Introduction
Apoptosis is an essential mechanism to eliminate
unwanted cells during the development and homeostasis of multi
cellular organisms (1,2). An imbalance between cell death and
proliferation may lead to the incidence of cancer. There are two
main apoptotic pathways in humans, the extrinsic pathway and the
intrinsic pathway (3). During the
apoptotic process, both of the two pathways use the caspase enzyme
cascade; the extrinsic pathway utilizes caspases-8 and -10, while
the intrinsic pathway employs caspase-9 and the converge to use
caspases-3, -6 and -7 as effector caspases, which lead to cell
death by nuclear membrane breakdown, DNA fragmentation, chromatin
condensation and the formation of apoptotic bodies (4–6).
Caspase-9, an apoptosis-related cysteine peptidase,
encoded by the CASP-9 gene, located on chromosome l at 1p36.21, is
a member of the caspase (cysteine aspartate protease) family of
proteins. It has been shown to be an executioner protein of
apoptosis. Caspase-9 plays a central role in the execution-phase of
cell apoptosis. Single nucleotide polymorphisms (SNPs) are the most
common form of human genetic variation and may contribute to an
individual’s susceptibility to cancer (7). In recent years, few studies have been
performed to investigate the associations between effector caspases
and cancer risk. Lan et al(8) found that CASP-9 was significantly
associated with a decreased risk for non-Hodgkin lymphoma. Hosgood
et al(9) found that
individuals with the AG and AA genotypes of CASP-9 Ex5+32 G>A
experienced a decreased risk of multiple myeloma. However, we are
still may unable to reach a consistent conclusion concerning the
association between the CASP-9 Ex5+32 G>A (rs1052576)
polymorphism and cancer risk according to previous studies.
Therefore, we performed a Human Genome Epidemiology (HuGE) review
and meta-analysis by including the most recent and relevant
articles to identify statistical evidence.
Materials and methods
Literature search
We performed an electronic search of the PubMed,
Cochrane library, Embase, Web of Science, SpringerLink, CNKI and
CBM databases extensively to identify relevant studies available up
to May 1, 2012. The search terms included [‘caspase-9’ or ‘CASP-9’
or ‘caspase-9’ (Mesh)] and [‘SNPs’ or ‘SNP’ or ‘polymorphism,
genetic’ (Mesh)] and [‘cancer’ or ‘tumor’ or ‘neoplasms’ (Mesh)].
References in the eligible studies or textbooks were also reviewed
through a manual search to identify other potentially eligible
studies.
Inclusion and exclusion criteria
The included studies were required to meet the
following criteria: i) case-control studies focusing on
associations between the CASP-9 Ex5+32 polymorphism and cancer
risk; ii) all patients were diagnosed with malignant tumors
confirmed by pathological examination of the surgical specimen;
iii) the frequencies of alleles or genotypes in case and control
groups could be extracted; iv) the publication was in English or
Chinese. Studies were excluded when they were: i) not case-control
studies concerning CASP-9 Ex5+32 polymorphism and cancer risk; ii)
based on incomplete data; iii) irrelevant or overlapping data were
reported; iv) meta-analyses, letters, reviews or editorial
articles.
Data extraction
Using a standardized form, data from published
studies were extracted independently by two reviewers (S. Yan and
Y.-Z. Li) to populate the necessary information. The following
information was extracted from each of the articles: first author,
year of publication, country, language, ethnicity, study design,
source of cases and controls, number of cases and controls, mean
age, sample, cancer type, genotype method, allele and genotype
frequency, and evidence of Hardy-Weinberg equilibrium (HWE) in
controls. In case of conflicting evaluations, an agreement was
reached following a discussion with a third reviewer (Y.-L.
Liu).
Quality assessment of included
studies
Two reviewers (S. Yan and Y.-Z. Li) independently
assessed the quality of papers according to modified STROBE quality
score systems (10,11). Forty assessment items related to
the quality appraisal were used in this meta-analysis, scores
ranging from 0 to 40. Scores of 0–20, 20–30 and 30–40 were defined
as low, moderate and high quality, respectively. Disagreement was
resolved by discussion.
Statistical analysis
The odds ratio (OR) and 95% confidence interval (95%
CI) were calculated using Review Manager Version 5.1.6 (provided by
the Cochrane Collaboration, available at: http://ims.cochrane.org/revman/download) and STATA
Version 12.0 (StataCorp, College Station, TX) softwares.
Between-study variations and heterogeneities were estimated using
Cochran’s Q-statistic (12,13)
(P≤0.05 was considered to be a manifestation of statistically
significant heterogeneity). We also quantified the effect of
heterogeneity by using I2 test, which ranges from 0 to
100% and represents the proportion of inter-study variability that
can be contributed to heterogeneity rather than by chance. When a
significant Q-test (P≤0.05) or I2>50% indicated that
heterogeneity among studies existed, the random effects model was
conducted for meta-analysis. Otherwise, the fixed effects model was
used. To establish the effect of heterogeneity on the conclusions
of the meta-analyses, subgroup analysis was carried out. We tested
whether genotype frequencies of controls were in HWE using the
χ2 test. Funnel plots are often used to detect
publication bias. However, due to limitations caused by varied
sample sizes and subjective reviews, Egger’s linear regression test
which measures funnel plot asymmetry using a natural logarithm
scale of OR was used to evaluate the publication bias (14). When the P-value was <0.1,
publication bias was considered significant. All the P-values were
two-sided. To ensure the reliability and the accuracy of the
results, two reviewers (S. Yan and Y.-Z. Li) populated the data in
the statistical software programs independently and obtained the
same results.
Results
Characteristics of the included
studies
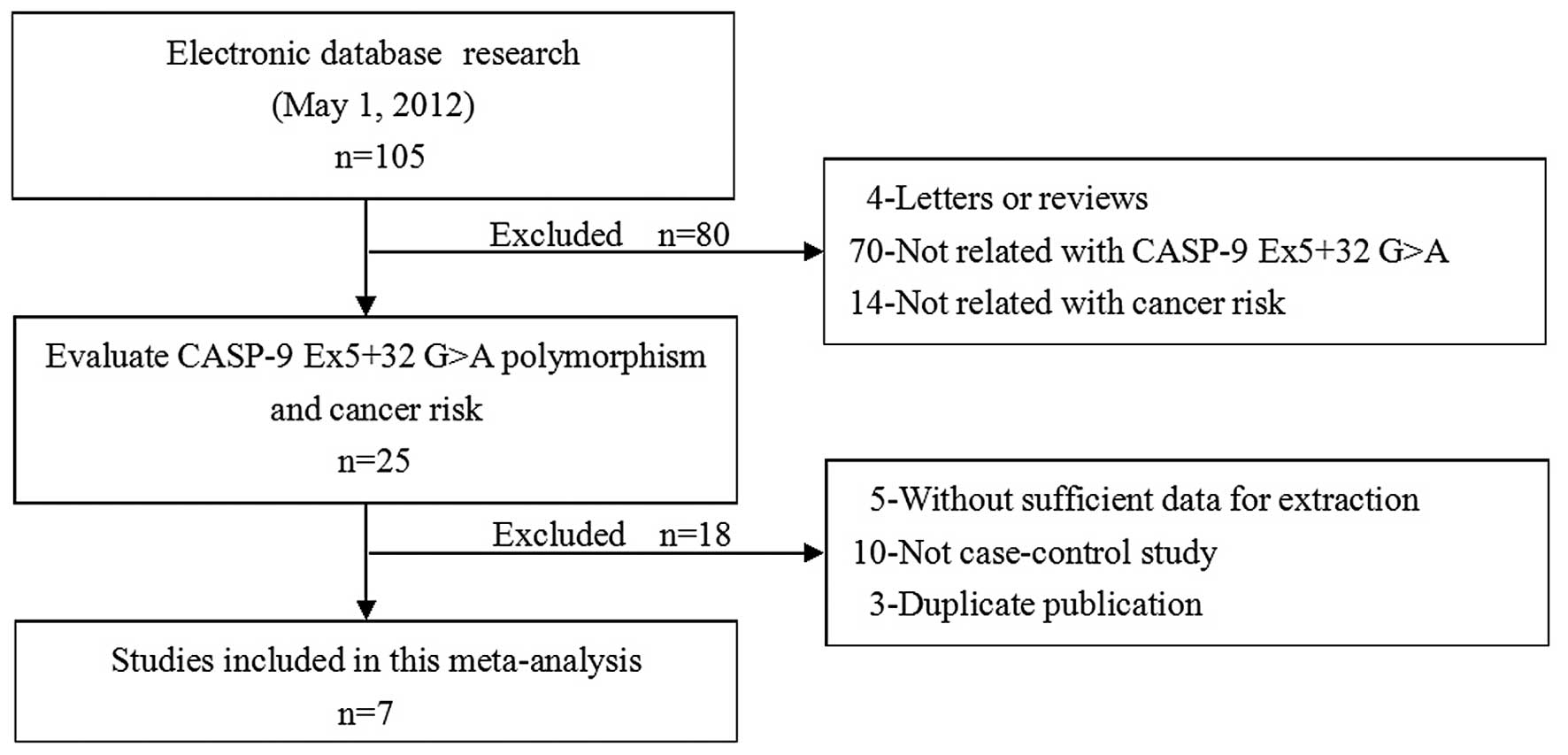
According to the inclusion criteria, seven studies
(8,9,15–19)
met the inclusion criteria and were subjected to further
examination. The flow chart of the study selection is shown in
Fig. 1. In total, 1668 cancer
cases and 2294 healthy controls from seven studies were included in
the pooled analysis. The publication year of involved studies
ranged from 2007 to 2009. Overall, there were six types of cancers
studied, including gastric cancer, lymphoma, lung cancer, colon
cancer, myeloma and liver cancer. One of these studies was
conducted in Russia, two in USA and four studies in China. The HWE
test was performed on the genotype distribution of the controls in
all included studies; all were in HWE (P>0.05). All quality
scores of the included studies were higher than 20 (moderate-high
quality). The characteristics and methodological quality of the
included studies are summarized in Table I. The genotype distributions of the
CASP-9 Ex5+32 G>A polymorphism in the case and control groups
are presented in Table II.
 | Table ICharacteristics of the individual
studies in this meta-analysis. |
Table I
Characteristics of the individual
studies in this meta-analysis.
| | | Number
| | | | |
|---|
| Author/(Ref.) | Year | Country | Case | Control | Sample | Genotype method | Cancer type | Quality score |
|---|
| Fang et
al(15) | 2007 | China | 70 | 100 | Blood | PCR-RFLP | Gastric cancer | 23 |
| Lan et
al(8) | 2007 | USA | 461 | 535 | Blood | DNA sequencing | Lymphoma | 22 |
| Lou et
al(16) | 2007 | China | 81 | 100 | Blood | PCR-RFLP | Lung cancer | 24 |
| He et
al(17) | 2008 | China | 170 | 100 | Blood | PCR-RFLP | Colon cancer | 21 |
| Hosgood et
al(9) | 2008 | USA | 128 | 516 | Blood/Tissue | DNA sequencing | Myeloma | 22 |
| Ulybina et
al(18) | 2009 | Russia | 111 | 110 | Blood | AS-PCR | Lung cancer | 24 |
| Wu et
al(19) | 2009 | China | 647 | 833 | Blood | PCR-RFLP | Liver cancer | 28 |
 | Table IIGenotype distribution of the CASP-9
Ex5+32 G>A polymorphism in the case and control groups. |
Table II
Genotype distribution of the CASP-9
Ex5+32 G>A polymorphism in the case and control groups.
| Case
| Control
| HWE test
|
|---|
| Author/(Ref.) | Total | G | A | GG | GA | AA | GA+AA | TA | Total | G | A | GG | GA | AA | GA+AA | TA | χ2 | P-value | Test |
|---|
| Fang et
al(15) | 70 | 71 | 69 | 16 | 39 | 15 | 54 | 140 | 100 | 68 | 132 | 13 | 42 | 45 | 87 | 200 | 0.412 | 0.521 | HWE |
| Lan et
al(8) | 455 | 497 | 413 | 137 | 223 | 95 | 318 | 910 | 530 | 534 | 526 | 137 | 260 | 133 | 393 | 1060 | 0.188 | 0.665 | HWE |
| Lou et
al(16) | 81 | 83 | 79 | 20 | 43 | 18 | 61 | 162 | 100 | 68 | 132 | 13 | 42 | 45 | 87 | 200 | 0.412 | 0.521 | HWE |
| He et
al(17) | 170 | 163 | 177 | 34 | 95 | 41 | 136 | 340 | 100 | 68 | 132 | 13 | 42 | 45 | 87 | 200 | 0.412 | 0.521 | HWE |
| Hosgood et
al(9) | 126 | 147 | 105 | 41 | 65 | 20 | 85 | 252 | 511 | 512 | 510 | 130 | 252 | 129 | 381 | 1022 | 0.096 | 0.757 | HWE |
| Ulybina et
al(18) | 111 | 88 | 134 | 16 | 56 | 39 | 95 | 222 | 110 | 93 | 127 | 20 | 53 | 37 | 90 | 220 | 0.018 | 0.893 | HWE |
| Wu et
al(19) | 100 | 130 | 70 | 43 | 44 | 13 | 57 | 200 | 60 | 78 | 42 | 25 | 28 | 7 | 35 | 120 | 0.039 | 0.843 | HWE |
Main results and subgroup analysis
A summary of the findings of the meta-analysis of
the association between CASP-9 Ex5+32 G>A polymorphism and
cancer risk is provided in Table
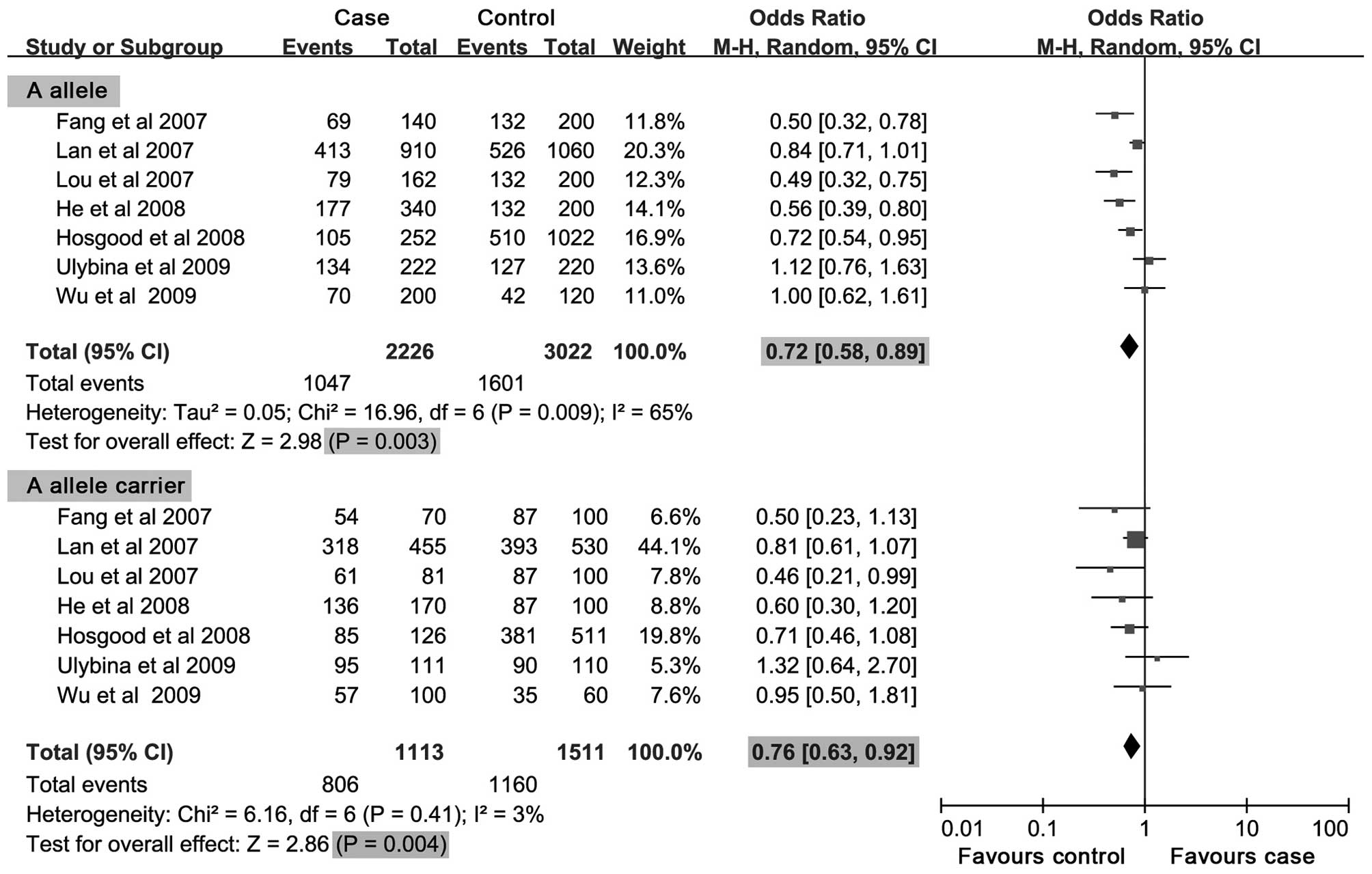
III. The meta-analysis results showed that the A allele and A
allele carrier of Ex5+32 G>A in the CASP-9 gene had negative
associations with cancer risk (OR= 0.72, 95% CI, 0.58–0.89, P=
0.003; OR= 0.76, 95% CI, 0.63–0.92, P= 0.004; respectively)
(Fig. 2). In the subgroup analysis
by country, we found that the A allele of Ex5+32 G>A was a
protective factor for cancer risk in Chinese and American
populations (OR= 0.60, 95% CI, 0.44–0.81, P<0.001; OR= 0.80, 95%
CI, 0.69–0.94, P=0.005; respectively), but no association was found
between the Ex5+32 G>A polymorphism with cancer risk in the
Russian population. For the A allele carrier of the Ex5+32 G>A
polymorphism, we also found positive associations with cancer risk
in Chinese and American populations (OR=0.63, 95% CI, 0.44–0.90, P=
0.01; OR= 0.78, 95% CI, 0.62–0.98, P=0.03; respectively).
Unfortunately, there was no significant difference between the
Ex5+32 G>A polymorphism with cancer susceptibility in the
Russian population. In the subgroup analysis by ethnicity, we found
that the A allele and A allele carrier of Ex5+32 G>A in CASP-9
might decrease the risk of cancer in the Asian population (OR=0.60,
95% CI, 0.44–0.81, P<0.001; OR= 0.63, 95% CI, 0.44–0.90, P=
0.01; respectively). However, there were no associations found
among A allele and A allele carrier of Ex5+32 G>A in CASP-9 with
risk in the Caucasian population (all P>0.05).
 | Table IIIMeta-analysis of the association
between the Ex5+32 G>A polymorphism and cancer risk. |
Table III
Meta-analysis of the association
between the Ex5+32 G>A polymorphism and cancer risk.
| Comparison | Case n/N | Control n/N | OR (95% CI) | P-value | Effect model |
|---|
| A allele | 1047/2226 | 1601/3022 | 0.72
(0.58–0.89) | 0.003 | Random |
| Subgroup analysis
by country | | | | | |
| Chinese | 395/842 | 438/720 | 0.60
(0.44–0.81) | <0.001 | |
| American | 518/1162 | 1036/2082 | 0.80
(0.69–0.94) | 0.005 | |
| Russian | 134/222 | 127/220 | 1.12
(0.76–1.63) | 0.57 | |
| Subgroup analysis
by ethnicity | | | | | |
| Caucasian | 652/1384 | 1163/2302 | 0.85
(0.70–1.04) | 0.11 | |
| Asian | 395/842 | 438/720 | 0.60
(0.44–0.81) | <0.001 | |
| A allele
carrier | 806/1113 | 1160/1511 | 0.76
(0.63–0.92) | 0.004 | Random |
| Subgroup analysis
by country | | | | | |
| Chinese | 308/421 | 296/360 | 0.63
(0.44–0.90) | 0.01 | |
| American | 403/581 | 774/1041 | 0.78
(0.62–0.98) | 0.03 | |
| Russian | 95/111 | 90/110 | 1.32
(0.64–2.70) | 0.45 | |
| Subgroup analysis
by ethnicity | | | | | |
| Caucasian | 498/692 | 864/1151 | 0.82
(0.66–1.02) | 0.08 | |
| Asian | 308/421 | 296/360 | 0.63
(0.44–0.90) | 0.01 | |
Publication bias
Publication bias of the literature was assessed by
Begger’s funnel plot and Egger’s linear regression test. Egger’s
linear regression test was used to measure the asymmetry of the
funnel plot. All graphical funnel plots of the included studies
appeared to be symmetrical (Fig.
3). Egger’s test also showed that there was no statistical
significance for all evaluations of publication bias (all
P>0.05).
Discussion
Apoptosis is a particular type of programmed cell
death which commonly occurs in the developing embryo, in normal
healthy adult tissues and in many pathological settings (20). The morphological features of
apoptosis include changes in plasma membrane asymmetry and
attachment, condensation of cytoplasm, nucleus and internucleosomal
cleavage of DNA (21). However,
excessive or failed apoptosis is a prominent morphological feature
of several human diseases (24).
Activation of caspases is of fundamental importance in cell death
commitment and hence substantial efforts have been devoted to the
understanding of mechanisms that underlie their activation
(22,23).
Caspase-9 is a key regulator of apoptosis or
programmed cell death, an essential defense mechanism against
hyper-proliferation and malignancy. Polymorphic variation in the
CASP-9 gene has been reported to influence cancer risk, especially
in the Ex5+32 variant. The published studies of an association
between the CASP-9 Ex5+32 variant and different cancers have
generated inconsistent results. Lou et al(16) reported that the rs1052576 which
locates in exon 5 of the CASP-9 gene was associated with non-small
cell lung cancer. However, a multi-center epidemiological
case-control study was not consistent with other previously
published data on non-Hodgkin lymphoma (8). This controversy might be due to
population and ethnicity of the corresponding studies. In this
meta-analysis, including a total of 1668 cancer cases and 2294
healthy controls from seven independent studies, we examined the
association of the Ex5+32 G>A polymorphism of the CASP-9 gene
with cancer risk. We demonstrated that A allele and A allele
carrier in the Ex5+32 G>A polymorphism had negative associations
with cancer susceptibility, which showed a protective effect of the
CASP-9 gene against cancer development. Ethnicity may influence
cancer susceptibility by different genetic backgrounds and
environmental exposures through gene-gene and gene-environmental
interactions. Subgroup analysis showed that A allele and A allele
carrier were protective factors for cancer risk in Chinese and
American populations. Contrary to our expectations, we found no
association between the Ex5+32 G>A polymorphism and cancer risk
in Russian population. In addition, we also identified that A
allele and A allele carrier of Ex5+32 G>A might decrease the
risk of cancer in the Asian population, but not in the Caucasian
population.
Similar to other meta-analyses, a number of
limitations of this study should be addressed. First, the relevant
research articles are not many and the sample size of this
meta-analysis was not large. In addition, some relevant studies
could not be included in our analysis due to incomplete raw data.
Thirdly, we were not able to address the sources of heterogeneity
among all studies. Fourthly, although all cases and controls of
each study were well defined with similar inclusion criteria, there
may be potential factors that were not taken into account that may
have influenced our results. Most important of all, our
meta-analysis was based on unadjusted OR estimates since not all
published presented adjusted ORs or when they did, the ORs were not
adjusted by the same potential confounders, such as ethnicity,
gender and geographic distribution. Given these results, additional
investigation in these areas is needed, and our conclusions should
be interpreted cautiously.
In conclusion, this meta-analysis of seven
case-control studies demonstrated that the CASP-9 Ex5+32 G>A
polymorphism is involved in the pathogenesis of variant cancer. The
A allele, A allele carrier and AA genotype of Ex5+32 G>A
polymorphism may be protective factors for cancer risk. As few
studies are available in this field current evidence remains
limited. Therefore, it is necessary to conduct large studies with
adequate methodological quality, properly controlling confounds in
order to obtain valid results.
Acknowledgements
We would like to thank Mr. J.L. Liu
(MedChina Medical Information Service Co., Ltd.) for his valuable
contribution and revision to the manuscript.
References
|
1
|
Raff M: Cell suicide for beginners.
Nature. 396:119–122. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobson MD, Weil M and Raff MC:
Programmed cell death in animal development. Cell. 88:347–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Theodoropoulos GE, Gazouli M, Vaiopoulou
A, Leandrou M, Nikouli S, Vassou E, Kouraklis G and Nikiteas N:
Polymorphisms of caspase 8 and caspase 9 gene and colorectal cancer
susceptibility and prognosis. Int J Colorectal Dis. 26:1113–1118.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hajra KM and Liu JR: Apoptosome
dysfunction in human cancer. Apoptosis. 9:691–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicholson DW and Thornberry NA: Caspases:
killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar
|
|
6
|
Kesarwani P, Mandal RK, Maheshwari R and
Mittal RD: Influence of caspases 8 and 9 gene promoter polymorphism
on prostate cancer susceptibility and early development of hormone
refractory prostate cancer. BJU Int. 107:471–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Son JW, Kang HK, Chae MH, Choi JE, Park
JM, Lee WK, Kim CH, Kim DS, Kam S, Kang YM and Park JY:
Polymorphisms in the caspase-8 gene and the risk of lung cancer.
Cancer Genet Cytogenet. 169:121–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan Q, Zheng T, Chanock S, Zhang Y, Shen
M, Wang SS, Berndt SI, Zahm SH, Holford TR, Leaderer B, et al:
Genetic variants in caspase genes and susceptibility to non-Hodgkin
lymphoma. Carcinogenesis. 28:823–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hosgood HD III, Baris D, Zhang Y, Zhu Y,
Zheng T, Yeager M, Welch R, Zahm S, Chanock S, Rothman N and Lan Q:
Caspase polymorphisms and genetic susceptibility to multiple
myeloma. Hematol Oncol. 26:148–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative: The
Strengthening the Reporting of Observational Studies in
Epidemiology (STROBE) statement: guidelines for reporting
observational studies. Epidemiology. 18:800–804. 2007.
|
|
11
|
Zhang L, Liu JL, Zhang YJ and Wang H:
Association between HLA-B*27 polymorphisms and
ankylosing spondylitis in Han populations: a meta-analysis. Clin
Exp Rheumatol. 29:285–292. 2011.PubMed/NCBI
|
|
12
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang CQ, Liu SL, Lou Y and Li JH:
Expression of the caspase 9 gene and its polymorphism distribution
in gastric cancer. Shijie Huaren Xiaohua Zazhi. 15:3190–3193.
2007.(In Chinese).
|
|
16
|
Lou Y, Fang CQ and Li JH: A study on the
expression of CASP9 gene and its polymorphism distribution in
non-small cell lung cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
24:59–62. 2007.(In Chinese).
|
|
17
|
He XM, Wang LL, Fang CQ, Liu SL, Lou Y and
Li JH: Expression of CASP9 gene and its polymorphism distribution
in colon cancer. Shijie Huaren Xiaohua Zazhi. 16:2371–2375.
2008.(In Chinese).
|
|
18
|
Ulybina YM, Kuligina ESh, Mitiushkina NV,
Rozanov ME, Ivantsov AO, Ponomariova DN, Togo AV, Levchenko EV,
Shutkin VA, Brenister SI, et al: Coding polymorphisms in Casp5,
Casp8 and DR4 genes may play a role in predisposition to lung
cancer. Cancer Lett. 278:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu H: Correlation between DNA pepair gene
XRCC1 single nucleotide polymorphism and susceptibility to
hepatocellular carcinoma in Fusui County of Guangxi. Guangxi
Medical University; 2009
|
|
20
|
Alison MR and Sarraf CE: Apoptosis: a
gene-directed programme of cell death. JR Coll Physicians Lond.
26:25–35. 1992.PubMed/NCBI
|
|
21
|
Doonan F and Cotter TG: Morphological
assessment of apoptosis. Methods. 44:200–204. 2008. View Article : Google Scholar
|
|
22
|
Kumar S: Measurement of caspase activity
in cells undergoing apoptosis. Methods Mol Biol. 282:19–30.
2004.PubMed/NCBI
|
|
23
|
Kumar S and Dorstyn L: Analyzing caspase
activation and caspase activity in apoptotic cells. Methods Mol
Biol. 559:3–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicholson DW: ICE/CED3-like proteases as
therapeutic targets for the control of inappropriate apoptosis. Nat
Biotechnol. 14:297–301. 1996. View Article : Google Scholar : PubMed/NCBI
|