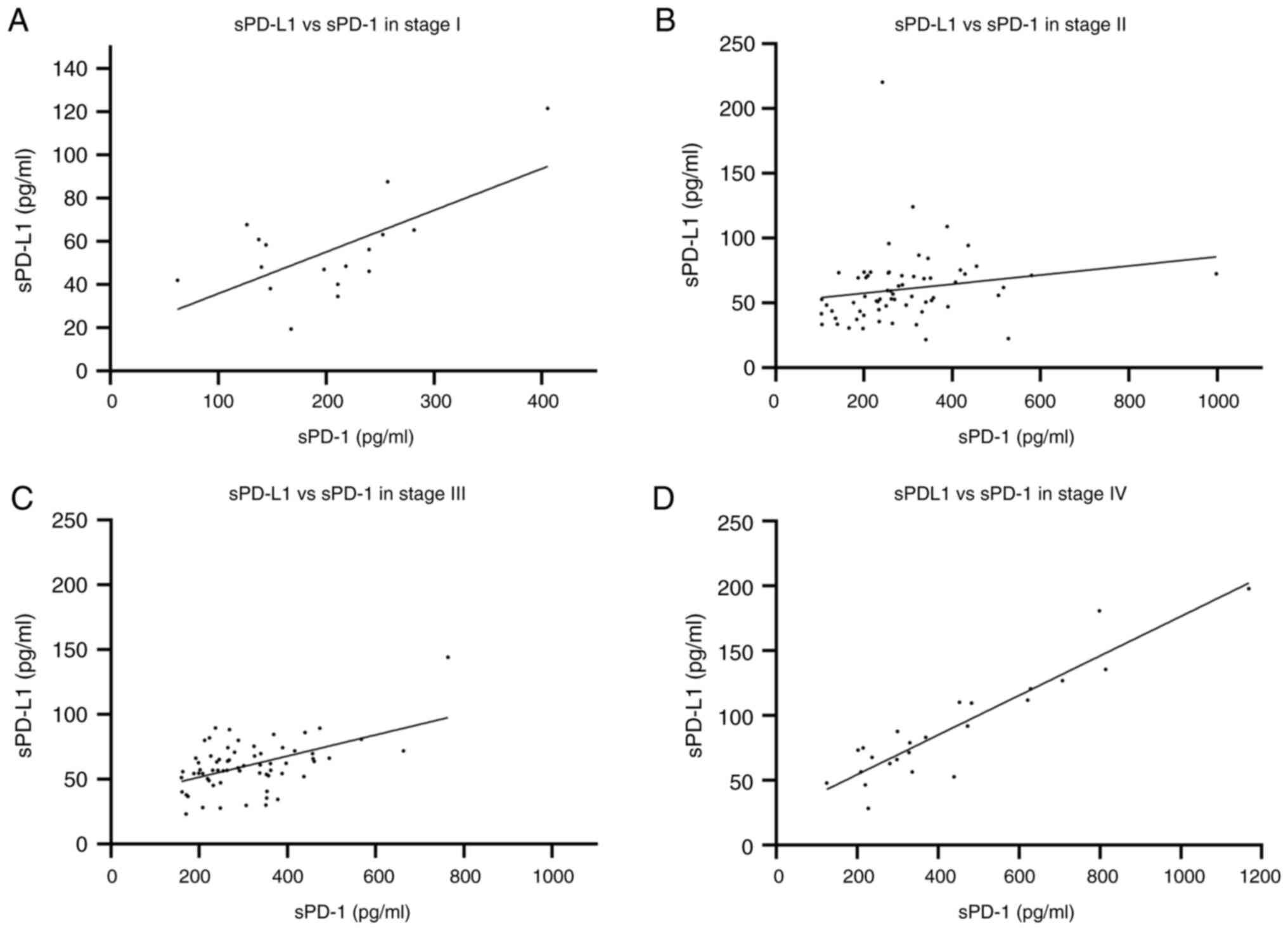
correlation was detected (r=0.3784) with P=0.017 (Fig. 6C). Clinical stage IV disease was
strongly correlated (r=0.8183 and P<0.0001) (Fig. 6D). The correlation of concentration
of sPD-1 and sPD-L1 increased with diseased progression suggesting
a potential role in the disease progression related with greater
immunosuppression.
Discussion
The present study evaluated the serum concentrations
of sPD-1 and sPD-L1 in patients with cervical cancer to determine
their diagnostic and prognostic value. The serum concentrations of
sPD-1 and sPD-L1 were greater in the cervical cancer group than in
the control group. In other cancer types, increased concentrations
of these soluble proteins were reported in patients with cancer
compared with healthy people. sPD-L1 levels are increased in
patients with Hodgkin lymphoma (23). sPD-1 was evaluated in patients with
associated liver diseases and hepatocellular carcinoma, with higher
levels reported in the cancer group (30,31).
In prostate and non-small cell lung cancer, both soluble proteins
were increased (32,33). These results showed that increased
levels of these sICs are related to cancer. The results of the ROC
curve analysis indicated that both soluble proteins are favorable
discriminators for patients with cervical cancer, with greater
specificity and sensitivity for sPD-L1. These results support the
potential use of these proteins as diagnostic markers and suggest a
role in cancer development as they may participate in immune
tolerance; however, these findings must be further explored.
Analysis of clinicopathological characteristics and
the serum concentrations of sPD-1 and sPD-L1 revealed associations
only with the clinical stage. sPD-1 levels were significantly
higher in patients with clinical stage II, III and IV than in those
with clinical stage I disease. On the other hand, sPD-L1 levels
were significantly higher in patients with clinical stage IV than
in those with clinical stage I, II and III. Additionally, when
early stages I and II were grouped and compared with advanced
stages III and IV, a significant increase was observed in the
advanced clinical stages. In other cancer types, such as extranodal
NK/T-cell lymphoma and gastric cancer, higher levels of sPD-L1 are
related to advanced-stage disease; in non-small cell lung cancer,
higher levels of sPD-1 and sPD-L1 are related to advanced clinical
stage but not to other pathological characteristics, such as the
histological type, as observed in the present study (34,35).
The clinical outcomes of patients with total
remission of the disease and those who succumbed were compared and
the levels of sPD-1 were significantly higher in the deceased
group. sPD-1 has shown inconsistent survival predictive value; for
example, in pancreatic cancer, higher levels of sPD-1 are
associated with survival (25); a
higher concentration of sPD-1 in hepatocellular carcinoma is a
favorable independent prognostic factor and in renal cell carcinoma
it is associated with longer progression-free survival (27,36).
It has been suggested that sPD-1 can promote antitumor immunity by
blocking PD-L1 in tumor cells (37); nonetheless, this result is not
supported by the results obtained in the present study; thus, the
role of sPD-1 in cervical cancer progression must be further
explored. For sPD-L1, increased levels were observed in the
deceased group, but the difference was not statistically
significant; however, in different cancer types, such as
hepatocellular carcinoma, gastric, prostate and renal cell
carcinoma, higher levels were associated with improved survival
(27,32,35,36).
Additionally, in some cancer types like gastric cancer, the
patients with tumors expressing PD-L1 showed low overall survival.
On this basis, it can be hypothesized that in cervical cancer,
sPD-L1 could be secreted by tumor cells and the serum levels be
related to an immunosuppressive tumor microenvironment (38). However, a larger sample size could
increase the accuracy of the present results.
In different types of cancer, analogous elevations
in sPD-1 and sPD-L1 were reported; therefore, the present study
determined the correlation of these soluble proteins in the control
and cervical cancer groups. In the control group, no correlation
was observed, while a favorable correlation was observed in the
cervical cancer group. Additionally, the correlation in the
clinical stage IV group was very strong. The authors of the present
study prefer not to hypothesize on the clinical implications of
these results; however, Hayasi et al (16) reported in 2024 that levels of sCIs
such as sPD-1, sPD-L1 and CTL-4 are related to the exhaustion of
antitumor immunity and the response to immunotherapy because higher
levels of sPD-1 and PD-L1 were associated with non-responsiveness
to PD-1/PD-L1 blockade therapy. An increase in sPD-L1 may
strengthen T-cell inhibition, promoting cancer immune evasion;
however, the roles of both soluble proteins must be studied in
patients with cancer.
Serum sPD-1 and sPD-L1 are potentially accessible
biomarkers that could be used in the diagnosis and prognosis of
patients with cervical cancer and for monitoring patients receiving
anti-PD-1 or anti-PD-L1 therapy, highlighting the importance of
studying sICs in patients with cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Mexican
Institute of Social Security (grant no. R-2023-2106-004).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SPP and ICR conducted investigation and data
collection. PMM and VVR analyzed data, and designed figures and
table. VVR and JRL conceptualized the study and wrote the
manuscript. ICR and SPP confirm the authenticity of all the raw
data. All authors drafted the manuscript, and read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
R-2023-2106-004) by the Human Ethics and Local Committee of the
Mexican Institute of Social Security (Puebla, Mexico). Written
informed consent was obtained from all the participants in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohamud A, Høgdall C and Schnack T:
Prognostic value of the 2018 FIGO staging system for cervical
cancer. Gynecol Oncol. 165:506–513. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hsu HC, Li X, Curtin JP, Goldberg JD and
Schiff PB: Surveillance epidemiology and end results analysis
demonstrates improvement in overall survival for cervical cancer
patients treated in the era of concurrent chemoradiotherapy. Front
Oncol. 5(81)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rocha Martins P, Luciano Pereira Morais K,
de Lima Galdino NA, Jacauna A, Paula SOC, Magalhães WCS, Zuccherato
LW, Campos LS, Salles PGO and Gollob KJ: Linking tumor immune
infiltrate and systemic immune mediators to treatment response and
prognosis in advanced cervical cancer. Sci Rep.
13(22634)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li C, Cang W, Gu Y, Chen L and Xiang Y:
The anti-PD-1 era of cervical cancer: Achievement, opportunity, and
challenge. Front Immunol. 14(1195476)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lou H, Cai H, Huang X, Li G, Wang L, Liu
F, Qin W, Liu T, Liu W, Wang ZM, et al: Cadonilimab combined with
chemotherapy with or without bevacizumab as first-line treatment in
recurrent or metastatic cervical cancer (COMPASSION-13): A phase 2
study. Clin Cancer Res. 30:1501–1508. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharpe AH, Wherry EJ, Ahmed R and Freeman
GJ: The function of programmed cell death 1 and its ligands in
regulating autoimmunity and infection. Nat Immunol. 8:239–245.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8(561)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen B, Hu J, Hu X, Chen H, Bao R, Zhou Y,
Ye Y, Zhan M, Cai W, Li H and Li HB: DENR controls JAK2 translation
to induce PD-L1 expression for tumor immune evasion. Nat Commun.
13(2059)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Twa DD, Chan FC, Ben-Neriah S, Woolcock
BW, Mottok A, Tan KL, Slack GW, Gunawardana J, Lim RS, McPherson
AW, et al: Genomic rearrangements involving programmed death
ligands are recurrent in primary mediastinal large B-cell lymphoma.
Blood. 123:2062–2065. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gupta S, Cheville JC, Jungbluth AA, Zhang
Y, Zhang L, Chen YB, Tickoo SK, Fine SW, Gopalan A, Al-Ahmadie HA,
et al: JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell
carcinomas with sarcomatoid transformation: Implications for
clinical management. Mod Pathol. 32:1344–1358. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gupta S, Vanderbilt CM, Cotzia P,
Arias-Stella JA III, Chang JC, Zehir A, Benayed R, Nafa K, Razavi
P, Hyman DM, et al: Next-generation sequencing-based assessment of
JAK2, PD-L1, and PD-L2 copy number alterations at 9p24.1 in breast
cancer: Potential implications for clinical management. J Mol
Diagn. 21:307–317. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ikeda S, Okamoto T, Okano S, Umemoto Y,
Tagawa T, Morodomi Y, Kohno M, Shimamatsu S, Kitahara H, Suzuki Y,
et al: PD-L1 is upregulated by simultaneous amplification of the
PD-L1 and JAK2 genes in non-small cell lung cancer. J Thorac Oncol.
11:62–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dezutter-Dambuyant C, Durand I, Alberti L,
Bendriss-Vermare N, Valladeau-Guilemond J, Duc A, Magron A, Morel
AP, Sisirak V, Rodriguez C, et al: A novel regulation of PD-1
ligands on mesenchymal stromal cells through MMP-mediated
proteolytic cleavage. Oncoimmunology. 5(e1091146)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hayashi H, Chamoto K, Hatae R, Kurosaki T,
Togashi Y, Fukuoka K, Goto M, Chiba Y, Tomida S and Ota T: Soluble
immune checkpoint factors reflect exhaustion of antitumor immunity
and response to PD-1 blockade. J Clin Invest.
134(e168318)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nielsen C, Ohm-Laursen L, Barington T,
Husby S and Lillevang ST: Alternative splice variants of the human
PD-1 gene. Cell Immunol. 235:109–116. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang W and Zhang T: Expression and
analysis of PD-L1 in peripheral blood circulating tumor cells of
lung cancer. Future Oncol. 17:1625–1635. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bailly C, Thuru X and Quesnel B: Soluble
programmed death ligand-1 (sPD-L1): A pool of circulating proteins
implicated in health and diseases. Cancers (Basel).
13(3034)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawasaki K, Noma K, Kato T, Ohara T,
Tanabe S, Takeda Y, Matsumoto H, Nishimura S, Kunitomo T, Akai M,
et al: PD-L1-expressing cancer-associated fibroblasts induce tumor
immunosuppression and contribute to poor clinical outcome in
esophageal cancer. Cancer Immunol Immunother. 72:3787–3802.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hira-Miyazawa M, Nakamura H, Hirai M,
Kobayashi Y, Kitahara H, Bou-Gharios G and Kawashiri S: Regulation
of programmed-death ligand in the human head and neck squamous cell
carcinoma microenvironment is mediated through matrix
metalloproteinase-mediated proteolytic cleavage. Int J Oncol.
52:379–388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo X, Wang J, Jin J, Chen H, Zhen Z,
Jiang W, Lin T, Huang H, Xia Z and Sun X: High serum level of
soluble programmed death ligand 1 is associated with a poor
prognosis in Hodgkin lymphoma. Transl Oncol. 11:779–785.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Krafft U, Olah C, Reis H, Kesch C, Darr C,
Grünwald V, Tschirdewahn S, Hadaschik B, Horvath O, Kenessey I, et
al: High serum PD-L1 levels are associated with poor survival in
urothelial cancer patients treated with chemotherapy and immune
checkpoint inhibitor therapy. Cancers (Basel).
13(2548)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bian B, Fanale D, Dusetti N, Roque J,
Pastor S, Chretien AS, Incorvaia L, Russo A, Olive D and Iovanna J:
Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As,
BTN3A1 and BTLA in patients with pancreatic adenocarcinoma.
Oncoimmunology. 8(e1561120)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fanale D, Incorvaia L, Badalamenti G, De
Luca I, Algeri L, Bonasera A, Corsini LR, Brando C, Russo A,
Iovanna JL, et al: Prognostic role of plasma PD-1, PD-L1,
pan-BTN3As and BTN3A1 in patients affected by metastatic
gastrointestinal stromal tumors: Can immune checkpoints act as a
sentinel for short-term survival? Cancers (Basel).
13(2118)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang B, Huang T, Wei H, Shen L, Zhu D, He
W, Chen Q, Zhang H, Li Y, Huang R, et al: The correlation and
prognostic value of serum levels of soluble programmed death
protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in
patients with hepatocellular carcinoma. Cancer Immunol Immunother.
68:353–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu C, Li X, Li A, Zou W, Huang R, Hu X,
Yu J, Zhang X and Yue J: Concurrent chemoradiotherapy increases the
levels of soluble immune checkpoint proteins in patients with
locally advanced cervical cancer. J Immunol Res.
2022(9621466)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
30
|
Cheng HY, Kang PJ, Chuang YH, Wang YH, Jan
MC, Wu CF, Lin CL, Liu CJ, Liaw YF, Lin SM, et al: Circulating
programmed death-1 as a marker for sustained high hepatitis B viral
load and risk of hepatocellular carcinoma. PLoS One.
9(e95870)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li N, Zhou Z, Li F, Sang J, Han Q, Lv Y,
Zhao W, Li C and Liu Z: Circulating soluble programmed death-1
levels may differentiate immune-tolerant phase from other phases
and hepatocellular carcinoma from other clinical diseases in
chronic hepatitis B virus infection. Oncotarget. 8:46020–46033.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zvirble M, Survila Z, Bosas P,
Dobrovolskiene N, Mlynska A, Zaleskis G, Jursenaite J, Characiejus
D and Pasukoniene V: Prognostic significance of soluble PD-L1 in
prostate cancer. Front Immunol. 15(1401097)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ancın B, Özercan MM, Yılmaz YM, Uysal S,
Kumbasar U, Sarıbaş Z, Dikmen E, Doğan R and Demircin M: The
correlation of serum sPD-1 and sPD-L1 levels with clinical,
pathological characteristics and lymph node metastasis in nonsmall
cell lung cancer patients. Turk J Med Sci. 52:1050–1057.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li JW, Wei P, Guo Y, Shi D, Yu BH, Su YF,
Li XQ and Zhou XY: Clinical significance of circulating exosomal
PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma,
nasal-type. Am J Cancer Res. 10:4498–4512. 2020.PubMed/NCBI
|
|
35
|
Chivu-Economescu M, Herlea V, Dima S,
Sorop A, Pechianu C, Procop A, Kitahara S, Necula L, Matei L, Dragu
D, et al: Soluble PD-L1 as a diagnostic and prognostic biomarker in
resectable gastric cancer patients. Gastric Cancer. 26:934–946.
2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Incorvaia L, Fanale D, Badalamenti G,
Porta C, Olive D, De Luca I, Brando C, Rizzo M, Messina C, Rediti
M, et al: Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1
predict response to nivolumab treatment in patients with metastatic
renal cell carcinoma: A step toward a biomarker for therapeutic
decisions. Oncoimmunology. 9(1832348)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qin YE, Tang WF, Xu Y, Wan FR and Chen AH:
Ultrasound-mediated co-delivery of miR-34a and sPD-1 complexed with
microbubbles for synergistic cancer therapy. Cancer Manag Res.
12:2459–2469. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hassen G, Kasar A, Jain N, Berry S, Dave
J, Zouetr M, Priyanka Ganapathiraju VLN, Kurapati T, Oshai S, Saad
M, et al: Programmed death-ligand 1 (PD-L1) positivity and factors
associated with poor prognosis in patients with gastric cancer: An
umbrella meta-analysis. Cureus. 14(e23845)2022.PubMed/NCBI View Article : Google Scholar
|