Introduction
The incidence of breast cancer (BC) in women always
is increasing and rank first according to statistics in China and
other countries, worldwide (1,2). The
mortality rate of patients with BC ranks second after lung cancer,
which account for ~30% of cancers in women (3,4).
According to the World Health Organization (WHO) report, ~2 million
new patients with BC are diagnosed per year, and this has been
attributed to the increased life span and improved medical care
worldwide (5-7).
Recently, the mortality rate of patients with BC has decreased
because of early screening with mammography and advanced
therapeutic methods, such as targeted and immune therapies
(8-13)
or the combination of ferroptosis with photodynamic therapy
(14). However, for those patients
with advanced BC, 5-year survival rate is only 5-10% (15); therefore, a sensitive and reliable
biomarker is critical for predicting the outcomes of patients with
BC.
Emerging evidence indicates that the detection of
circulating tumor cells (CTCs) in peripheral blood are markedly
related to the recurrence, metastasis and the outcomes of numerous
cancers (16-18).
CTCs originate from primary tumor or metastatic tumors and enter
peripheral circulation, potentially giving rise to tumor cell
proliferation in other parts of the body undergoing
epithelial-mesenchymal transition (EMT) (16,19-21).
CTCs can be divided into epithelial CTCs (eCTCs), mesenchymal CTCs
(MCTCs) and hybrid CTCs (HCTCs), which express EpCAM and ck8/18/19
as epithelial marker and vimentin combined twist as mesenchymal
marker, respectively (19,22). Initially, EpCAM was considered as a
major marker for epithelial cell because it is a transmembrane
glycoprotein and mediates cell-cell adhesion of epithelial tissues
(23,24), but later cytokeratin 8/18/19CTCs
combined EpCAM measurements to enhance epithelial cell specificity
because cytokeratin 8/18/19 proteins are essential components of
the cytoskeleton in epithelial cells (25,26).
In addition, vimentin was identified as a mesenchymal cell marker
and twist was a relevant transcription factor for mesenchymal cells
(27). Therefore, EMT markers are
more likely to reflect biological functions of CTCs. CTCs'
detecting methods were varied dependent on available equipment and
patients' status, including density-gradient centrifugation,
filtration approaches, flow cytometry, immunohistochemistry (IHC),
glycolysis-associated long non-coding RNAs (IncRNAs) (20), long non-coding RNA detection
(28), and RNA in situ
hybridization (RNA-ISH) (29-32).
Among these techniques, RNA-ISH has numerous advantages over other
methods, such as high sensitivity, less CTCs loss, detecting CTCs
undergoing EMT (33).
The relationship between the numbers of CTCs and the
prognosis of patients with cancer were extensively investigated
(34-37).
Recent studies have revealed that the overall survival (OS) of
patients with higher CTCs number was significantly shorter than
that of patients with low CTCs number (38,39).
Therefore, measurement of the CTCs number is very helpful for
predicting the outcomes of patients with cancer and guiding
treatment decisions.
Recently, Ebright et al (40) identified a ribosomal submit,
ribosomal protein L15 (RPL15) gene, that plays a role in BC
metastasis during an in vivo genome-wide CRISPR screening of
CTCs from patients with hormone receptor positive (HR+)
BC. It was found that RPL15 overexpression was strongly
associated with the metastatic growth of BC in numerous organs of
patients and a poor prognosis. RPL15 gene encodes the 60S
RPL15, which catalyzes protein synthesis (41). A previous study showed that
RPL15 also enhances drug resistance to chemotherapy in
patients with gastric cancer (42);
however, the exact mechanism of RPL15 action in cancer
development remains unclear. It was hypothesized that RPL15
overexpression in CTCs promotes recurrence and metastasis of BC. To
address this hypothesis, the CanPatrol technique was used to detect
RPL15 gene expression in CTCs of patients with BC and
evaluate the relationship between RPL15 expression and
patient outcomes. Therefore, patients with BC were recruited to
measure their CTCs and RPL15 expression before treatment.
The results provide insights into the metastatic mechanisms of BC
and can be helpful for guiding clinical treatment decision.
Materials and methods
Study design and subjects
The present study recruited 170 patients with BC to
evaluate the outcomes of patients with various CTCs levels. Female
patients who were 27-83 years-old and admitted to the Hubei Cancer
Hospital from November 2007 to July 2022 were included in the
present study. The criteria for enrollment were as follows: i) age
>18 years; ii) BC diagnosis confirmed by two independent
clinical pathologists in tumor biopsy or fine-needle aspiration
samples and combined computerized tomography (CT) scan images; iii)
tumor-node-metastasis (TNM) stages I-III determined according to
proposal criteria in AACR-8th edition (43); iv) with estrogen receptor (ER),
progesterone receptor (PR) and epidermal growth factor receptor 2
(HER2) expression detected via IHC before surgery; and v) complete
medical data and follow-up record. The following exclusion criteria
were applied to the present study: i) incomplete clinical data; ii)
lost to follow-up; and iii) no previous therapies, such as surgery,
chemotherapy, or radiotherapy before the study. In addition, 10
patients with breast benign nodule were also recruited as a
control. The present study was reviewed and approved (approval no.
2024-264) by the Ethical Committee of the Hubei Cancer Hospital
(Wuhan, China). Written informed consent was obtained from all
patients before the study. The present study was conducted in
accordance with the principles of Declaration of Helsinki (2013
version).
CTCs enrichment and analysis
During EMT transition of CTCs, eCTCs, MCTCs and
HCTCs subtypes can be differentiated using EpCAM and
CK8/18/19 for eCTCs biomarker, Vimentin and
Twist for mesenchymal biomarker measurement. In the present
study, CanPatrol™ technology was utilized to detect
these biomarker-branched DNA (bDNA) (24). Briefly, CTCs were enriched via a
filter-based process, then different CTCs subtypes were identified
using an RNA-ISH technique.
CTCs enrichment was performed as previously
described (19), and none of the
patients received any therapy before the present study. To enrich
CTCs in the peripheral blood of patients with BC, 5 ml of whole
blood was collected from enrolled patients and was transferred into
an EDTA-coated tube one day just before treatment. Blood samples
was immediately processed or stored at 4˚C for <4 h before the
next step. To obtain white blood cells, erythrocytes were mixed
with 15 ml red blood lysis buffer and incubated for 30 min at room
temperature (RT). After centrifugation for 5 min with 300 x g at
RT, the supernatant was discarded. The cells were then washed twice
with phosphate-buffered saline (PBS). The collected cells were
transferred into a filter tube with 8-µM pore size membrane and
connected with the vacuum filtration system at 0.08 MPa. The
filtered cells were fixed in 4% paraformaldehyde (PFA) for 1 h at
RT.
CTCs and RPL15 gene detection using
mRNA probes in RNA-ISH
To evaluate total CTCs and subtypes as well as
RPL15 gene expression in the peripheral blood of patients
with BC, the RNA-ISH technique was employed to detect specific
genes based on the bDNA signal amplification technique because this
technique is more powerful for rare gene expression. The bDNA
signal amplification employs specific capture probes to bind the
target gene sequences and combine bDNA signal amplification probes,
including preamplifier sequence, the amplifier sequences and the
label probes (24,44). The pre-amplifier sequence was
designed to recognize the capture and bDNA amplifier sequences. The
label probes were conjugated with fluorescent dye and counted under
fluorescence scanning microscope (24). Briefly, previous fixed samples were
digested with 0.1 mg/ml proteinase K for 30 min at 4˚C to enhance
the cell membrane permeability. After rinsing twice with PBS
solution, the following capture probes were added to the
hybridization solution and incubated for 2 h at 40˚C: EpCAM
and CK8/18/19 for epithelial biomarker; Vimentin and
Twist for mesenchymal biomarker; RPL15 bDNA probes.
These bDNA specific probes were custom-made and purchased from
Invitrogen; Thermo Fisher Scientific; Inc. based on their gene
sequences. To deduce background signals, the cells were washed
twice with 0.1X SSC eluent (MilliporeSigma). Cells were then added
pre-amplification and amplification solution and incubated at 40˚C
for 90 min to obtain a strong signal after adding Alexa Fluor (AF)
594 conjugated EpCAM and CK8/18/19 detection probe, AF488
conjugated Vimentin and Twist probe, and AF750 conjugated
RPL15 probe for 60 min incubation. Finally, DAPI was added
into samples for cellular nucleus staining. Images were obtained
using fluorescence scanning microscope at x100 magnification
(Olympus BX53; Olympus Corporation).
Criteria for CTCs and RPL15 gene
expression positivity
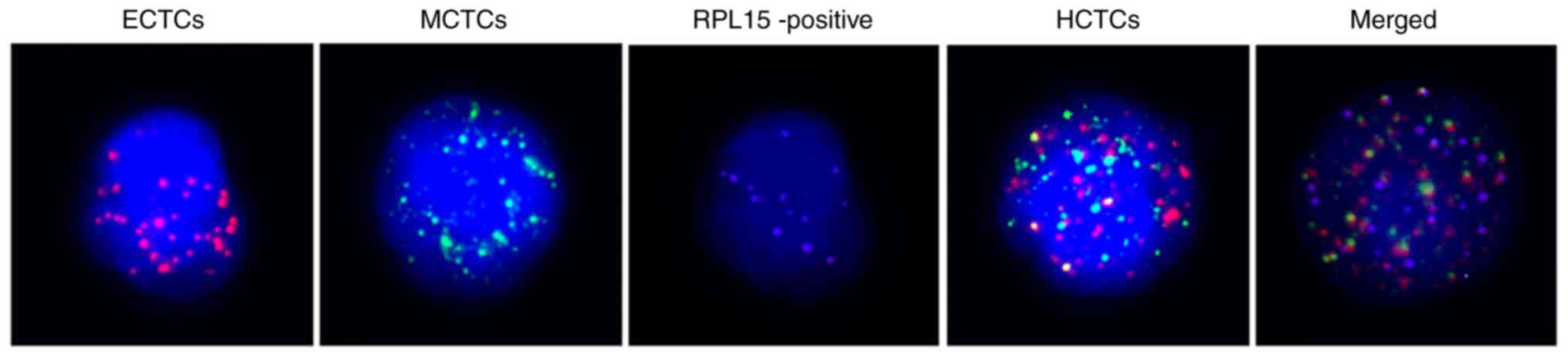
Positive eCTCs, MCTCs, HCTCs, and RPL15 cells
were determined and counted under a fluorescence microscope,
following the manufacturer's instructions (SurExam; http://www.surexam.com). Only red or green
fluorescence signal spots on cell surface represent eCTCs and
MCTCs, respectively. If there were both red and green spots on cell
surface, these cells were classified as HCTCs. The purple
fluorescence signal dots represent RPL15 gene on cellular
surface. The positive RPL15 gene was counted with ≥1 purple
dots/5 ml peripheral blood on CTC, MCTCs, or HCTCs. No any purple
dots on CTCs were observed and defined as RPL15 negative.
Images of 5 microscopy fields were randomly captured and average
number of CTCs in each field was counted. These cell-specific
images and characteristics are presented in Fig. 1 and Table I.
 | Table ICTCs and RPL15 classification
features. |
Table I
CTCs and RPL15 classification
features.
| Subtypes | Spot color | DAPI |
|---|
| Epithelial
CTCs | Red | + |
| Mesenchymal
CTCs | Green | + |
| Hybrid CTCs | Red and green | + |
| RPL15+, | Purple | + |
HR detection using IHC and
fluorescence in-situ hybridization (FISH)
HR expression levels, including ER, PR and HER2,
were detected via IHC for ER and PR or FISH for HER2. Tumor samples
were prepared as 4-µM wide sections and mounted into slides. The
primary antibodies at 1:1,000 dilution were incubated overnight at
4˚C and the secondary antibody at 1:1,000 dilution were incubated
at RT for 1 h according to the manufacturer's instructions (Roche
Diagnostics). ER- and PR-positive cells were identified by at least
three certified pathologists. Positive HER2 cells were quantified
using FISH method (Sinomdgene; https://www.sinogenepets.com). Briefly, the
deparaffinized sections of formalin-fixed paraffin-embedded tumor
tissue were incubated with a serial of reagents. HER2 specific DNA
probe labeled with spectrum orange and chromosome enumeration probe
(CEP17) labeled with spectrum green were added to slides for
staining. The cells were observed and counted under a fluorescence
microscope following the 2018 ASCO/CAP criteria (45).
Disease status follow-up
All patients were followed up to 24 months by
visitor phone call by every three months in the first half year,
then every six months thereafter. Follow-up data included disease
symptoms, chest computed tomography (CT), skull magnetic resonance
imaging, whole-body bone scan, abdominal color ultrasound, and
positron emission tomography. Signs of recurrence and metastasis
were determined by image data showing space-occupying lesions in
the chest and other organs. Progression-free survival (PFS) was
defined as time from treatment to recurrence.
Statistical analysis
All data analysis was performed using GraphPad Prism
9.0 version (Dotmatics). A comparison of the relationship between
continuous or categorical variables and patient demographics was
performed using the paired t-test and χ2 tests. Cox
proportional hazard regression model were used to investigate the
factors (age, MCTCs, HCTCs, RPL15, ER, PR and HER2)
impacting patients' outcomes. In this model, age, MCTCs, HCTCs,
RPL15, ER, PR and HER2 were as variables and PFS was as
events at 24 months cut-off. Hazard ratio (HR), 95% confidence
intervals (CI), and P-values were compared in varous groups. The
assessment of Schoenfeld's residuals was used to ensure the
assumptions have not been violated. The PFS curve of patients was
plotted by the Kaplan-Meier method after the log-rank test. A power
calculation was used to determine differences in PFS of the
subgroups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient clinico-pathological
charateristics
All patients were female, and ages ranged from 27-83
years-old (median, 59 years-old; mean ± SD, 50.95±10.76 years-old).
A total of 29 patients were ≥65 years-old (range 61-63) (29/170,
17.1%) and 141 patients were <60 years-old (range 27-60,
141/170, 82.9%). A total of 152 patients had invasive ductal
carcinoma (IDC) (89.4%), and the rest of the study cohort included
3 patients with invasive lobular carcinoma (ILC, 1.8%), 8 with
ductal carcinoma in situ (DCIS, 4.8%), 2 with mucinous
carcinoma (MC, 1.1%), 2 with metaplastic carcinoma (MeC, 1.1%) and
3 with papillary carcinoma (PC, 1.8%), r espectively. Regarding
patient TNM stages, 70 were stage I (70/170, 41.2%), 72 were stage
II (72/170, 42.3%), 20 were stage III (20/170, 11.7%), and 8
patients had DCIS (8/170,4.8%) based on criteria recommended by
AACR-8th edition (43). ER, PR and
HER2 were measured using IHC and FISH methods. Regarding
HR+ status, 114 were ER+ (114/170, 67.1%),
105 were PR+ (105/170, 61.8%), 88 were HER2+
(88/170, 51.8%), and 12 triple-negative BC (TNBC, 25/170, 14.7%).
All patients performed surgery, 162 cases (95.3%) of which added
chemotherapy. Additionally, 90 patients (52.9%) experienced
immunotherapy using anti-PD1 administration (Table II).
 | Table IIBaseline clinical characteristics of
170 patients with breast cancer. |
Table II
Baseline clinical characteristics of
170 patients with breast cancer.
|
Characteristics | Median | Range | Case number | Percentage (%) |
|---|
| Age, years | | | | |
|
≥60 | 65 | 61-83 | 29 | 17.1 |
|
<60 | 49 | 27-60 | 141 | 82.9 |
| Histologic
type | | | | |
|
Invasive
ductal carcinoma | NA | NA | 152 | 89.4 |
|
Invasive
lobular carcinoma | NA | NA | 3 | 1.8 |
|
DCIS | NA | NA | 8 | 4.8 |
|
Papillary
carcinoma | NA | NA | 3 | 1.8 |
|
Metaplastic
carcinoma | NA | NA | 2 | 1.1 |
|
Mucinous
carcinoma | NA | NA | 2 | 1.1 |
|
Tumor-node-metastasis stage | | | | |
|
DCIS | NA | NA | 8 | 4.8 |
|
I | NA | NA | 70 | 41.2 |
|
II | NA | NA | 72 | 42.3 |
|
III | NA | NA | 20 | 11.7 |
| Hormone receptor
positive | | | | |
|
Estrogen
receptor | NA | NA | 114 | 67.1 |
|
Progesterone
receptor | NA | NA | 105 | 61.8 |
|
Epidermal
growth factor receptor 2 | NA | NA | 88 | 51.8 |
|
Triple
negative breast cancer | NA | NA | 25 | 14.7 |
| Therapies | | | | |
|
Surgery | NA | NA | 170 | 100 |
|
Surgery +
chemotherapy | NA | NA | 162 | 95.3 |
|
Surgery +
immunotherapy | NA | NA | 90 | 52.9 |
CTCs and RPL15 expression in patients
with BC
CTCs and RPL15 gene expression are strongly
associated with tumorigenesis and the outcome of patients with BC.
CTCs, CTCs' subtypes and RPL15 expression level in patients
with BC were detected in the present study using CanPatrol
technology combined triple color in situ RNA hybridization
(Table I and Fig. 1). These specific biomarkers possess
unique cellular surface molecules that can be labeled with
differentiated fluorochromes and counted under a fluorescence
microscope. For positive RPL15 expression, CTC, MCTCs, or
HCTCs were firstly counted, then RPL15 gene expression was
calculated on these CTCs. As can be observed in Table III, no significant differences
were found in total CTCs, eCTCs, MCTCs, HCTCs and RPL15 on
TCTCs between patients aged ≥60 years and those <60 years.
Furthermore, the CTCs in patients with different pathological
subtypes, including IDC, ILC, DCIS, PC, MeC and MC, did not
significantly differ. Interestingly, CTCs and RPL15 on the total
CTCs of patients with BC were strongly associated with TNM stage
(P=0.021) and HR levels (P=0.13). Positive CTCs and RPL15 on CTC
rates were significantly increased in advanced TNM. Furthermore,
patients with TNBC had significantly higher CTCs and PRL15
expression than those with other HR types. Recent data have shown
that CTCs and RPL15 expression are significantly associated
with TNM stages and HR expression levels in patients with BC.
 | Table IIICTCs and RPL15 prevalence of patients
with breast cancer (170 cases). |
Table III
CTCs and RPL15 prevalence of patients
with breast cancer (170 cases).
|
Characteristics | Total CTCs
(>7/≤7, n %) | Epithelial CTCs
(P/N, n %) | Mesenchymal CTCs
(>2/≤2, n %) | Hybrid CTCs
(>5/≤5, n %) | RPL15 on total CTCs
(P/N, n %) | P-value |
|---|
| Age, years | | | | | | 0.763 |
|
≥60 | 15 (60.0) | 2(8) | 14(56) | 18(72) | 7 (46.7) | |
|
<60 | 70 (49.6) | 40 (28.3) | 50 (35.5) | 75 (53.2) | 40 (57.1) | |
| Histologic
type | | | | | | 0.384 |
|
Invasive
ductal carcinoma | 50 (32.9) | 60 (39.5) | 70(46) | 55 (36.2) | 40 (26.3) | |
|
Invasive
lobular carcinoma | 1 (33.3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |
|
Ductal
carcinoma in situ | 2(25) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 0 (0) | |
|
Papillary
carcinoma | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 1 (33.3) | |
|
Metaplastic
carcinoma | 1(50) | 0 (0) | 0 (0) | 1(50) | 1(50) | |
|
Mucinous
carcinoma | 1(50) | 0 (0) | 0 (0) | 1(50) | 1(50) | |
|
Tumor-node-metastasis stages | | | | | | 0.021 |
|
I | 30 (42.9) | 3 (4.3) | 16 (22.9) | 11 (15.7) | 10 (33.3) | |
|
II | 50 (69.4) | 5 (6.9) | 26 (36.1) | 19 (26.3) | 35 (48.6) | |
|
III | 15(75) | 7(35) | 13(65) | 12(60) | 15(75) | |
| Hormone
receptor | | | | | | 0.013 |
| Estrogen
receptor | 20(18) | 17 (15.3) | 15 (13.5) | 17 (15.3) | 25 (22.5) | |
|
Progesterone
receptor | 23 (22.3) | 20(19) | 23 (21.9) | 25 (23.8) | 30 (28.7) | |
|
Epidermal
growth factor receptor 2 | 13 (14.8) | 21 (23.9) | 31 (35.2) | 29 (32.9) | 35 (39.7) | |
|
Triple-negative
breast cancer | 15(60) | 5(20) | 16(64) | 20(80) | 19(76) | |
The prediction of recurrence and
metastaisis in patients with BCusing multivariate COX regression
analysis
To further evaluate the outcomes of patients with
different clinical characteristics, recurrence and metastasis in
patients were predicted via multivariate COX proportional hazard
regression analysis using age, MCTCs, HCTCs, RPL15 on TCTCs,
ER, PR and HER2 as co-variates. The follow-up time was variable,
and PFS as well as relapse were defined as events. Age, CTC cut-off
values, RPL15, ER, PR, HER2 positive or negative expression were
used as the other variable, and Graphpad Prism softare was used for
statitical analysis. A total of 24 months follow-up was set as
cut-off duration for recurrence,metastasis and PFS time (Table IV). These cut-off values of
biomarker expressive levels in patients with BC were determined
according to RPL15 CTCs and levels of 10 patients with
breast benign nodule, which can distinguish benign and malignant
breast mass with >80% sensitivity and specificity at these
cut-off values. The patients with ≥/<60 years of age,
ER+/- and
PR+/- expression were not
determining factors for recurrenece and metastasis. By contrast,
high MCTCs, HCTCS, positive RPL15 on CTCs had 2.73, 2.38,
1.83 and 2.76 HR for recurrence and metastasis, which mean that
relapse and metastasis chances of patients with high MCTCs (>2),
HCTCs (>5), and positive RPL15 were 2.73,2.38 and
2,76-fold than that of patients with low MCTCs (≤2), HCTCs (≤5) and
negative RPL15, respectively. The PFS of patients
with low MCTCs, low HCTCs, negative RPL15 gene expression and
negative HER2 expression was significantlylonger than that of the
patients with positive expression levels, respectively
(P<0.05).
 | Table IVMultivariate analysis of risk factors
for recurrence and metastasis in patients with breast cancer. |
Table IV
Multivariate analysis of risk factors
for recurrence and metastasis in patients with breast cancer.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age | 1.004 | 0.978-1.031 | 0.505 |
| Mesenchymal
circulating tumor cells | 2.73 | 1.25-116.9 | 0.014 |
| Hybrid circulating
tumor cells | 2.38 | 2.14-3.27 | 0.027 |
| Ribosomal protein L
15 on circulating tumor cells | 1.83 | 1.87-2.79 | 0.0032 |
| Estrogen
receptor-positive | 1.097 | 0.5456-2.3 | 0.801 |
| Progesterone
receptor-positive | 1.09 | 0.558-2.27 | 0.807 |
| Human epithelial
growth factor receptor 2-positive | 2.76 | 2.83-3.57 | 0.013 |
Survival comparison of patients with
various CTCs, RPL15 and HR expression by Kaplan-Meier analysis
The multivariate COX regress analysis showed that
MCTCs, HCTCs, RPL15 and positive HER2 were critical risk
factors for recurrence and metastasis of patients with BC;a
survival analysis of patient with different CTCs and RPL15
levels was further carried out via a Kaplan-Meier survival analysis
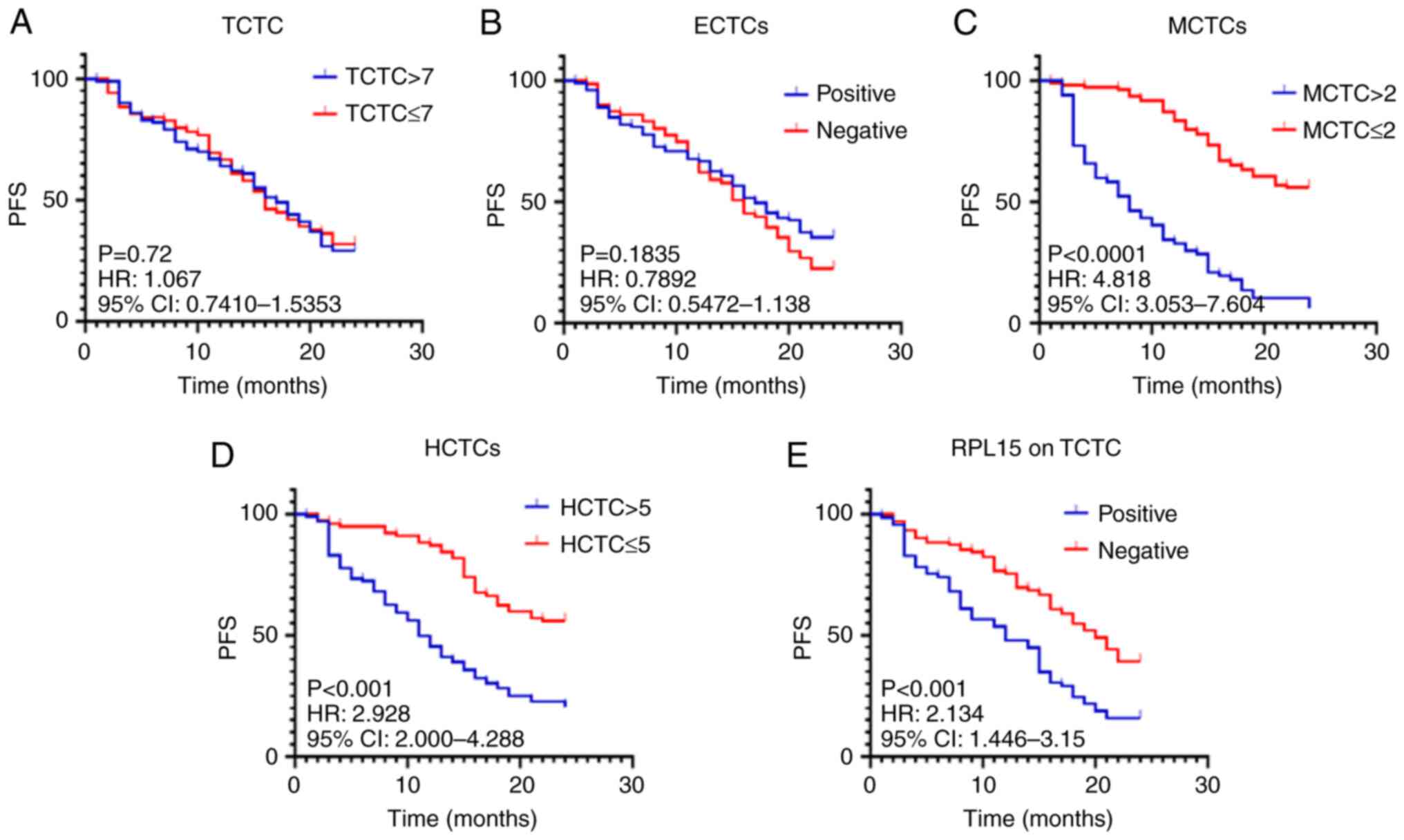
(Fig. 2). Different cut-off values
for total CTCs and subtypes were set to compare PFS duration. The
results demonstrated that PFS of patients with >7/≤7 TCTCs
(Fig. 2A), positive/negative eCTCs
(Fig. 2B) did not significatly
differ (P>0.05). By contrast, the PFS of patients with low MCTCs
(Fig. 2C), HCTCs (Fig. 2D) and RPL15 on TCTCs
(Fig. 2E) was significantly longer
than that of patients with high MCTCs, HCTCs, and positive
RPL15 expression. The associated HRs, 95% CIs, and P-value
were: HR, 4.818; 95% CI, 3.053-7.604; P<0.0001 for MCTCs; HR,
2.928; 95% CI, 2-4.288; P<0.001 for HCTCs; HR, 2.134; 95% CI,
1.446-3.15; P<0.001 for RPL15 (Fig. 2 and Table V). These data revealed that high
MCTCs, HCTCs, and positive RPL15 expression on CTCs were
critical risk factors for prognosis of patients with BC.
 | Table VSurvival comparison of CTCs and
RPL15 expression in patients with breast cancer. |
Table V
Survival comparison of CTCs and
RPL15 expression in patients with breast cancer.
| Variables | Hazard ratio | 95% confidence
interval | P-value |
|---|
| TCTCs | 1.067 | 0.741-1.535 | 0.72 |
| Epithelial
CTCs | 0.7892 | 0.5472-1.138 | 0.1835 |
| Mesenchymal
CTCs | 4.818 | 3.053-7.604 | <0.0001 |
| Hybrid CTCs | 2.928 | 2-4.288 | <0.0001 |
| RPL15 on
TCTCs | 2.134 | 1.446-3.15 | <0.0001 |
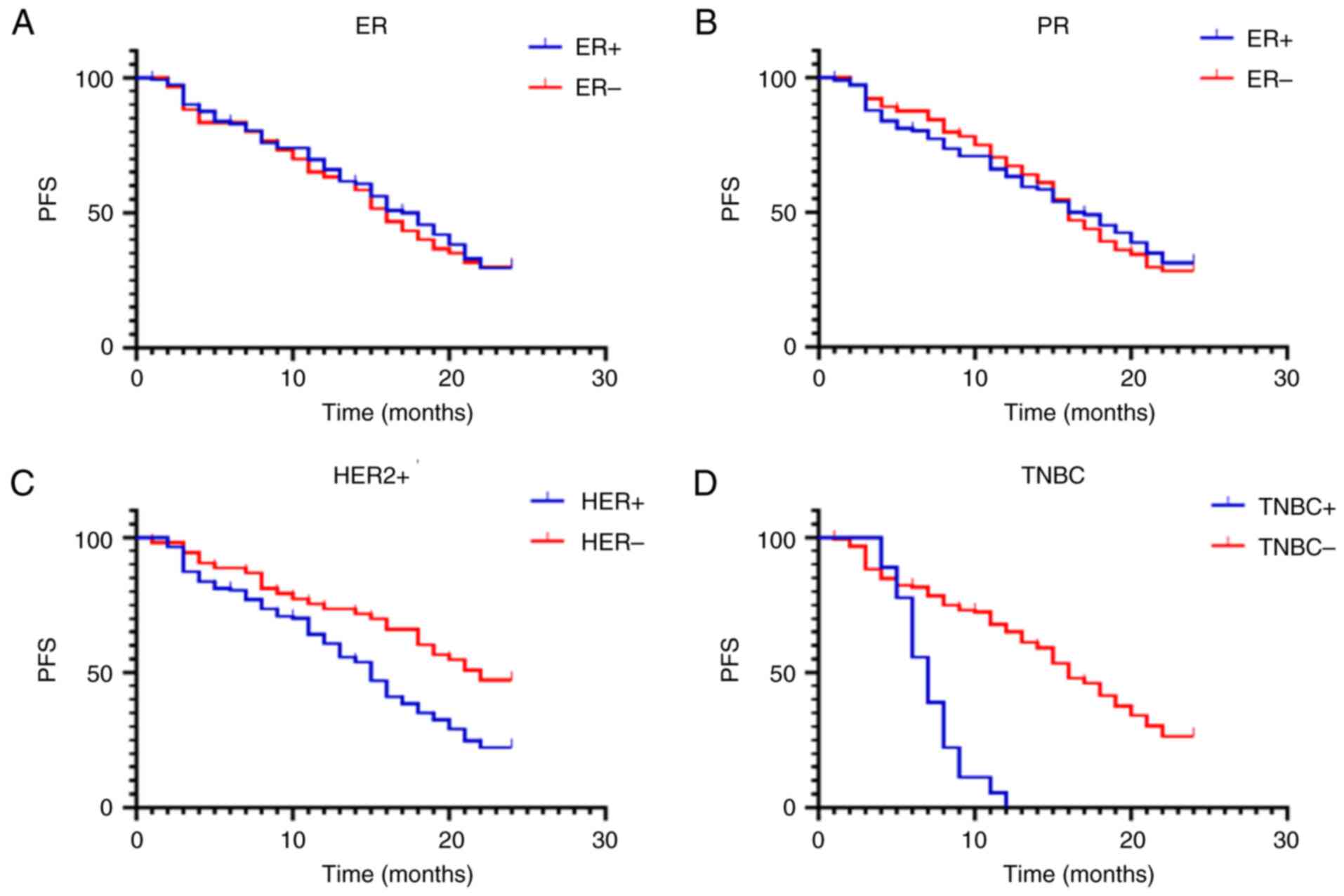
The PFS durations of patients with different HR
expression levels was also compared (Fig. 3 and Table VI). The results identified that PFS
duration of patients with positive and negative ER (Fig. 3A) or PR (Fig. 3B) was not significant differences
(P>0.05). By contrast, the PFS of patients with positive HER2
expression was significantly shorter than that of patients with
negative HER2 (Fig. 3C; HR, 1.929;
95% CI, 1.33-2.797; P=0.0014). The PFS of patients with and without
TNBC were also compared (Fig. 3D),
and the following results were observed: HR, 3.734; 95% CI,
1.573-8.862; P<0.001 (Table
VI).
 | Table VISurvival comparison of hormone
receptor expression in patients with breast cancer. |
Table VI
Survival comparison of hormone
receptor expression in patients with breast cancer.
| Variables | | 95% confidence
interval | P-value |
|---|
| Estrogen
receptor-positive | 0.9575 | 0.6558-1.398 | 0.8159 |
| Progesterone
receptor-positive | 0.9578 | 0.6611-1.388 | 0.814 |
| Human epithelial
growth factor receptor 2-positive | 1.929 | 1.33-2.797 | 0.0014 |
| Triple-negative
breast cancer | 3.734 | 1.573-8.862 | <0.0001 |
Discussion
Numerous prior studies have indicated that CTCs are
critical biomarkers for predicting tumor recurrence and metastasis.
Notably, the results of the present study showed that patients with
high MCTCs, HCTCs, RPL15 positivity, and HER2 positivity had
significantly poorer survival times than those without. In
addition, the PFS of patients with TNBC was significanlty shorter
than that of patients without TNBC.
BC is an exceedingly prevalent disease in women and
can frequently recur (46,47). Chemotherapy is a critical
therapeutic option for patients with advanced-stage cancers;
however, the pathologic types of BC are very heterogeneous and
occur in various locations (48).
Most patients eventually experience relapse owing to drug
resistance; therefore, there is an urgent need to identify
sensitive and reliable biomarkers for predicting patient prognosis.
Recently, CTCs have received increasing attention because they have
the same features at the primary and metastatic sites (49), and CTC detection has been
extensively used to predict the outcomes of numerous types of
cancers (19,22,49,50).
CanPatrol combined with RNA-ISH has been utilized to detect CTCs
levels in patients with non-small cell lung cancer (NSCLC) and
achieved 81.6% sensitivity and 86.8% specificity at 0.5 CTCs/5 ml
cut-off (19). This technique has
also been used to determine CTCs and programmed cell death-ligand 1
(PD-L1) expression in patients with NSCLC (50). The results indicated high levels of
TCTCs, MCTCs and PD-L1 (+) CTCs levels in patients correlate with
poor survival. The present data are consistent with these findings
and indicate that patients with high levels of MCTCs and HCTCs had
a shorter PFS. Prior results also suggest that the EMT is really
involved in BC tumorigenesis, because MCTCs exhibit greater
invasive ability (51).
Prior studies revealed that CTCs levels were
positively correlated with cancer stage. Dong et al
(50) revealed that OS of patients
with NSCLC at stage III was significantly shorter that those at
stage I-II. Wang et al (52)
indicated that positive CTCs' detection was most likely to occur in
patients with NSCLC at stage IV. Actually, the Food and Drug
Administration (FDA) approved CellSearch® system use in
clinical practice in 2004, which measured expression of CTCs in
patients with monitor metastatic BC based on the results of
Cristofanilli et al (23).
The present data showed that CTCs and positive RPL15 of
patients at stage III were significantly higher than those at stage
I-II, indicating that CTCs and RPL15 arereally associated
with BC stage. It was also identified that PFS of patients with
high MCTCs (>2) and HCTCs (>5) was significantly shorter than
those with low MCTCs (≤2) and HCTCs (≤5) (P<0.001), suggesting
that MCTCs may play more crucial roles in EMT transition in
patients with BC. In addition, some positive CTCs and RPL15
were also found at early stage of BC, illustrating early CTCs and
RPL15 detection have great clinical significances. These
results drive physicians to measure CTCs and RPL15
expression and guide optimal treatments before BC treatments.
RPL15 is a subunit of the large ribosomal
complex and is involved in rRNA processing (40). Previous studies showed that high
RPL15 levels are associated with a poor prognosis in
esophageal cancer (53), skin
squamous cell carcinoma (54) and
pancreatic cancer (55).
Furthermore, Shi and Liu (56)
reported that elevated RPL15 expression in patients with
hepatocellular carcinoma was associated with shorter survival
times. The present study showed that the PFS of patients with
positive RPL15 on CTCs was shorter (2.134-fold) than that of
patients with negative RPL15 expression on CTCs. This result
further confirms that RPL15 expression in CTCs is also a key
biomarker for predicting prognosis of patients with BC. Other
studies also revealed that RPL15 is involved in antitumor
immune activation (57,58), indicating that RPL15 plays
crucial roles in occurrence, progression and prognosis of cancer.
Therefore, RPL15 detection may have great benefits for
guiding treatment of patients with BC, which encourages clinicians
to routinely perform these tests to improve evaluation of the
status of patients and predict their prognosis. If RPL15 of
patients is positive on CTCs, chemotherapy, immune therapy and
hormone therapy should be performed as soon as possible after
surgery because these patients have high relapse chances.
The relationship between HR expression and outcome
in patients with BC has been extensively investigated (49,59,60),
and HR expression has been found to strongly associated with the
therapeutic methods used by patients (61,62).
For example, HER2+ patients with Enhertu treatment have
significantly longer survival times than those treated with regular
chemotherapy (61). The outcomes of
BC with HER2+ are poor because HER2 amplification is
closely associated with HER2 protein overexpression, indicating
breast cancer with HER2+ had more invasive ability than
those with HER2- (63).
The present data indicate that HER2+ expression before
treatment strongly associates with the prognosis of patients with
BC, although ER+ and PR+ were not risk
factors for recurrence and metastasis. The PFS of patients with
HER2+ was significantly shorter than those with HER2-,
indicating that targeting HER2 has significant benefits on the
outcomes of patients with BC. Therefore, if HER2 in BC will be
detected before treatment, immediate Enhertu administration may
prolong survival time of patients. In the present study,
HER2+ expression was detected before treatment, and
targeting HER2+ therapy was not adjusted for survival
analysis. This may give rise to bias based on targeting
HER2+ treatment. Further analysis will compare patient
survival rates in the patients with and without targeting
HER2+ therapy.
The present findings are noteworthy; however, the
following observations and limitations should be noted when
interpreting our results: i) CTC number was found to drive BC
tumorigenesis; ii) RPL15 expression was associated with the
prognosis of patients with BC; iii) the sample size patient
subtypes is small, which may have resulted in a selection bias; iv)
the lack of validation of cellular or clinical samples; and v)
sub-analysis of RPL15 on TCTC was unclear. To overcome these
limitations, the authors plan to recruit more patients and perform
additional biological, molecular biology and biochemical
experiments to explore CTC biology in patients with BC. The
prognosis of patients with RPL15-combined MCTCs or HCTCs
will be explored in the future to further understand biological
significance of RPL15. The clinical significances of
sub-analysis of RPL15 on TCTC will undoubtfully have great
benefits for BC survival.
In conclusion, the PFS of patients with >2 MCTC,
>5HCTCs, and positive RPL15expression was shorter than
that of those with ≤2 MCTCs, ≤5 HCTCs, and negative RPL15
expression, and the prognosis of BC patients with negative HER2
expression was favorable, compared with that of patients with
positive HER2 expression.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Health
Commission of Hubei (grant no. WJ2019F190), the Beijing Kangmeng
Charity Federation (grant no. TB212045) and the Administration of
Traditional Chinese Medicine of Hubei (grant no. ZY2023F024).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ and WZ contributed to manuscript drafting and the
design of the study. YZ, SSL and WF carried out experiments. YZ,
KS, SSL and HL contributed to the acquisition and analysis of the
data. KS and WF confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved
(approval no. 2024-264) by the Ethical Committee of Hubei Cancer
Hospital (Wuhan, China). Written informed consent was obtained from
all patients before the study. The present study was conducted in
accordance with the principles of Declaration of Helsinki (2013
version).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zhang S, Zeng H, Xia C,
Zuo T, Yang Z, Zou X and He J: Cancer incidence and mortality in
China, 2013. Cancer Lett. 401:63–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Balasubramanian R, Rolph R, Morgan C and
Hamed H: Genetics of breast cancer: Management strategies and
risk-reducing surgery. Br J Hosp Med (Lond). 80:720–725.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Collaborative Group on Hormonal Factors in
Breast Cancer. Breast cancer and breastfeeding: collaborative
reanalysis of individual data from 47 epidemiological studies in 30
countries, including 50302 women with breast cancer and 96973 women
without the disease. Lancet. 360:187–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gage M, Wattendorf D and Henry LR:
Translational advances regarding hereditary breast cancer
syndromes. J Surg Oncol. 105:444–451. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oeffinger KC, Fontham ET, Etzioni R,
Herzig A, Michaelson JS, Shih YC, Walter LC, Church TR, Flowers CR,
LaMonte SJ, et al: Breast cancer screening for women at average
risk: 2015 Guideline update from the American cancer society. JAMA.
314:1599–1614. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fleshner L, Lagree A, Shiner A, Alera MA,
Bielecki M, Grant R, Kiss A, Krzyzanowska MK, Cheng I, Tran WT and
Gandhi S: Drivers of emergency department use among oncology
patients in the era of novel cancer therapeutics: A systematic
review. Oncologist. 28:1020–1033. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Egelston CA, Guo W, Yost SE, Ge X, Lee JS,
Frankel PH, Cui Y, Ruel C, Schmolze D, Murga M, et al:
Immunogenicity and efficacy of pembrolizumab and doxorubicin in a
phase I trial for patients with metastatic triple-negative breast
cancer. Cancer Immunol Immunother. 72:3013–3027. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao J, Li GY, Lu XY, Zhu LR and Gao Q:
Landscape of m6A RNA methylation regulators in liver
cancer and its therapeutic implications. Front Pharmacol.
15(1376005)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Du C, Wu X, He M, Zhang Y, Zhang R and
Dong CM: Polymeric photothermal agents for cancer therapy: Recent
progress and clinical potential. J Mater Chem B. 9:1478–1490.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumar V, Garg V and Dureja H:
Nanomedicine-based approaches for delivery of herbal compounds.
Tradit Med Res. 7(48)2022.
|
|
14
|
Gao Z, Zheng S, Kamei K and Tian C: Recent
progress in cancer therapy based on the combination of ferroptosis
with photodynamic therapy. Acta Mater Med. 1:411–426. 2022.
|
|
15
|
Huober J and Thurlimann B: The role of
combination chemotherapy in the treatment of patients with
metastatic breast cancer. Breast Care (Basel). 4:367–372.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cohen EN, Jayachandran G, Moore RG,
Cristofanilli M, Lang JE, Khoury JD, Press MF, Kim KK, Khazan N,
Zhang Q, et al: A multi-center clinical study to harvest and
characterize circulating tumor cells from patients with metastatic
breast cancer using the Parsortix® PC1 system. Cancers
(Basel). 14(5238)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fina E: Signatures of breast cancer
progression in the blood: What could be learned from circulating
tumor cell transcriptomes. Cancers (Basel). 14(5668)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li J, Liao Y, Ran Y, Wang G, Wu W, Qiu Y,
Liu J, Wen N, Jing T, Wang H and Zhang S: Evaluation of sensitivity
and specificity of CanPatrol™ technology for detection
of circulating tumor cells in patients with non-small cell lung
cance. BMC Pulm Med. 20(274)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ho KH, Huang TW, Shih CM, Lee YT, Liu AJ,
Chen PH and Chen KC: Glycolysis-associated lncRNAs identify a
subgroup of cancer patients with poor prognoses and a
high-infiltration immune microenvironment. BMC Med.
19(59)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lu H, Li Z, Liu L, Tao Y, Zhou Y, Mao X,
Zhu A, Wu H and Zheng X: A pan-cancer analysis of the oncogenic
roles of RAD51 in human tumors. Adv Gut Microbiome Res.
2022(1591377)2022.
|
|
22
|
Mäurer M, Schott D, Pizon M, Drozdz S,
Wendt T, Wittig A and Pachmann K: Increased circulating epithelial
tumor cells (CETC/CTC) over the course of adjuvant radiotherapy is
a predictor of less favorable outcome in patients with early-stage
breast cancer. Curr Oncol. 30:261–273. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J,
Yang D, Deng H, Yang N and Xu J: Classification of circulating
tumor cells by epithelial-mesenchymal transition markers. PLoS One.
10(e0123976)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jung R, Krüger W, Hosch S, Holweg M,
Kröger N, Gutensohn K, Wagener C, Neumaier M and Zander AR:
Specificity of reverse transcriptase polymerase chain reaction
assays designed for the detection of circulating cancer cells is
influenced by cytokines in vivo and in vitro. Br J Cancer.
78:1194–1198. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giuliano M, Giordano A, Jackson S, Hess
KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De
Laurentiis M, et al: Circulating tumor cells as prognostic and
predictive markers in metastatic breast cancer patients receiving
first-line systemic treatment. Breast Cancer Res.
13(R67)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mego M, Cierna Z, Janega P, Karaba M,
Minarik G, Benca J, Sedlácková T, Sieberova G, Gronesova P,
Manasova D, et al: Relationship between circulating tumor cells and
epithelial to mesenchymal transition in early breast cancer. BMC
Cancer. 15(533)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mancheng AD and Ossas U: How does lncrna
regulation impact cancer metastasis. Cancer Insight. 1(6)2022.
|
|
29
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Balic M, Dandachi N, Hofmann G, Samonigg
H, Loibner H, Obwaller A, van der Kooi A, Tibbe AG, Doyle GV,
Terstappen LW and Bauernhofer T: Comparison of two methods for
enumerating circulating tumor cells in carcinoma patients.
Cytometry B Clin Cytom. 68:25–30. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cruz I, Ciudad J, Cruz JJ, Ramos M,
Gómez-Alonso A, Adansa JC, Rodríguez C and Orfao A: Evaluation of
multiparameter flow cytometry for the detection of breast cancer
tumor cells in blood samples. Am J Clin Pathol. 123:66–74.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Meng S, Tripathy D, Frenkel EP, Shete S,
Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et
al: Circulating tumor cells in patients with breast cancer
dormancy. Clin Cancer Res. 10:8152–8162. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang J, Ma J, Jin Y, Cheng S, Huang S,
Zhang N and Wang Y: Development and validation for prognostic
nomogram of epithelial ovarian cancer recurrence based on
circulating tumor cells and epithelial-mesenchymal transition. Sci
Rep. 11(6540)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou H, Shen H, Xiang F, Yang X, Li R,
Zeng Y and Liu Z: Correlation analysis of the expression of
mesenchymal circulating tumor cells and CD133 with the prognosis of
colorectal cancer. Am J Transl Res. 15:3489–3499. 2023.PubMed/NCBI
|
|
35
|
Rath B, Plangger A, Klameth L, Hochmair M,
Ulsperger E, Boeckx B, Neumayer C and Hamilton G: Small cell lung
cancer: Circulating tumor cell lines and expression of mediators of
angiogenesis and coagulation. Explor Target Antitumor Ther.
4:355–365. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Magri V, Marino L, Nicolazzo C, Gradilone
A, De Renzi G, De Meo M, Gandini O, Sabatini A, Santini D, Cortesi
E and Gazzaniga P: Prognostic role of circulating tumor cell
trajectories in metastatic colorectal cancer. Cells.
12(1172)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen H, Li H, Shi W, Qin H and Zheng L:
The roles of m6A RNA methylation modification in cancer stem cells:
New opportunities for cancer suppression. Cancer Insight.
1(10)2022.
|
|
38
|
Gao T, Mao J, Huang J, Luo F, Lin L, Lian
Y, Bin S, Zhao L and Li S: Prognostic significance of circulating
tumor cell measurement in the peripheral blood of patients with
nasopharyngeal carcinoma. Clinics (Sao Paulo).
78(100179)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang HT, Bai LY, Wang YT, Lin HJ, Yang HR,
Hsueh PR and Cho DY: Circulating tumor cells positivity provides an
early detection of recurrence of pancreatic cancer. J Formos Med
Assoc. 122:653–655. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ebright RY, Lee S, Wittner BS,
Niederhoffer KL, Nicholson BT, Bardia A, Truesdell S, Wiley DF,
Wesley B, Li S, et al: Deregulation of ribosomal protein expression
and translation promotes breast cancer metastasis. Science.
367:1468–1473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kenmochi N, Kawaguchi T, Rozen S, Davis E,
Goodman N, Hudson TJ, Tanaka T and Page DC: A map of 75 human
ribosomal protein genes. Genome Res. 8:509–523. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma Y, Xue H, Wang W, Yuan Y and Liang F:
The miR-567/RPL15/TGF-β/Smad axis inhibits the stem-like properties
and chemo-resistance of gastric cancer cells. Transl Cancer Res.
9:3539–3549. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Amin MB, Edge SB, Greene FL and Brierley
JD: AJCC cancer staging manual. 8th edition. New York: Springer,
2017.
|
|
44
|
Tsongalis GJ: Branched DNA technology in
molecular diagnostics. Am J Clin Pathol. 126:448–453.
2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Joseph C, Papadaki A, Althobiti M,
Alsaleem M, Aleskandarany MA and Rakha EA: Breast cancer
intratumour heterogeneity: Current status and clinical
implications. Histopathology. 73:717–731. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ji X, Tian X, Feng S, Zhang L, Wang J, Guo
R, Zhu Y, Yu X, Zhang Y, Du H, et al: Intermittent F-actin
perturbations by magnetic fields inhibit breast cancer metastasis.
Research (Wash DC). 6(0080)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Navin N, Krasnitz A, Rodgers L, Cook K,
Meth J, Kendall J, Riggs M, Eberling Y, Troge J, Grubor V, et al:
Inferring tumor progression from genomic heterogeneity. Genome Res.
20:68–80. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li Y, Jiang X, Zhong M, Yu B and Yuan H:
Whole genome sequencing of single-circulating tumor cell
ameliorates unraveling breast cancer heterogeneity. Breast Cancer
(Dove Med Press). 14:505–513. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dong J, Zhu D, Tang X, Qiu X, Lu D, Li B,
Lin D and Zhou Q: Detection of circulating tumor cell molecular
subtype in pulmonary vein predicting prognosis of stage I-III
non-small cell lung cancer patients. Front Oncol.
9(1139)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang X, Ma K, Yang Z, Cui J, He H, Hoffman
AR, Hu JF and Li W: Systematic correlation analyses of circulating
tumor cells with clinical variables and tumor markers in lung
cancer patients. J Cancer. 8:3099–3104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Q, Yang C, Zhou J, Wang X, Wu M and
Liu Z: Cloning and characterization of full-length human ribosomal
protein L15 cDNA which was overexpressed in esophageal cancer.
Gene. 263:205–209. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nindl I, Dang C, Forschner T, Kuban RJ,
Meyer T, Sterry W and Stockfleth E: Identification of
differentially expressed genes in cutaneous squamous cell carcinoma
by microarray expression profiling. Mol Cancer.
5(30)2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yan TT, Fu XL, Li J, Bian YN, Liu DJ, Hua
R, Ren LL, Li CT, Sun YW, Chen HY, et al: Downregulation of RPL15
may predict poor survival and associate with tumor progression in
pancreatic ductal adenocarcinoma. Oncotarget. 6:37028–37042.
2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shi R and Liu Z: RPL15 promotes
hepatocellular carcinoma progression via regulation of RPs-MDM2-p53
signaling pathway. Cancer Cell Int. 22(150)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kitai Y: Elucidation of the mechanism of
topotecan-induced antitumor immune activation. Yakugaku Zasshi.
142:911–916. 2022.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
58
|
Yamada S, Kitai Y, Tadokoro T, Takahashi
R, Shoji H, Maemoto T, Ishiura M, Muromoto R, Kashiwakura JI, Ishii
KJ, et al: Identification of RPL15 60S ribosomal protein as a novel
topotecan target protein that correlates with DAMP secretion and
antitumor immune activation. J Immunol. 209:171–179.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Feng H, Liu H, Wang Q, Song M, Yang T,
Zheng L, Wu D, Shao X and Shi G: Breast cancer diagnosis and
prognosis using a high b-value non-Gaussian continuous-time
random-walk model. Clin Radiol. 78:e660–e667. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mai N, Abuhadra N and Jhaveri K:
Molecularly targeted therapies for triple negative breast cancer:
History, advances, and future directions. Clin Breast Cancer.
23:784–799. 2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bartsch R and Bergen E: ASCO 2018:
Highlights in HER2-positive metastatic breast cancer. Memo.
11:280–283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Papadaki MA, Stoupis G, Theodoropoulos PA,
Mavroudis D, Georgoulias V and Agelaki S: Circulating tumor cells
with stemness and epithelial-to-mesenchymal transition features are
chemoresistant and predictive of poor outcome in metastatic breast
cancer. Mol Cancer Ther. 18:437–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ahn S, Woo JW, Lee K and Park SY: HER2
status in breast cancer: Changes in guidelines and complicating
factors for interpretation. J Pathol Transl Med. 54:34–44.
2020.PubMed/NCBI View Article : Google Scholar
|