Introduction
Alzheimer's disease is a neurodegenerative disease
caused by amyloid-β accumulation in brain cells combined with
oxidative stress and inflammation (1,2).
Neurotoxic amyloid-β is an approximately 4-kDa peptide generated
via cleavage of the amyloid-β precursor protein (APP) by the
γ-secretase proteolytic complex (3). Presenilin is the catalytic member of
the γ-secretase complex, and mutations in presenilin are the major
cause of early-onset familial Alzheimer's disease. Presenilin is
involved in several biological functions, but is well known for its
role in the generation of the amyloid-β peptide in Alzheimer's
disease and is therefore thought to be an important drug target for
this disorder. Thus, an increased understanding of Presenilin may
help to improve the development of drugs for Alzheimer's disease
and for other neurological diseases (4).
Certain herbs have been demonstrated to possess a
multitude of beneficial activities, and herbal medications are
currently being used for widespread clinical use in disease
therapy, as herbs exhibit relatively mild bioavailability and low
toxicity (5). As for Alzheimer's
disease, polyphenols extracted from grape seeds have been found to
inhibit amyloid-β aggregation, reduce amyloid-β production and
protect against amyloid-β neurotoxicity in vitro (6,7). In
addition, cryptotanshinone (CTS), an active component of the
medicinal herb Salvia miltiorrhiza, has been shown to
improve learning and memory in several pharmacological models of
Alzheimer's disease (8). Further
research demonstrated that CTS improved the cognitive ability and
promoted APP metabolism involving the non-amyloidogenic product
pathway in rat cortical neuronal cells. Moreover, as the Indian
diet is rich in herbs and spices, the incidence of Alzheimer's
disease in India is considerably low (9,10).
However, the precise molecular mechanisms of the therapeutic
effects of medicinal herbs and spices are largely undefined, and
limited data and a small body of convincing evidence exist at the
molecular level (11). Therefore,
basic research and development aimed at elucidating the mechanism
of action underlying herbal effects should have high priority. We
hypothesized that various herbs or spices may affect the
γ-secretase proteolytic function. Therefore, we investigated the
in vitro effect of several herbs on the expression of
presenilin 1 in cultured human cells.
Materials and methods
Cell culture
The human cell lines, Jurkat, Daudi, U937 and K562,
were maintained in RPMI-1640 supplemented with 10% fetal bovine
serum (FBS), penicillin and streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
Extract preparation
Herb and spice powders were purchased at a food
market in Japan. The powders were dissolved in 80% ethanol and
subsequently diluted in 40% ethanol at a stock concentration of 50
mg/ml. The mixtures were vortexed rigorously for 3 min followed by
3-min sonication. After centrifugation (1,500 × g, 5 min), the
supernatants were collected and stored at −20°C until use. For the
cell treatments, a range of 0.5–10 μl was added to 1 ml of the cell
culture medium.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Presenilin 1 and GAPDH mRNAs were analyzed by
semi-quantitative RT-PCR. Total RNA was extracted by an RNA
isolation kit (Takara, Japan). Total RNA (2 μg) was
reverse-transcribed using the Phusion RT-PCR kit (NEB) as described
in the manufacturer's protocol. Cycle-based PCR was used to
semi-quantitate the presenilin 1 gene level. GADPH was also used as
an internal loading control. All the samples were evaluated within
3 months after collection. The primers used for the PCR were:
presenilin 1 forward, GGTCCACTTCGTATGCTGGT and reverse,
GCTGTTGCTGAGGCTTTACC (expected size 404 bp); GAPDH forward,
TCCCATCACCATCTTCCA and reverse, CATCACGCCACAGTTTCC (expected size
376 bp). For real-time PCR, the reactions were performed in a
real-time PCR system (Illumina, USA) using Kapa SYBR Fast reaction
mix (Genetics, Japan). Thermocycling was performed according to the
instructions at an annealing temperature of 60°C in a final volume
of 10 μl, including Taq DNA polymerase. To correct for
differences in both RNA quality and quantity between samples, data
were normalized using the ratio of the target cDNA concentration to
that of GAPDH.
Western blot analysis
Equal amounts of protein samples were used for
Western blot analysis using anti-presenilin 1 (Genscript) and
anti-Erk2 (Epitomics) antibodies, and quantified by densitometry.
The Western blotting was repeated at least three times, and the
representative data are shown.
Results and discussion
To investigate the possibility of using medicinal
herbs to down-regulate presenilin 1 protein, extracts of many herbs
and spices, including garlic, red pepper, cinnamon, phakchi,
turmeric, basil and black pepper, were added to the cell culture
medium of Jurkat, Daudi, U937 or K562 cells, and the levels of
genes, including presenilin 1, were examined. RT-PCR analysis was
employed to quantify the expression level of the genes. Total RNA
was isolated 24 h after herbal extract treatment for the detection
of presenilin 1, and the levels of presenilin 1 mRNA were
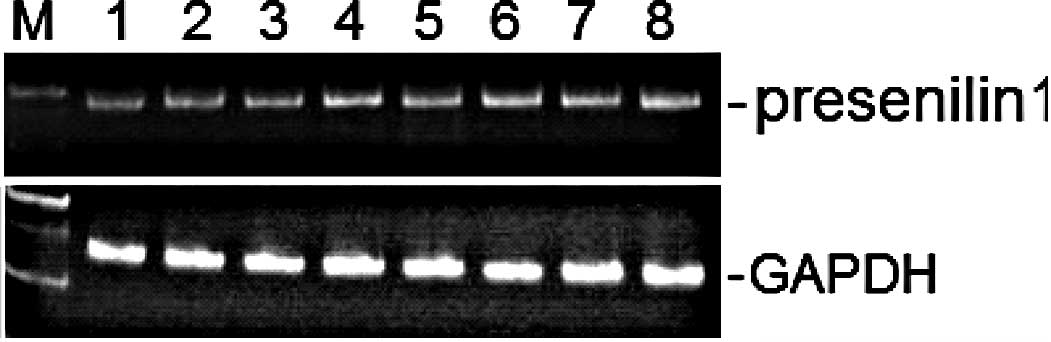
determined by conventional semi-quantitative RT-PCR. As shown in
Fig. 1, the expression level of
the presenilin 1 gene was not altered by treatment of the herbal
and spice extracts at a final concentration of 50 μg/ml, compared
to the untreated ethanol vehicle. Expression of presenilin 2 (data
not shown) and the housekeeping gene GAPDH were also unaltered
(Fig. 1). In addition, similar
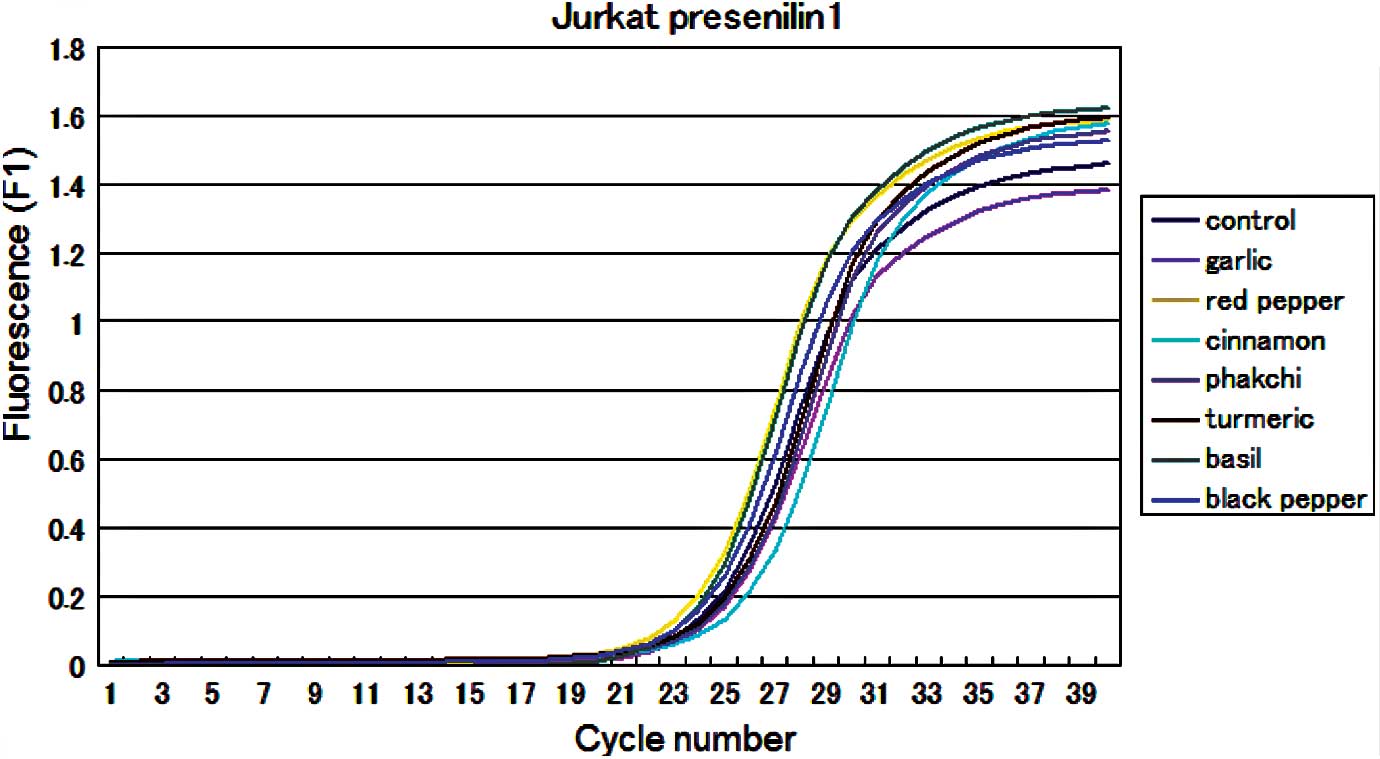
results were also obtained from the quantitative real-time PCR
analysis (Fig. 2). There was
little difference in the results of the gene expressional profile
among the Jurkat, Daudi, U937 and K562 cells (data not shown). To
exclude the possibility of carry-over DNA contamination, reactions
containing all RT-PCR reagents, including primers without sample
RNA, were performed as negative controls. No such RNA contamination
was detected (data not shown).
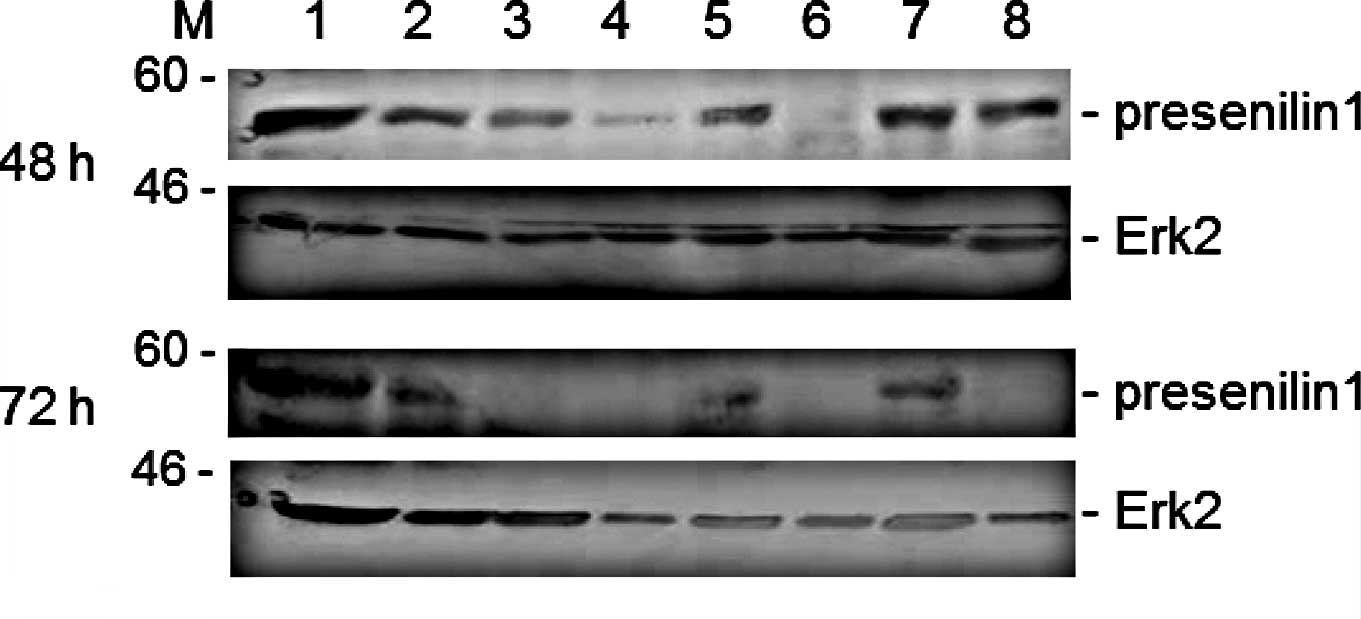
To further examine the status of the level of
protein expression, Western blotting was performed to analyze the
presenilin 1 protein in the cells stimulated by the herbs and
spices. As shown in Fig. 3, the
turmeric and cinnamon extract dramatically reduced the protein
expression of presenilin 1 in the Jurkat cells when the cell
cultures were treated with the spices for 48 h. The down-regulation
of presenilin 1 protein expression by the several herbal extracts
was in approximate accord with the result of the long-term-treated
cells (72 h after herbal stimulation) (Fig. 3, lower panels). After long-term
stimulation, it was evident that red and black pepper, in addition
to turmeric and cinnamon, down-regulated presenilin 1 protein
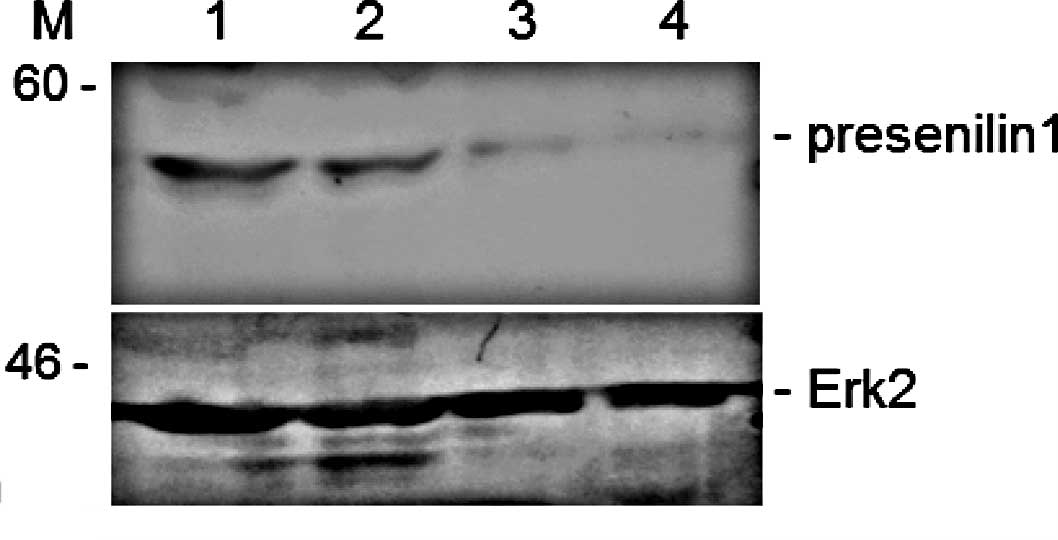
expression. We then investigated whether curcumin, a major
component of turmeric, reduces presenilin 1 expression. After
treating the cells with different concentrations of curcumin,
presenilin 1 protein, but not Erk2 protein expression was decreased
with increasing concentrations of the curcumin extract. A final
concentration of 100 μg/ml of the curcumin extract inhibited
expression of presenilin 1 by >95% in the Jurkat cells (Fig. 4). The turmeric extract also
down-regulated presenilin 1 in a dose-dependent manner in the K562
cells (data not shown).
A recent in vivo study demonstrated that
curcumin was able to reduce amyloid-β-related pathology in
transgenic Alzheimer's disease mouse models via unknown molecular
mechanisms (12). Curcumin is a
small-molecule fluorescent compound found in the widely used
culinary spice, turmeric, which possesses potent biological
activities, including anti-inflammatory, anti-fibrilogenic and
antioxidant, as well as chemopreventative effects and effects on
protein trafficking. Curcumin has also been found to lower
amyloid-β protein levels by attenuating the maturation of APP in
the secretory pathway. Curcumin was demonstrated to promote a
significant reversal of structural changes in dystrophic dendrites,
including abnormal curvature and dystrophy size (13). The computed ionization potential
and electron affinity show that curcumin has a low-molecular
hardness, and the resulting charge undergoes delocalization
throughout the structure, resulting in excitonic features. This
feature appears to be important for its binding capability to human
proteins, such as human serum albumin and amyloid-β (14). Together, these data suggest that
curcumin reverses Alzheimer's disease pathology and propose a
mechanism of action for the ability of curcumin to attenuate
amyloid-β pathology. This approach may lead to more effective
clinical therapies for the prevention of oxidative stress,
inflammation and neurotoxicity associated with Alzheimer's
disease.
In the present study, curcumin and the other spice
components dose-dependently down-regulated presenilin 1 protein,
which plays a pivotal role in γ-secretase activity. Recently,
ubiquilin-1 was reported to regulate the proteasomal degradation of
proteins, including presenilin (15). Since the proteasome is responsible
for the removal of oxidatively damaged proteins in the cytosol and
nucleus during oxidative stress (16), it is plausible that presenilin 1
protein may be degraded by the ubiquitin proteasome pathway
(17). Various herbs and spices
may accelerate this degradation. Further study including in
vivo experiments must be undertaken to elucidate the precise
molecular mechanisms of these herbs particularly in regard to the
treatment of Alzheimer's disease.
Acknowledgements
This study was supported by
grants-in-aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan, and the Nara Women's University
Intramural Grant for Project Research.
References
|
1.
|
Bekris LM, Yu CE, Bird TD and Tsuang DW:
Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol.
23:213–227. 2010. View Article : Google Scholar
|
|
2.
|
Ittner LM and Götz J: Amyloid-β and tau –
a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci.
12:65–72. 2011.
|
|
3.
|
Woo HN, Baik SH, Park JS, Gwon AR, Yang S,
Yun YK and Jo DG: Secretases as therapeutic targets for Alzheimer's
disease. Biochem Biophys Res Commun. 404:10–15. 2011.
|
|
4.
|
Crews L, Rockenstein E and Masliah E: APP
transgenic modeling of Alzheimer's disease: mechanisms of
neurodegeneration and aberrant neurogenesis. Brain Struct Funct.
214:111–126. 2010.
|
|
5.
|
Al-Mofleh IA: Spices, herbal xenobiotics
and the stomach: friends or foes? World J Gastroenterol.
16:2710–2719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang YJ, Thomas P, Zhong JH, Bi FF,
Kosaraju S, Pollard A, Fenech M and Zhou XF: Consumption of grape
seed extract prevents amyloid-beta deposition and attenuates
inflammation in brain of an Alzheimer's disease mouse. Neurotox
Res. 15:3–14. 2009.PubMed/NCBI
|
|
7.
|
Thomas P, Wang YJ, Zhong JH, Kosaraju S,
O'Callaghan NJ, Zhou XF and Fenech M: Grape seed polyphenols and
curcumin reduce genomic instability events in a transgenic mouse
model for Alzheimer's disease. Mutat Res. 661:25–34.
2009.PubMed/NCBI
|
|
8.
|
Mei Z, Zhang F, Tao L, Zheng W, Cao Y,
Wang Z, Tang S, Le K, Chen S, Pi R and Liu P: Cryptotanshinone, a
compound from Salvia miltiorrhiza modulates amyloid
precursor protein metabolism and attenuates beta-amyloid deposition
through upregulating alpha-secretase in vivo and in vitro. Neurosci
Lett. 452:90–95. 2009.PubMed/NCBI
|
|
9.
|
Kelloff GJ, Crowell JA, Steele VE, Lubet
RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R,
Lawrence JA, Ali I, Viner JL and Sigman CC: Progress in cancer
chemo-prevention: development of diet-derived chemopreventive
agents. J Nutr. 130:S467–S471. 2000.
|
|
10.
|
Ganguli M, Chandra V, Kamboh MI, Johnston
JM, Dodge HH, Thelma BK, Juyal RC, Pandav R, Belle SH and DeKosky
ST: Apolipoprotein E polymorphism and Alzheimer disease: the
Indo-US Cross-National Dementia Study. Arch Neurol. 57:824–830.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Okumura N, Nishimura Y, Yoshida H, Nagata
Y, Kitagishi Y and Matsuda S: Ethanol extract of herb Sage
suppressed the TIMP-1 expression in lymphoid culture cells. Asia J
Sci Technol. 3:64–66. 2010.
|
|
12.
|
Zhang C, Browne A, Child D and Tanzi RE:
Curcumin decreases amyloid-beta peptide levels by attenuating the
maturation of amyloid-beta precursor protein. J Biol Chem.
285:28472–28480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Garcia-Alloza M, Borrelli LA, Rozkalne A,
Hyman BT and Bacskai BJ: Curcumin labels amyloid pathology in vivo,
disrupts existing plaques, and partially restores distorted
neurites in an Alzheimer mouse model. J Neurochem. 102:1095–1104.
2007. View Article : Google Scholar
|
|
14.
|
Balasubramanian K: Molecular orbital basis
for yellow curry spice curcumin's prevention of Alzheimer's
disease. J Agric Food Chem. 54:3512–3520. 2006.PubMed/NCBI
|
|
15.
|
Viswanathan J, Haapasalo A, Böttcher C, et
al: Alzheimer's disease-associated ubiquilin-1 regulates
presenilin-1 accumulation and aggresome formation. Traffic.
12:330–348. 2011.
|
|
16.
|
Breusing N and Grune T: Regulation of
proteasome-mediated protein degradation during oxidative stress and
aging. Biol Chem. 389:203–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Magini A, Urbanelli L, Ciccarone V,
Tancini B, Polidoro M, Timperio AM, Zolla L, Tedde A, Sorbi S and
Emiliani C: Fibroblasts from PS1-mutated pre-symptomatic subjects
and Alzheimer's disease patients share a unique protein level
profile. J Alzheimers Dis. 21:431–444. 2010.PubMed/NCBI
|