Introduction
Periodontal diseases present a notable challenge in oral health management, demanding continual exploration and assessment of potential therapeutic interventions. Periodontal disease is characterized by several different forms, with two of the more common forms being gingivitis and periodontitis (1), as well as a number of symptoms, including deep periodontal pockets (2). Increasing evidence indicates that systemic conditions, including cardiovascular disease and diabetes, are connected to periodontal diseases (3).
Several studies have assessed plant-based components, including garlic, for preventing and treating periodontal diseases, and they have reported that herbal extracts and polyphenols are effective when used as mouthwashes or toothpastes in oral care (4). Garlic is a plant in the Allium family, which has historically been used throughout the world for centuries as a remedy, including by the Ancient Greeks, Romans, Chinese, Egyptians and Japanese (5). Aged garlic extract (AGE) is an odorless compound derived from the prolonged extraction of fresh garlic at room temperature (6). It is available both biologically and commercially, and has exhibited substantial biological activity in both animals and humans, with numerous studies exploring its potent antioxidant capabilities and its possible role in promoting health-by reducing oxidative stress (7), prevention of cardiovascular disease (8), protection from oxidative injury and pathogenesis of some diseases (9).
The results of our previous study demonstrated that regular intake of AGE can improve oral health by markedly reducing gingival inflammation and bleeding (10). Furthermore, AGE is potentially effective in treating periodontitis in the long term by decrease measure of periodontitis such as probing pocket depth (PPD) (11). The present study aimed to evaluate the impact of AGE on periodontal pockets and assess the potential benefits of AGE in the treatment of periodontitis.
Patients and methods
Study design
The present study was a randomized, controlled, examiner-blind trial with four parallel treatment groups. The study was performed from April 2021 to September 2022 at the Faculty of Dental Medicine of the Hebrew University and Hadassah Ein Kerem Hospital (Jerusalem, Israel). A comprehensive screening process was employed to enroll 300 generally healthy adult volunteers presenting with moderate to severe periodontal pockets. Participants were classified and randomly assigned in equal numbers to three treatment groups with varying doses of AGE, or to a control group (placebo). The clinical protocol was reviewed and approved by the Helsinki committee of Hadassah Hospital (Jerusalem, Israel; approval no. HMO-0536-20). Prior to screening, participants were required to review and sign an informed consent form, after which they were provided with a signed copy.
Participants
Statistical power calculations were performed and a total sample size of 256 participants was computed for the completion of the study, with 64 participants assigned to each group. There was ≥90% power to detect a statistically significant difference between treatment groups, estimating a mean difference of 7 bleeding sites and a standard deviation of 14. These power calculations utilized a 2-sided 5% significance level. A suitable number of participants were screened to enroll ~300 generally healthy adult volunteers with mild to severe periodontitis with ≥3 eligible periodontal pockets sites and a PPD of 3.5-6 mm. The participants were divided into four groups of 75 each, to account for a projected dropout rate of ≤15%.
The key inclusion criteria were as follows: Aged 30-60 years old; overall favorable health (assessed through a health questionnaire); ≥16 natural teeth (excluding third molars); facial and lingual surfaces suitable for scoring; ≥20 bleeding sites [sum of sites with a score of 1 or 2 on the Gingival Bleeding Index (GBI) (12)]; ≥3 periodontal pocket sites with a PPD of 3.5-6 mm; and agreement to postpone any elective dental procedures until the conclusion of the study, adhere to the study protocols and attend all scheduled visits. The exclusion criteria were as follows: Signs of advanced periodontal disease, such as purulent discharge, widespread tooth mobility or severe gum recession; current active periodontitis treatment; medical conditions necessitating antibiotic prophylaxis before dental procedures; presence of fixed orthodontic appliances on the facial or lingual surfaces; use of removable partial dentures; use of antibiotics, chlorhexidine or anti-inflammatory drugs within 2 weeks prior to the initial visit; pregnancy; recent dental prophylaxis within 2 months before the screening visit; presence of any condition likely to compromise the ability of the participant to safely complete the study.
Examination data
Participants were categorized and randomly distributed in equal proportions between the regimen groups and the control group, considering factors such as sex, age, tobacco consumption, baseline mean of PPD of above or below 2 mm and mean GBI of above or below 0.44. The study coordinator performed the random allocation of subjects to one of the treatment groups using an encoded program. This allocation process, along with the distribution of test products, was performed in a controlled environment to ensure the blinding of the examiner to the identity of the test products.
Procedure
Participants were instructed to use the provided products at home throughout the study period, following both the written and verbal guidelines provided at the time of distribution. Oral soft tissue examinations and periodontal measurements were performed on participants at baseline and at months 6, 12 and 18, with the exception of PPD, which was not assessed at the 6-month visit.
The study and control groups were given the following instructions: Group A, two AGE tablets daily with a meal twice a day; Group B, three AGE tablets daily with a meal twice a day; Group C, four AGE tablets daily with a meal twice a day; and Group D, four placebo tablets daily with a meal twice a day.
Materials
The provided products were AGE tablets (Kyolic®; reserve formula; Wakunaga Pharmaceutical, Co., Ltd.) containing 300 mg AGE powder per tablet or placebo tablet (Microcrystalline Cellulose; Agar Powder; Hydroxypropyl Methylcellulose Carboxymethylcellulose Calcium). AGE was produced under a license granted by the Ministry of Health, Labor and Welfare of Japan, following a series of steps: Organically cultivated raw garlic (Allium sativum L.) was sliced, soaked in aqueous ethanol and aged at ambient temperature. The process and specifications adhered to the garlic fluid extract monograph outlined in the US Pharmacopeia/Natural Formula. S-allyl-cysteine (SAC) was used as the quality control standard of garlic extract. The content of SAC was defined as ≥0.05% per dried basis of the garlic extract.
All groups received a supra-gingival dental prophylaxis every 6 months in accordance with local guidelines and standards. Products were replenished approximately every 6 months after the baseline visit.
Participant and clinical guidelines
Throughout the study, participants showing signs of advancing periodontal disease (such as a ≥3 mm increase in pocket depth, attachment loss or recession) were withdrawn from the study and were provided treatment based on local protocols and standards. Participants who missed a single examination (excluding the first or final visit) were still considered as members of the study group. Subjects were instructed to avoid all oral hygiene activities for 4 h before each examination. Additionally, they were asked to abstain from consuming medicated lozenges, breath mints, food or beverages (except water), as well as from smoking or chewing gum for 4 h prior the visit.
Clinical observations excluded teeth that had crowns, extensive restorations covering ≥50% of the tooth surface, bridges, orthodontic devices or implants. In the end, the participant cohort consisted of 138 men (53%) and 123 women (47%) (median age, 46 years; age range, 29-61 years).
GBI
GBI was assessed using lightly air-dried gums and a periodontal probe with a 0.5 mm diameter tip, following the established GBI protocol as described by Saxton and Van Der Ouderaa (12). GBI full-mouth score was calculated by summing the sites scores and dividing by the total number of scorable sites. Bleeding sites were identified as those with a GBI score of either 1 or 2.
PPD
PPD at each site was measured in mm using a World Health Organization Community Periodontal Index probe as the distance from the gingival margin to the apical end of the pocket. Measurements were rounded to the nearest mm. The full-mouth PPD score was determined by summing all site measurements scores and dividing by the total number of scorable sites assessed.
Data analysis
Demographic data, along with baseline and post-treatment scores, were calculated for each treatment group and visit. For each efficacy variable and visit, paired t-tests were performed to compare mean values to those recorded at screening for each group. Linear regression analysis was used to identify predictors for improved periodontal status. All statistical tests were two-tailed and P<0.05 was considered to indicate a statistically significant difference. The analyses were performed using SPSS software (version 27.0; IBM Corp.).
Results
A total of 311 individuals were screened, of which 302 met the inclusion criteria. By the end of the 18-month study, 261 participants completed the trial, reflecting an attrition rate of 14%. The baseline randomization of the treatment groups is presented in Table I. No significant differences were observed between the AGE and control groups with regards to sex, age, tobacco usage, mean GBI or mean PPD (Figs. 1 and 2, respectively). Male participation was 49%, with a mean age of 45.6±8.5 years. Tobacco use was reported at 23.5%, baseline mean GBI was 0.81±0.40 (P=0.917) and baseline mean PPD was 2.21±0.44 (P=0.974).
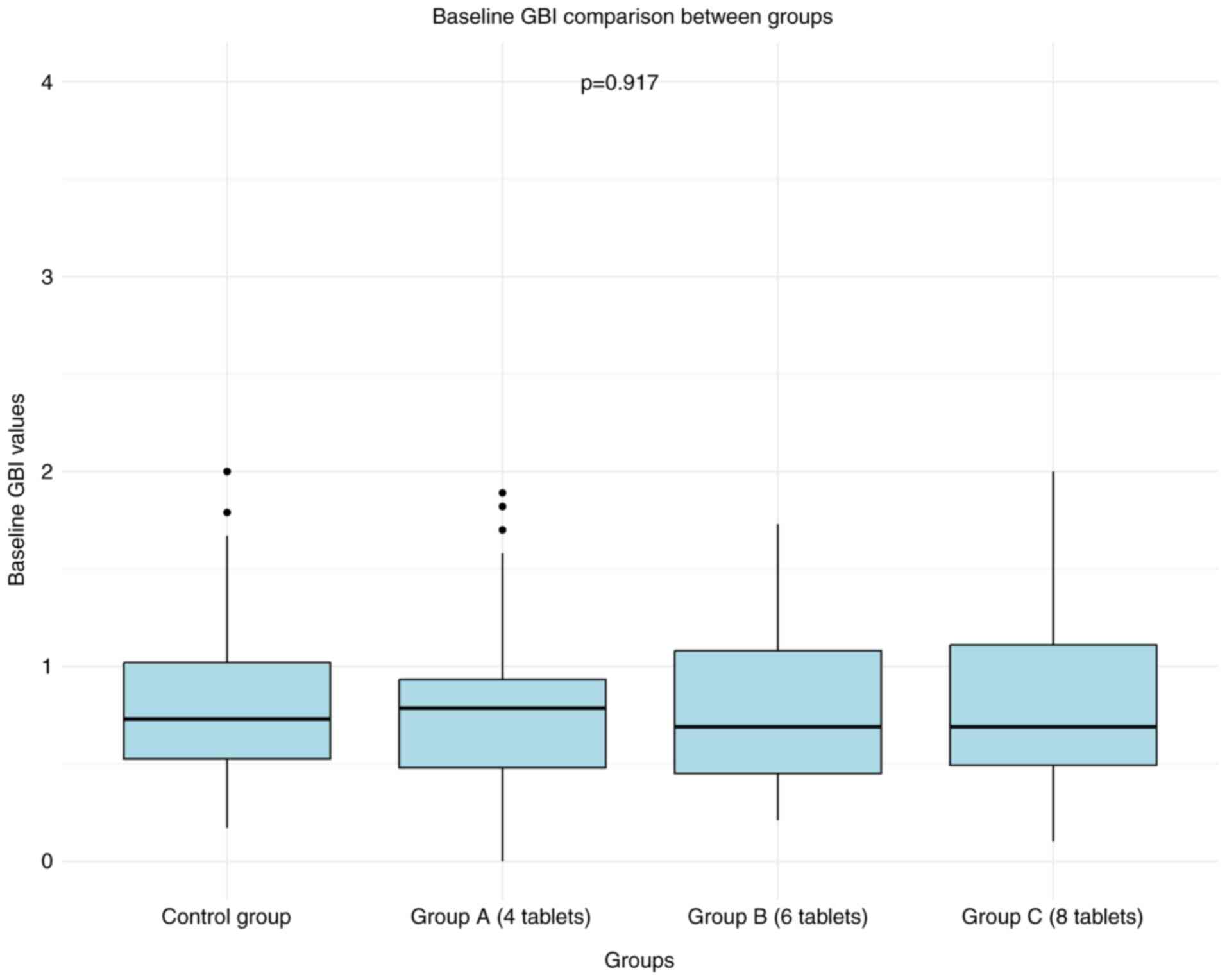 |
Figure 1
Baseline GBI comparison between groups. Box plots represent the GBI values for the control group and aged garlic extract groups (Group A: 4 tablets, Group B: 6 tablets, Group C: 8 tablets). P=0.917, indicating no significant differences between the groups at baseline. GBI, gingival bleeding index.
|
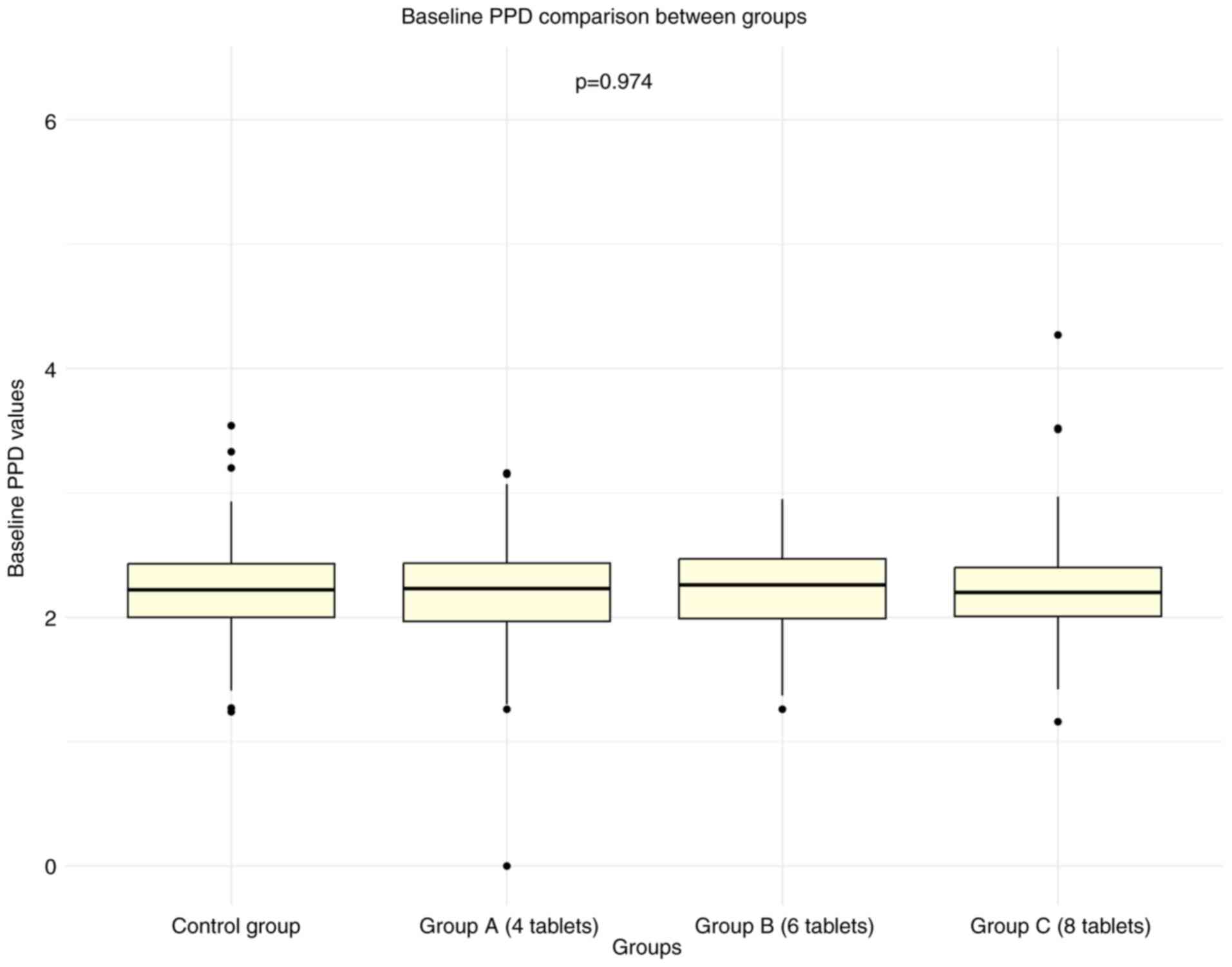 |
Figure 2
Baseline PPD comparison between groups. Box plots represent the PPD values for the control group and AGE groups (Group A: 4 tablets, Group B: 6 tablets, Group C: 8 tablets). P-value of 0.974, indicating no significant differences between the groups at baseline. PPD, probing pocket depth.
|
 |
Table I
Randomization of the treatment groups.
|
Table I
Randomization of the treatment groups.
| |
|
N |
Mean |
Standard deviation |
P-value |
| Age |
Placebo |
76 |
44.96 |
8.75 |
0.86 |
| |
AGE-4 tablets |
79 |
45.47 |
8.78 |
|
| |
AGE-6 tablets |
76 |
45.51 |
8.81 |
|
| |
AGE-8 tablets |
80 |
46.12 |
7.58 |
|
| |
Total |
311 |
45.52 |
8.46 |
|
| Baseline GBI |
Placebo |
75 |
0.82 |
0.38 |
0.92 |
| |
AGE-4 tablets |
76 |
0.78 |
0.38 |
|
| |
AGE-6 tablets |
75 |
0.80 |
0.42 |
|
| |
AGE-8 tablets |
76 |
0.81 |
0.43 |
|
| |
Total |
302 |
0.81 |
0.40 |
|
| Baseline PPD |
Placebo |
75 |
2.22 |
0.44 |
0.97 |
| |
AGE-4 tablets |
76 |
2.19 |
0.48 |
|
| |
AGE-6 tablets |
75 |
2.21 |
0.37 |
|
| |
AGE-8 tablets |
76 |
2.22 |
0.47 |
|
| |
Total |
302 |
2.21 |
0.44 |
|
The GBI results over the 18-month study period are presented in Table II and Fig. 3. There were no statistically significant differences in GBI scores between the AGE groups and the placebo group at the 6-, 12- and 18-month evaluations. A multiple linear regression analysis was performed to evaluate the final GBI scores adjusted by age, sex, tobacco use, baseline GBI score and study group (Table III). The regression model identified baseline GBI scores (P<0.001) and tobacco use (P=0.031) as the only variables significantly associated with the final GBI levels.
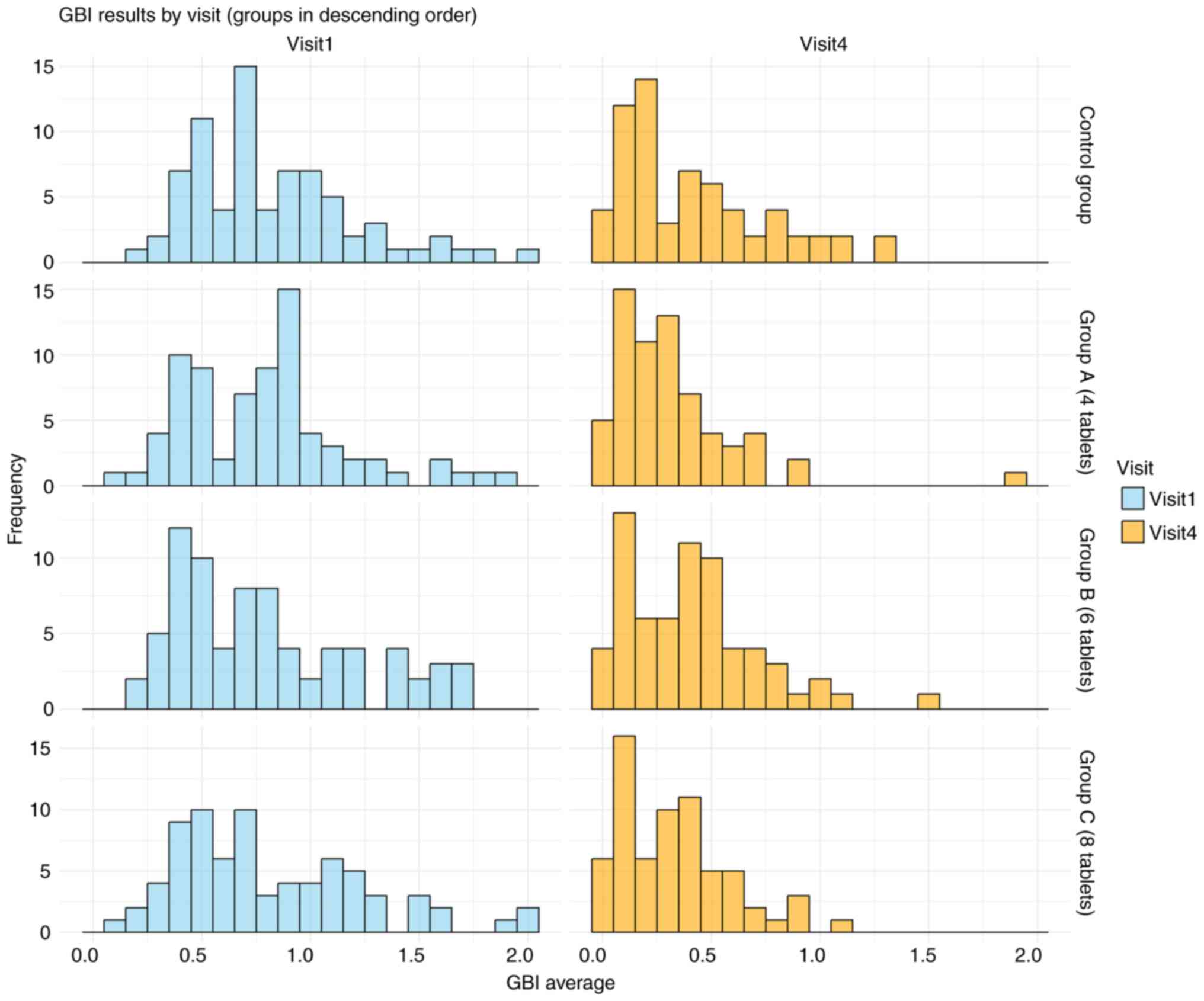 |
Figure 3
GBI data across visits. The histogram illustrates the effect of aged garlic extract tablets throughout the study period, showing a reduction in GBI values over time. GBI, gingival bleeding index.
|
 |
Table II
GBI data.
|
Table II
GBI data.
| |
|
N |
Mean |
Standard deviation |
P-value |
| Baseline data |
Placebo |
75 |
0.83 |
0.38 |
0.92 |
| |
AGE-4 tablets |
76 |
0.78 |
0.38 |
|
| |
AGE-6 tablets |
75 |
0.80 |
0.42 |
|
| |
AGE-8 tablets |
76 |
0.82 |
0.43 |
|
| |
Total |
302 |
0.81 |
0.40 |
|
| GBI 6 months data |
Placebo |
63 |
0.65 |
0.40 |
0.48 |
| |
AGE-4 tablets |
63 |
0.63 |
0.36 |
|
| |
AGE-6 tablets |
62 |
0.67 |
0.42 |
|
| |
AGE-8 tablets |
63 |
0.65 |
0.40 |
|
| |
Total |
251 |
0.65 |
0.40 |
|
| GBI 12 months data |
Placebo |
62 |
0.51 |
0.39 |
0.13 |
| |
AGE-4 tablets |
64 |
0.44 |
0.28 |
|
| |
AGE-6 tablets |
63 |
0.48 |
0.32 |
|
| |
AGE-8 tablets |
65 |
0.45 |
0.29 |
|
| |
Total |
254 |
0.47 |
0.32 |
|
| GBI 18 months data |
Placebo |
64 |
0.42 |
0.34 |
0.12 |
| |
AGE-4 tablets |
65 |
0.33 |
0.30 |
|
| |
AGE-6 tablets |
66 |
0.41 |
0.30 |
|
| |
AGE-8 tablets |
66 |
0.34 |
0.26 |
|
| |
Total |
261 |
0.37 |
0.30 |
|
 |
Table III
GBI regression analysis.
|
Table III
GBI regression analysis.
| |
Coefficients unstandardized |
Standardized coefficients |
|
| |
B |
Standard error |
Beta |
t |
Sig. |
| Constant |
0.15 |
0.12 |
N/A |
1.22 |
0.23 |
| Groups |
-0.02 |
0.02 |
-0.06 |
-0.99 |
0.32 |
| Age |
<0.01 |
<0.01 |
0.02 |
0.35 |
0.72 |
| Smoking |
-0.08 |
0.04 |
-0.12 |
-2.17 |
0.03 |
| Sex |
0.03 |
0.03 |
0.05 |
0.84 |
0.41 |
| Baseline GBI |
0.35 |
0.04 |
0.47 |
8.48 |
<0.01 |
The PPD results over the 18-month study period are summarized in Table IV and Fig. 4. At 18 months, a statistically significant trend emerged, with the AGE groups showing significant differences in pocket depth scores compared with that of the placebo group. The final mean pocket depth was 1.6±0.33 (P=0.023 vs. placebo) for the group taking 4 AGE tablets per day (Group A), 1.59±0.33 (P=0.022 vs. placebo) for the 6-tablet group (Group B) and 1.55±0.34 (P=0.005 vs. placebo) for the 8-tablet group (Group C). These values were significantly lower than the final mean pocket depth of the placebo group (1.72±0.41; P=0.003 for the comparison of all AGE groups to placebo).
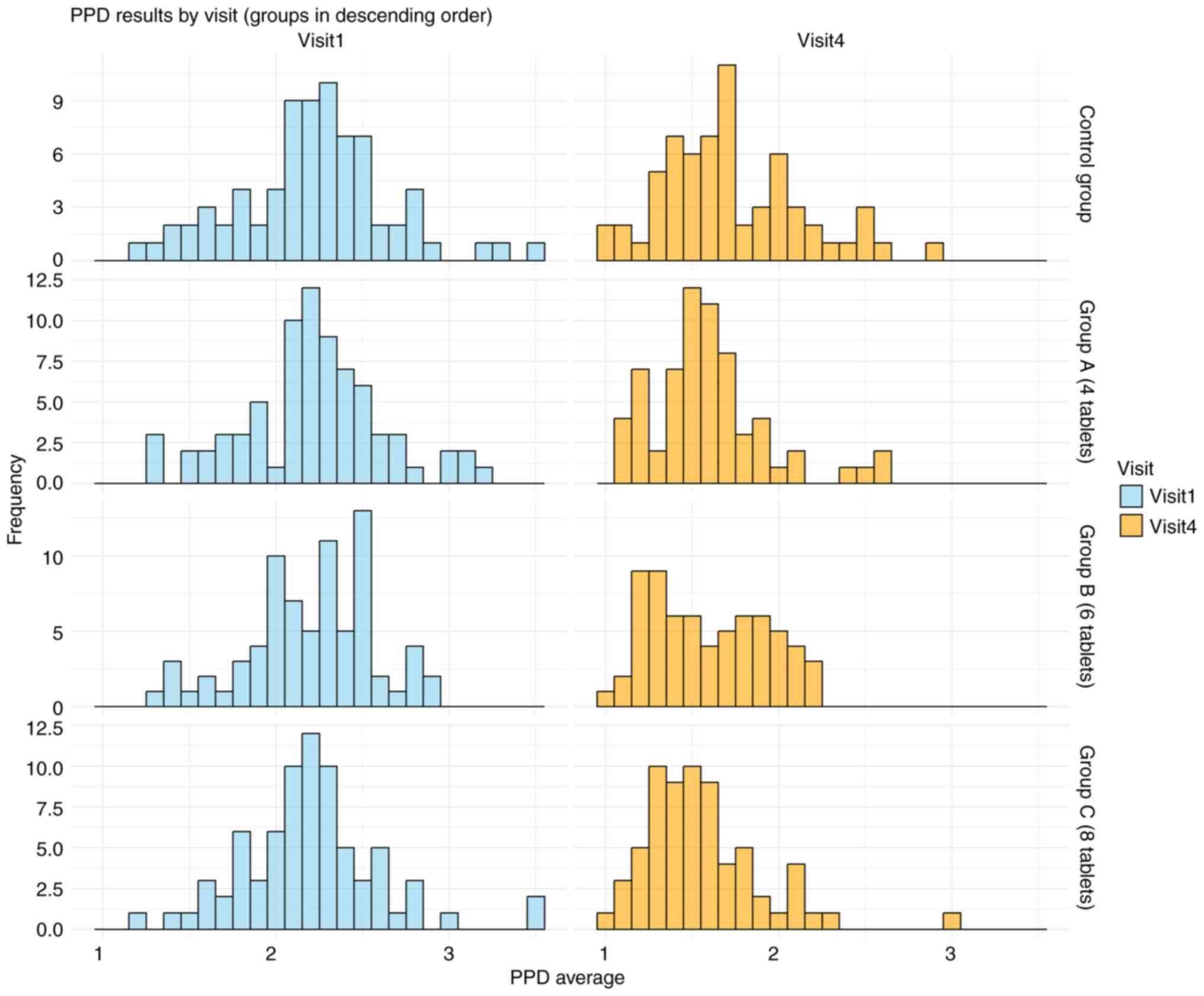 |
Figure 4
PPD data across visits. The histogram illustrates the effect of aged garlic extract tablets throughout the study period, showing a reduction in PPD values over time. PPD, probing pocket depth.
|
 |
Table IV
Probing Pocket Depth data.
|
Table IV
Probing Pocket Depth data.
| |
|
N |
Mean |
Standard deviation |
P-value |
| Baseline data |
Placebo |
75 |
2.22 |
0.44 |
0.97 |
| |
AGE-4 tablets |
76 |
2.19 |
0.48 |
|
| |
AGE-6 tablets |
75 |
2.21 |
0.37 |
|
| |
AGE-8 tablets |
76 |
2.22 |
0.47 |
|
| |
Total |
302 |
2.21 |
0.44 |
|
| 12 months data |
Placebo |
62 |
1.60 |
0.44 |
0.73 |
| |
AGE-4 tablets |
64 |
1.53 |
0.42 |
|
| |
AGE-6 tablets |
63 |
1.54 |
0.34 |
|
| |
AGE-8 tablets |
65 |
1.53 |
0.40 |
|
| |
Total |
254 |
1.55 |
0.40 |
|
| 18 months data |
Placebo |
64 |
1.72 |
0.41 |
<0.01 |
| |
AGE-4 tablets |
65 |
1.60 |
0.33 |
|
| |
AGE-6 tablets |
66 |
1.59 |
0.33 |
|
| |
AGE-8 tablets |
66 |
1.56 |
0.34 |
|
| |
Total |
261 |
1.62 |
0.36 |
|
A multiple linear regression analysis was performed to assess the PPD, adjusted by age, sex, tobacco use, baseline PPD and study group (Table V). The regression model identified three variables as statistically significant predictors of the final PPD levels: Baseline PPD scores (P<0.001), tobacco use (P=0.044) and the daily dose of AGE consumed (P=0.012). Participants in the AGE groups demonstrated a marked reduction in PPD scores, with a mean decrease of ~0.15 units.
 |
Table V
PPD regression analysis.
|
Table V
PPD regression analysis.
| |
Coefficients unstandardized |
Standardized coefficients |
|
| |
B |
Standard error |
Beta |
t |
Sig. |
| Constant |
0.97 |
0.18 |
N/A |
5.38 |
<0.01 |
| Groups |
-0.05 |
0.02 |
-0.14 |
-2.54 |
0.01 |
| Age |
0.00 |
<0.01 |
-0.01 |
-0.18 |
0.86 |
| Smoking |
-0.10 |
0.05 |
-0.12 |
-2.03 |
0.04 |
| Sex |
0.01 |
0.04 |
0.01 |
0.21 |
0.84 |
| Baseline PPD |
0.38 |
0.05 |
0.44 |
7.64 |
<0.01 |
Discussion
Periodontitis, a chronic inflammatory condition, is the leading cause of tooth loss among adults. It represents a major oral health issue notably contributing to the global burden of chronic diseases (13). Severe periodontitis impacts ~11% of the adult population, ranking as the sixth most widespread disease worldwide and posing a substantial public health challenge (1,14). Numerous studies have suggested a potential link between periodontitis and several conditions, including diabetes, atherosclerotic vascular disease, rheumatoid arthritis, adverse pregnancy outcomes, obesity and Alzheimer's disease. Additionally, periodontitis markedly deteriorates oral health, impacting quality of life and self-esteem (11,13). AGE has been reported to offer a wide range of advantages, including antimicrobial properties and cardio-protective, anticancer and anti-inflammatory effects (15). Previous meta-analysis findings demonstrated its ability to reduce blood pressure in individuals with hypertension and indicated its potential benefits for Alzheimer's disease (16). Moreover, a previous review on the preventive and therapeutic use of plant-based ingredients for treating periodontal diseases emphasized the efficacy of herbal extracts and polyphenols when used as mouthwashes or dentifrices for the oral cavity (4). In particular, two previous studies evaluated the effectiveness of AGE in treating periodontal diseases. The first reported that a daily intake of AGE improves oral health by reducing gingival inflammation and gingival bleeding (10), whilst the second reported that AGE is effective in preventing periodontitis (11).
AGE serves as a beneficial supplement for the prevention and management of periodontal disease. Based on evidence from previous studies, it is hypothesized that SAC, S-1-propenylcysteine (S1PC™) and S-allyl-mercapto-cysteine (SAMC) are the active components in AGE. SAC, S1PC and SAMC have been reported to suppress inflammatory responses induced by TNF-α in human gingival epithelial cells (17). Additionally, S1PC has been reported to inhibit the expression of matrix metalloproteinase-1 induced by lipopolysaccharides from Porphyromonas gingivalis in human gingival fibroblasts (18). AGE beneficial effect on periodontitis was also demonstrated in a previous randomized controlled trial in which the importance of performing further research to determine the exact duration and appropriate dosage for its use was emphasized (11). Therefore, the present 18-month dose response clinical study, which evaluated the efficacy of AGE on periodontal pockets, aimed to achieve this.
Following baseline clinical examination, the present study performed assessments after 12 and 18 months, and the results revealed that 18 months of daily consumption of the product was associated with a marked improvement in periodontal health in comparison with a placebo. Furthermore, regarding the daily dose of tablets, four regimens were utilized: i) Four AGE tablets per day; ii) six AGE tablets per day; iii) eight AGE tablets per day; and iv) four placebo tablets per day. The results of the present study revealed that the PPD of the three AGE groups was significantly reduced compared with that of the placebo group, with a dose response trend. Furthermore, a daily dose of only four tablets yielded a significant periodontal health improvement, compared with that of the placebo.
However, a notable limitation of the present study was the inability to monitor participant compliance to the assigned regimens or their consumption patterns at home. Whilst participants were instructed on proper usage, individual variability in compliance may have influenced the outcomes.
In conclusion, in addition to strengthening the already well-established benefits of AGE for oral health, the present research provides research-based evidence for the use of AGE as a mean of promoting public health. Furthermore, the present study provides practical, valid and important data concerning the effective duration of usage and dosage of AGE, thereby tackling the challenge of dealing with chronic diseases, which is a common practice and interest of dentistry, medicine and public health.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Wakunaga Pharmaceutical Co., Ltd. (grant no. 3144000683).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
AZ and YV initiated and designed the study concept, and confirm the authenticity of all the raw data. AZ, LZ, HG and YV participated in data collection and manuscript writing. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The clinical protocol was reviewed and approved by the Helsinki committee of Hadassah Hospital (Jerusalem, Israel; approval no. HMO-0536-20). Prior to screening, participants were required to review and sign an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
Financial support was received from Wakunaga Pharmaceutical Co., Ltd.
References
|
1
|
Mann J, Bernstein Y and Findler M: Periodontal disease and its prevention, by traditional and new avenues. Exp Ther Med. 19:1504–1506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim J and Amar S: Periodontal disease and systemic conditions: A bidirectional relationship. Odontology. 94:10–21. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H and Ojcius DM: Association between periodontal pathogens and systemic disease. Biomed J. 27-35:2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ohtani M and Nishimura T: The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp Ther Med. 19:1507–1510. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Petrovska BB and Cekovska S: Extracts from the history and medical properties of garlic. Pharmacogn Rev. 4:106–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amagase H, Petesch BL, Matsuura H, Kasuga S and Itakura Y: Intake of garlic and its bioactive components. J Nutr. 131:955S–962S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ide N and Lau BH: Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa B activation. J Nutr. 131:1020S–1026S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morihara N, Sumioka I, Moriguchi T, Uda N and Kyo E: Aged garlic extract enhances production of nitric oxide. Life Sci. 71:509–517. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim KM, Chun SB, Koo MS, Choi WJ, Kim TW, Kwon YG, Chung HT, Billiar TR and Kim YM: Differential regulation of NO availability from macrophages and endothelial cells by the garlic component S-allyl cysteine. Free Radic Biol Med. 30:747–756. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zini A, Mann J, Mazor S and Vered Y: The efficacy of aged garlic extract on gingivitis-a randomized clinical trial. J Clin Dent. 29:52–56. 2018.PubMed/NCBI
|
|
11
|
Zini A, Mann J, Mazor S and Vered Y: Beneficial effect of aged garlic extract on periodontitis: A randomized controlled double-blind clinical study. J Clin Biochem Nutr. 67:297–301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saxton CA and van der Ouderaa FJ: The effect of a dentifrice containing zinc citrate and Triclosan on developing gingivitis. J Periodontal Res. 24:75–80. 1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eke PL, Borgnakke WS and Genco RJ: Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. 82:257–267. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dumitrescu AL: Editorial: Periodontal disease-a public health problem. Front Public Health. 3(278)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang HP, Yang J, Qin LQ and Yang XJ: Effect of garlic on blood pressure: A meta-analysis. J Clin Hypertens (Greenwich). 17:223–231. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rohner A, Ried K, Sobenin IA, Bucher HC and Nordmann AJ: A systematic review and metaanalysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 28:414–423. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ohtani M and Nishimura T: Sulfur-containing amino acids in aged garlic extract inhibit inflammation in human gingival epithelial cells by suppressing intercellular adhesion molecule-1 expression and IL-6 secretion. Biomed Rep. 12:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nango H and Ohtani M: S-1-propenyl-L-cysteine suppresses lipopolysaccharide-induced expression of matrix metalloproteinase-1 through inhibition of tumor necrosis factor-α converting enzyme-epidermal growth factor receptor axis in human gingival fibroblasts. PLoS One. 18(e0284713)2023.PubMed/NCBI View Article : Google Scholar
|


















