Introduction
Coronary heart disease is one of the most common and
serious diseases affecting human health. Since Gruentzig (1) completed the first percutaneous
transluminal coronary angioplasty (PTCA) in Zurich, Switzerland in
1977, interventional therapy has become an effective means of
treating coronary heart diseases; however, PTCA has a high
incidence of restenosis (2).
Coronary stent implantation effectively suppresses the vascular
elastic recoil, negative remodeling and thrombosis; however, 15–30%
cases present in-stent restenosis (3). The higher incidence of restenosis
increases patients’ suffering and the economic burden imposed on
society, and affects the long-term efficacy and clinical
application of PTCA. Research into the mechanisms of restenosis to
assist in the effective prevention of restenosis is the key to
improving the effects of percutaneous coronary intervention, which
is an issue of serious concern to cardiovascular physicians.
The mechanisms of restenosis following PTCA involve
the synthesis, release and chemical chemotaxis of inflammatory
cytokines and growth factors, the degradation and synthesis of
extracellular matrix (ECM), phenotypic switching, proliferation and
migration of smooth muscle cells, neointimal thickening and
vascular remodeling (VR). Using histology and microscopy, studies
have focused on the medial membrane and intima of the arteries
(4). However, few studies have
considered the vascular adventitia. The role of the vascular
adventitia is gradually gaining attention (5). Shi et al (6) reported that the adventitia of pig
coronary arteries change following balloon injury. A study of
restenosis following angioplasty demonstrated that in addition to
the smooth muscle cells of the vascular intima, the vascular
adventitial fibroblasts were also involved in restenosis (7), indicating that the adventitia is not
only a ‘bystander’ in a traditional sense, but also combines with
the smooth muscle cells of the intima and medial membrane to
participate in the restenosis of blood vessels. However, the
mechanism of the vascular adventitia in restenosis following
angioplasty remains unclear. This study aimed to explore the
mechanism of the vascular adventitia, particularly the role of
fibroblasts, in vascular restenosis following balloon injuries.
Pure line Sprague-Dawley (SD) rats were used to establish the
restenosis models following angioplasty based on balloon-induced
intimal injuries. The rats underwent pathological hematoxylin and
eosin (H&E) staining and immunohistochemical staining of the
injured blood vessels and image analysis processing in the second
and sixth postoperative week to explore the VR in the restenosis
models of injured blood vessels, as well as the mechanisms of
activation, proliferation and phenotypic switching in vascular
adventitia cells. The effect and mechanism of the vascular
adventitia in vascular restenosis following balloon-induced intimal
injuries were observed based on whole-animal experiments, and may
lead to a new approach in the prevention and treatment of vascular
restenosis.
Materials and methods
Animal groups
A total of 32 healthy female SD rats weighing 200±20
g were provided by the Experimental Animal Research Center of
Ruijin Hospital affiliated to Shanghai Jiaotong University. The
6-week-old pure line SD rats were divided into two groups,
including the control group (n=16) and the balloon-injured group
(n=16). This study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of Shandong University.
Establishment of animal models
The rats in the balloon-injured group underwent
intraperitoneal anesthesia with ketamine and were fixed in the
supine position. A midline incision of the abdomen was made under
sterile conditions, through which the small intestine and part of
the large intestine were removed and wrapped with physiological
saline gauzes. The abdominal aorta and inferior vena cava were
bluntly dissected and a transverse incision was made ∼1 cm below
the renal artery branch of the abdominal aorta. A balloon catheter
was inserted retrogradely along the abdominal aorta into the aortic
arch, to a depth of ∼7–8 cm. The balloon was filled with 8–10 atm
heparin saline solution and then slowly pulled back to the
incision, reducing the pressure inside the balloon at the same time
to maintain a certain resistance. Thereafter, the balloon catheter
was inserted into the aortic arch again and then was finally
withdrawn after being repeated three times. Following balloon
injury surgery, the incision and intravascular thromboses were
washed with heparin saline solution. The incision was sutured using
4–5 needles with an 8/0 vascular suture under a surgical
microscope. Before the last two stitches of suturing, intravascular
bubbles were discharged with heparin saline solution. The rats
underwent postoperative intraperitoneal injection of 800,000 U/kg
body weight penicillin for 3 days.
Production of paraffin sections
Each group of rats was randomly narcotized and
sacrificed (n=16) on the second and sixth postoperative weeks.
Their aortas from the aortic arches to the horizontal segments of
the abdominal aorta in the openings of the renal arteries were
immediately removed and washed with physiological saline to remove
the attached blood cells. The abdominal aortic segments were
clipped with surgical scissors and fixed in 4% neutral
paraformaldehyde to prepare the paraffin sections.
Immunohistochemical detection and
histomorphological analysis
The vascular ring of the paraffin sections underwent
immunohistochemical staining using the SABC method. Firstly, mouse
anti-rat proliferating cell nuclear antigen (PCNA; Zymed Lab Inc.,
San Diego, CA, USA) and α-smooth muscle actin (α-SMA; Neomarkers
Inc., Fremont, CA, USA) antibodies were added (1:200 dilution) and
the sections were incubated overnight at 4°C, with
phosphate-buffered saline (PBS) as the negative control. Then, the
goat anti-rabbit (Wuhan Boster Biological Technology Ltd., Hubei,
China) and the goat anti-mouse secondary antibodies (Fuzhou Maixin
Biological Technology Co., Fujian, China) were added and the
sections were incubated for 30 min at 37°C, followed by washing
with PBS and 3,3′-diaminobenzidine (DAB) chromogenesis. The
expression levels of PCNA and α-SMA in the vascular adventitia,
medial membrane and intima were observed in each group. The
paraffin sections underwent H&E staining and measurement of the
lumen area (LA), external elastic lamina area (EELA), internal
elastic lamina area (IELA) and the area of vascular adventitia and
surrounding adipose tissue at each time point using a Leica
software image analysis system (Leica, Munich, Germany). VR was
evaluated according to the changes of vascular remodeling index
(VRI), IELA and EELA, while vascular stenosis was evaluated
according to the changes in residual stenosis rate and vessel
LA.
Statistical analysis
SPSS v11.5 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Measurement data are presented
as mean ± standard deviation. Comparisons between the two groups
were performed using t-test and inter-group comparisons using
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of the restenosis model
following balloon injury
Among the 34 rats, 2 rats died due to excessive
bleeding during balloon injury of the endothelium. Therefore, 32
rats were included in the study.
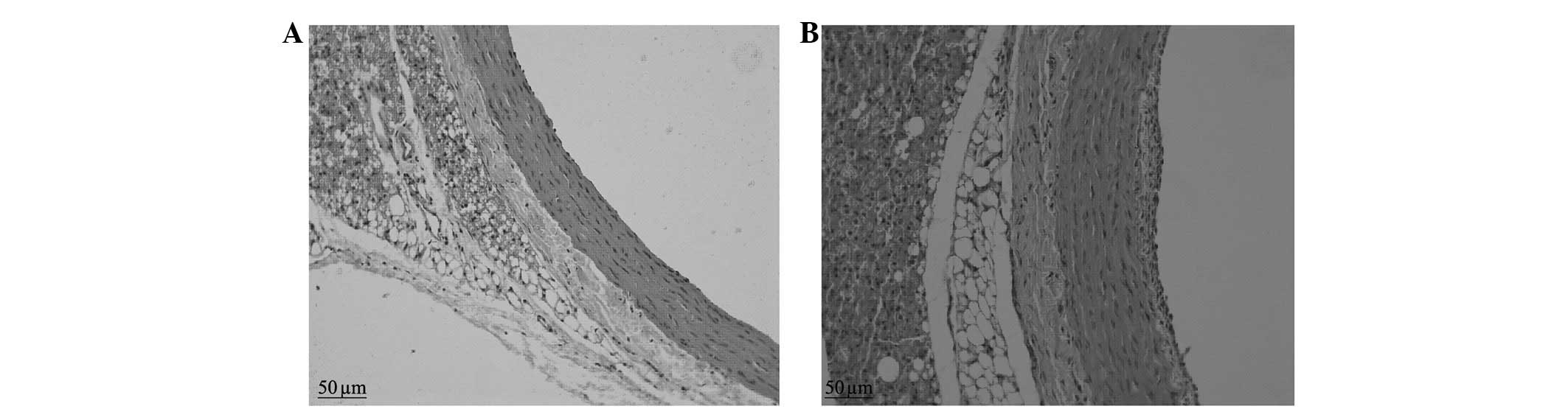
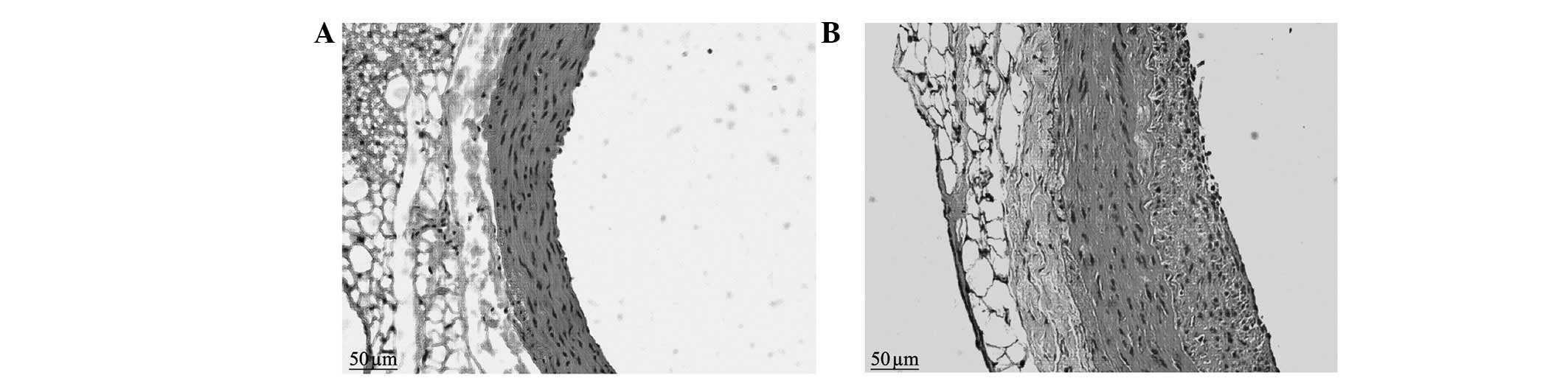
Results of H&E staining
The control group presented vascular walls of even
thickness, a single layer of endothelial cells in the vessel lumen
surface and large round nuclei which were stained blue-violet.
There were no new smooth muscle cells visible in the intima;
however, there was a clear and complete undulating internal and
external elastic lamina and the intima comprised a single layer of
endothelial cells and an extremely small amount of ECM. There were
6–10 layers of wavy elastic fibers visible in the even thickness of
the medial membrane, between which smooth muscle cells were
visible, with fusiform or almost round nuclei of approximately the
same size. The adventitia was located between the external elastic
lamina and the perivascular adipose tissue, constituted by loose
connective tissue, including helical or longitudinal distribution
of elastic fibers, collagen fibers and fibroblasts (Figs. 1 and 2).
The neointimal formation was visible under a
microscope in the balloon-injured group in the second postoperative
week. The LA was reduced compared with that of the control group
and the internal elastic lamina presented multiple fractures, the
vascular adventitia was thickened compared with that of the control
group and the number of nuclei was increased. In the sixth week, a
large amount of neointima was visible on the intimal surface of
injured blood vessels under a microscope. The proliferous neointima
had an extremely uneven thickness, uneven vascular walls and
irregular vessel lumen surfaces. The vessel LAs were clearly
reduced compared with those of the control group. There was
abundant cell proliferation in the neointima, with a clear increase
in the number of blue nuclei, which were disordered and arranged in
different directions. The nuclei varied in size and shape, and
multiple fractures were visible in the internal elastic lamina. The
medial membrane was of uneven thickness and there was no clear
change in the cell morphology. The vascular adventitia was thicker
than that of the control group, with an increased number of nuclei
and thickened vascular walls (Figs.
1 and 2).
Histomorphological analysis
A Leica image analysis system was used to measure
the area and thickness of the vascular adventitia in the two
groups, as well as the number of nuclei following H&E staining.
The results revealed that in the second postoperative week: the
area of the vascular adventitia (0.389±0.048 vs. 0.255±0.030
mm2; P<0.05), the vascular adventitia thickness
(0.152±0.023 vs. 0.096±0.011 mm; P<0.05), and the number of
nuclei (28.63±3.50 vs. 22.25±3.69; P<0.05) were increased
compared with those of control group. In the sixth postoperative
week: the area of the vascular adventitia (0.337±0.066 vs.
0.255±0.029 mm2; P<0.05); the vascular adventitia
thickness (0.130±0.014 vs. 0.095±0.013 mm; P<0.05); and the
number of nuclei (44.88±5.62 vs. 15.75±3.50; P<0.05) were also
increased compared with those of the control group.
Changes in vessel LA, IELA, EELA and
VR
The LA, IELA, EELA, VRI and the residual stenosis
rate of the two groups were measured on the second and sixth weeks
after balloon injury. The results revealed that in the injury group
in the second week compared with the control group in the second
and sixth weeks: the LA was significantly reduced (1.299±0.011 vs.
1.592±0.046 and 1.608±0.001 mm2, respectively;
P<0.05); the IELA was significantly reduced (1.349±0.109 vs.
1.592±0.046 and 1.608±0.001 mm2, respectively;
P<0.05); and the EELA was significantly reduced (1.740±0.106 vs.
2.012±0.037 and 2.005±0.047 mm2, respectively;
P<0.05). In the injured group in the sixth week compared with
the control group in the second and sixth weeks: the LA was
significantly reduced (1.101±0.007 vs. 1.592±0.046 and 1.608±0.001
mm2, respectively; P<0.05); the IELA was
significantly reduced (1.199±0.003 vs. 1.592±0.046 and 1.608±0.001
mm2, respectively; P<0.05); and the EELA was
significantly reduced (1.504±0.001 vs. 2.012±0.037 and 2.005±0.047
mm2, respectively; P<0.05). The intimal area in the
injured group in the sixth week was significantly thickened
compared with that of the injured group in the second week
(0.099±0.007 vs. 0.050±0.011 mm2; P<0.05).
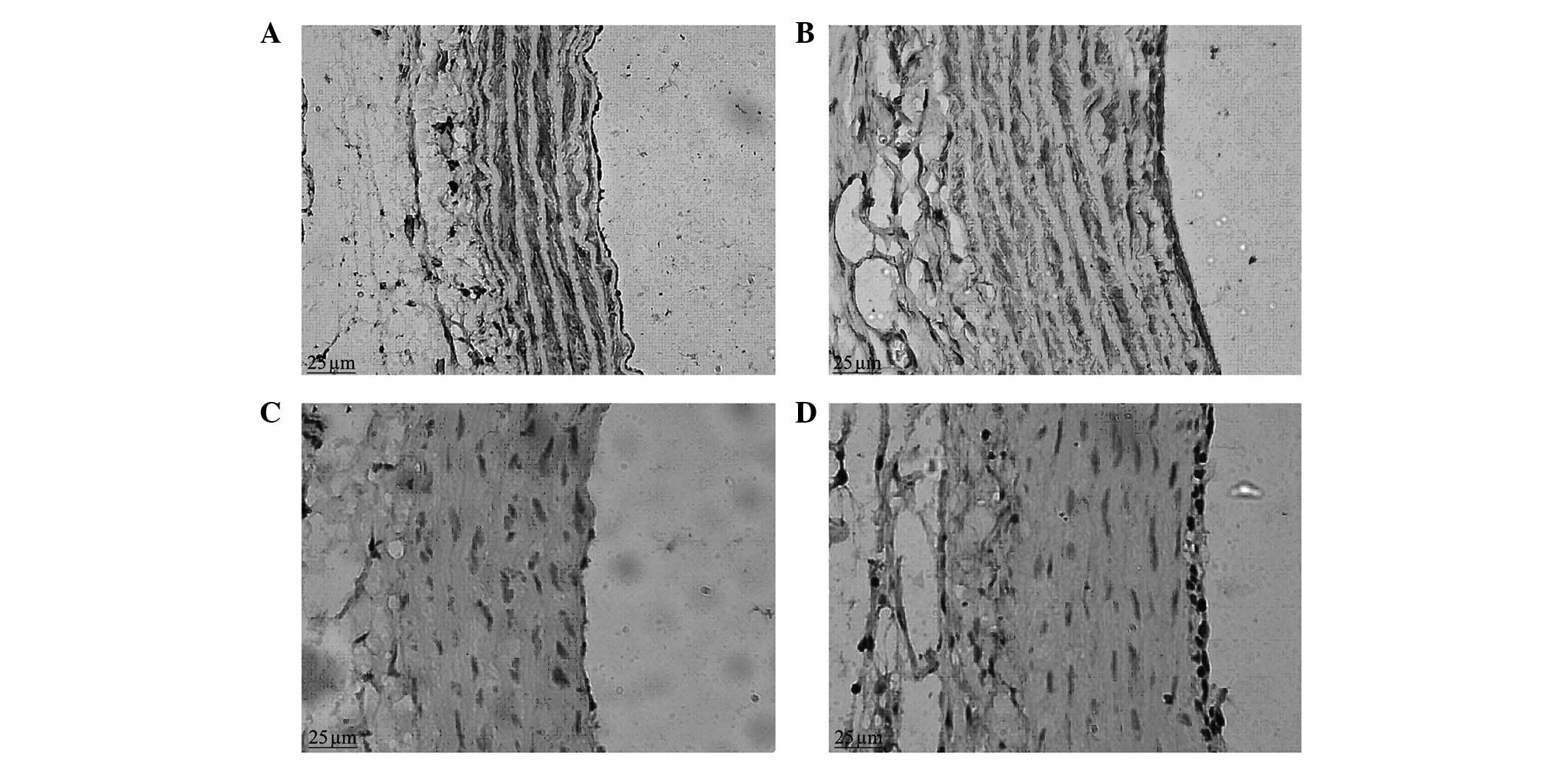
Immunohistochemical staining
In the control group, when observed under a light
microscope, a weak positive expression of α-SMA was observed in the
vascular adventitia; a strong brown positive expression of α-SMA
was clearly visible in the vascular medial membrane, evenly
distributed in the cytoplasm of vascular smooth muscle cells
(VSMCs), with blue nuclei, and there was no positive expression of
α-SMA in the vascular intima. In the balloon-injured group in the
second week, a positive expression of α-SMA was visible in the
vascular adventitia; there was a stronger positive expression of
α-SMA in the neointimal layer and a lower level of expression in
the medial membrane, with variable intensity and uneven
distribution, significantly reduced compared with that of the
normal control group (Fig. 3). In
the balloon-injured group in the sixth week, the expression of
α-SMA in the vascular adventitia and medial membrane was similar to
that of the control group and positive expression of α-SMA was
visible in the thicker intima.
The positive expression of PCNA was observed to be
located in the nucleus, the positive staining was brown and the
negative staining was blue. Under a light microscope, brown
positive expression indicative of PCNA was occasionally observed in
the vascular adventitia, medial membrane and intima in the control
group. In the balloon-injured group in the second week, there was a
large amount of strong brown positive expression of PCNA visible in
the vascular adventitia; there was also strong positive expression
of PCNA visible in the neointima, which tended to be aggregated at
the luminal surface (Fig. 3). In
the balloon-injured group in the sixth week, the vascular
adventitia cells were fewer in number than in the second week; the
proliferating cells with brown nuclei were hardly visible;
proliferating cells were occasionally visible in the medial
membrane; and there was positive expression of PCNA visible in the
thicker neointima. There was a statistical difference in the
vascular proliferation index between the balloon-injured and
control groups (22.59±5.29 vs. 6.90±1.10; P<0.05).
Discussion
Glagov et al (8) identified that with the growth of
intracoronary atherosclerotic plaques in humans, the lumen expands
in a compensatory effect. This has also been observed in a large
number of animal experiments and autopsies (9). As research has progressed, the
concept of VR has been proposed and it is considered that VR may
play an important role in lumen stenosis. VR is a chronic change in
the vascular diameter or a structural change in the vascular wall;
therefore, it changes the ratio of the size of the vascular lumen
to the vascular wall or the geometrical shape, including an
increase or reduction of the vascular cross-sectional area. VR is
associated with corresponding adjustments to hemodynamic functions
and vascular wall structures under the conditions of continuous
adaptation to different physiological and pathological states,
which are the pathophysiological basis of vascular diseases.
Remodeling processes include the expansionary remodeling of blood
vessels (also known as positive remodeling, outward remodeling or
compensatory remodeling) with a VRI >1 and contractile
remodeling (also known as negative remodeling, inward remodeling or
decompensatory remodeling) with a VRI <1. Expansionary
remodeling may prevent the vascular lumen from stenosis through
vascular compensatory expansion, while contractible remodeling may
cause inadequate compensation due to plaque formation or
vasoconstriction, resulting in vascular lumen stenosis (10).
A quantitative histological method is the most
common method used to evaluate VR. In the current study, multiple
parameters of morphological changes in rat VR following balloon
injury were quantitatively analyzed using an image analysis system.
VR was evaluated according to the changes of VRI, IELA and EELA,
while vascular stenosis was evaluated according to the changes of
residual stenosis rate and vessel LA. In this study, compared with
the control group, the injured group in the second week presented
clear retraction of the blood vessels and a reduction in the area
of the vascular lumen (P<0.05), as well as reduced EELA and IELA
of blood vessels (P<0.05). The area of the neointima was
0.050±0.011 mm2. The VRI was 0.865, indicating
contractile remodeling. The injured group in the sixth week
presented a greater reduction in the area of the vascular lumen
(P<0.05) and significantly reduced EELA and IELA of blood
vessels (P<0.05) compared with that in the second week. The area
of the neointima increased to 0.099±0.007 mm2. The VRI
was 0.750, indicating a further role of the VR in the vascular
lumina narrowing process. Vascular contractile remodeling may cause
the vascular walls to lose their compensatory abilities to undergo
retraction, indicating that the role of contractile remodeling in
the vascular luminal narrowing process should not be ignored. One
study demonstrated that the main factors affecting the late changes
in vascular LA and causing vascular contractile remodeling and
restenosis are the imbalance of synthesis and degradation of ECM
collagen, as well as the anomalous collagen structure and the
fibrosis of the vascular adventitia (11).
In this study, in the blood vessels of the
balloon-injured group in the second week compared with those of the
control group, the adventitia demonstrated not only a marked
increase in the number of cells (P<0.05), but the thickness and
area were also significantly increased (P<0.01). There was
neointimal hyperplasia; the thickness and area of the adventitia
and the number of cells in the balloon-injured group in the sixth
week remained elevated compared with those of the control group
(P<0.05). There was more apparent neointimal hyperplasia in the
lumen in the sixth week than in the the second week. This indicates
that when the artery is injured, cell proliferation occurs in the
adventitia, leading to thickening in the intima and loss of the
vicarious expansion ability, which is a significant cause of
vascular restenosis. In this study, the thickness and area of the
vascular adventitia gradually increased following balloon injury to
the blood vessels, as well as the number of the adventitial cells
and the thickness of the intima, which were more evident in the
sixth week than the second week. Shi et al (12) identified that in a pig model of
vascular restenosis following coronary artery injury, the change in
vascular adventitial thickness was positively correlated with the
change in cell number, which was consistent with our experimental
result. This phenomenon may indicate a change in the structure of
the vascular adventitia (including connective tissue and
fibroblasts), which may be involved in a change in the
characteristics of cell phenotype or function. We consider that it
may be caused by cell aggregation induced by the phenotypic change
and enhanced proliferation ability of the adventitial cells. It may
also be related to the deposition of ECM caused by increased
collagen synthesized by the adventitial fibroblasts. The
proliferation, synthesis and secretion of ECM in the muscle fiber
cells of the vascular adventitia may cause the injured arteries to
undergo adventitial cicatrization and elastic rebound, causing
vascular adventitial remodeling, which is an important factor of
vascular restenosis following balloon injury.
Fibroblasts are the main cell type in the vascular
adventitia in mammals and humans. It is considered that following
arterial injury, fibroblasts participate in vascular repair. In the
early stages of balloon injury, the proliferating cells in the
vascular adventitia and on the medial membrane are in fusiform
hypertrophy, similar to VSMCs but differing from fibroblasts in
function (13). Certain scholars
consider that these non-muscular cells in the adventitia are
derived from fibroblasts; therefore, they are called myofibroblasts
(MFs), which synthesize and secrete α-actin (14). This protein was previously
considered a specific expressive substance of muscular cells;
therefore, the fusiform cells that synthesize and secrete α-actin
are called muscle cells (MCs) here. Therefore, myofibroblast is
another name for VSMC in a different environment (15). In the present study, the vascular
adventitia presented actin protein expression according to
immunohistochemical analysis. We observed under a light microscope
that in the control group: there was weak positive expression of
α-SMA in the vascular adventitia; there was strong brown positive
expression of α-SMA clearly visible in the vascular medial
membrane; and there was no positive expression of α-SMA in the
vascular intima. In the balloon-injured group in the second week:
there was positive expression of α-SMA visible in the vascular
adventitia; there was a lower level of expression in the medial
membrane, with variable intensity and uneven distribution,
significantly reduced compared with that of the normal control
group; and there was stronger positive expression of α-SMA in the
neointimal layer. In the balloon-injured group in the sixth week,
the expression of α-SMA in the vascular adventitia and medial
membrane was similar to that in the control group and positive
expression of α-SMA was visible in the thicker intima. Following
balloon injury in the blood vessels, the adventitial fibroblasts
underwent phenotypic changes to form MFs, fibroblasts with
characteristics of muscle fiber cells, which also secrete ECM and
numerous types of biologically active factors to participate in
tissue repair. They also have a strong migration ability (migrating
to the medial membrane and intima through the lacerated external
elastic lamina), as well as synthesis, secretion and shrinkage
properties, thus causing restenosis in injured blood vessels.
However, further investigation of the expression of desmin and
myosin is required to determine the properties of MFs.
A previous study has demonstrated that the
proliferation of VSMCs is a characteristic change associated with
vascular restenosis formation following balloon injury (16). Therefore, it is important to look
for a simple, sensitive and specific evaluation index of cell
proliferation. PCNA, a 36 kD nucleoprotein, is an auxiliary DNA
polymerase-δ protein in mammals. When resting cells begin to
proliferate, the synthesis of PCNA is activated and significantly
increased, which is an important biological indicator of
proliferation of responding cells (17). Additionally, immunohistochemical
staining of PCNA is more sensitive than labeling with
3H-thymine or bromodeoxyuridine, the latter of which
only measures the cells in the S phase while PCNA also exists in G1
and G2. PCNA has been used in clinical and basic studies of
vascular restenosis following balloon injury (18), since its analysis is simple and
sensitive. PCNA detection is a relatively reliable index for
evaluating the state of cell proliferation (19), since it objectively reflects the
activity and distribution of cell proliferation.
Using immunohistochemistry, we identified that there
was a large amount of positive expression of PCNA in the vascular
adventitia in the balloon-injured group in the second week compared
with the control group; the expression levels of PCNA in the
vascular adventitia and the medial membrane in the balloon-injured
group in the sixth week were similar to those in the control group
and there was positive expression of PCNA visible in the thicker
neointima. This indicates that the adventitial fibroblasts were in
an active state of proliferation and migrated to the intima to form
the neointima, which is a similar result to that reported in
previous studies (20,21). One study demonstrated that the
protein expression of PCNA is closely related to intimal
hyperplasia and that the expression level is proportional to the
degree of cell proliferation (22).
We identified that the proliferation index of PCNA
in the balloon-injured group in the second week was significantly
increased compared with that of the control group, with a
statistically significant difference, indicating that the number of
adventitial cells was increased. Fibroblast proliferation, an early
response to vascular injury, relies on reactive oxygen species
(ROS), particularly the H2O2 effect derived
from reduced nicotinamide adenine dinucleotide phosphate oxidase II
(NADPH). In an animal model of arterial injury, fibroblasts labeled
with 5-bromo-2 deoxyuridine (BrdU) were mainly concentrated in the
vascular adventitia outside the vascular external elastic layer.
Following vascular injury, intima was ischemic and hypoxic. The
vascular adventitial fibroblasts quickly adapt to the requirements
of the local blood vessels, initiate Gq protein signal transduction
pathways, activate protein kinase C (PKC) and promote mitogen
activated protein kinase (MAPK) family members, leading to
extensive fibroblast proliferation (23). The proliferated vascular
adventitial cells have a similar function to muscle cells, which
migrate to the intima through the internal and external elastic
lamina to constitute the cell components of the new intima. There
was also strong positive expression of PCNA visible in the
neointima in the vascular injury group, which tended to be
aggregated at the lumen surface. Therefore, the proliferation of
adventitial cells was confirmed to be one of the causes of vascular
restenosis, consistent with the conclusion of Diez-Juan and Andrés
(24).
In conclusion, following vascular injury, the
adventitia area was increased. At the same time, EELA, IELA and LA
were reduced, with a remodeling index <1 and contractile
remodeling in the vessels, which was more apparent in the sixth
postoperative week compared with the second postoperative week. The
neointimal area was gradually increased as the LA reduced,
indicating aggravated vascular stenosis. Therefore, in the
different periods following balloon injury, the changes in the
expression of α-SMA and PCNA in the vascular adventitia
demonstrated that the vascular adventitial cells were activated to
cause proliferation and phenotypic switching, participating in VR
and vascular restenosis following balloon injury. Fully
understanding and using this mechanism may provide new ideas for
the future development of new drugs for the treatment of
cardiovascular diseases. This study requires further investigation
with regard to the adventitial cell factor activation signal
pathway, with a larger number of cases.
References
|
1.
|
Gruentzig AK: Transluminal dilatation of
coronary-artery stenosis. Lancet. 1:2631978. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Gasterell PJ and Teirstein PS: Prevention
of coronary restenosis. Cardio Rev. 7:219–231. 1999. View Article : Google Scholar
|
|
3.
|
Smith SC Jr, Dove JT, Jacobs AK, et al:
ACC/AHA guidelines of percutaneous coronary intervention (revision
of the 1993 PTCA guidelines)-executive summary. A report of the
American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (committee to revise the 1993
guidelines for percutaneous transluminal coronary angioplasty). J
Am Coll Cardiol. 37:2215–2239. 2001.
|
|
4.
|
Raines EW: The extracellular matrix can
regulate vascular cell migration, Proliferation and survival:
relationships to vascular disease. Int J Exp Pathol. 81:173–182.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shen K, Liu FJ, Yuan GY, et al: Effect of
paclitaxel on the expression of vascular fibroblast of matrix
metalloproteinases-2 and α-smooth muscle actin. Zhongguo Lin Chuang
Yao Li Xue Za Zhi. 26:665–667. 2010.(In Chinese).
|
|
6.
|
Shi Y, Pieniek M, Fard A, O’Brien J,
Mannion JD and Zalewski A: Adventitial remodeling after coronary
arterial injury. Circulation. 93:340–348. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Herrmann J, Samee S, Chade A, Rodriguez
Porcel M, Lerman LO and Lerman A: Differential effect of
experimental hypertension and hypercholesterolemia on adventitial
remodeling. Arteroscler Thromb Vasc Biol. 25:447–453. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Glagov S, Weisenberg E, Zarins CK,
Stankunavicius R and Kolettis GJ: Compensatory enlargement of human
atherosclerotic coronary arteries. N Engl J Med. 316:1371–1375.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lafont AM, Chisolm GM, Wlaidow PL, et al:
Postangioplasty restenosis in the atherosclerotic rabbit:
Proliferative response or chronic constriction? Circulation.
88:1–521. 1993.
|
|
10.
|
Hong MK, Mintz GS, Abizaid AS, et al:
Intravascular ultrasound assessment of the presence of vascular
remodeling in diseased human saphenous vein bypass grafts. Am J
Cardiol. 84:992–998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Durand E, Addad F, Boulanger C, et al:
Mechanical and functional predictive factors for restenosis and
arterial remodeling after experimental angioplasty. Arch Mal Coeur
Vaiss. 94:605–611. 2001.(In French).
|
|
12.
|
Shi Y, O’Brien JE Jr, Mannion JD, et al:
Remodeling of autologous saphenous vein grafts. The role of
perivascular myofibroblasts. Circulation. 95:2684–2693. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Berrutti L and Silverman JS: Cardiac
myxoma is rich in FXIIIa positive dendrophages: immunohistochemical
study of four cases. Histopathology. 28:529–536. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Qiu ZB, Chen X and Wan S: Phenotypic
modulation of vascular smooth muscle cells and fibroblasts in
vascular remodeling of pig vein grafts. Chinese Journal of
Arteriosclerosis. 16:532–536. 2008.(In Chinese).
|
|
15.
|
Narvaez D, Kanitakis J, Faure M and Claudy
A: Immunohistochemical study of CD34-positive dendritic cells of
human dermis. Am J Dermatopathol. 18:283–288. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sun AJ, Cao PJ, Liu JJ, et al: Osteopontin
enhance migratory ability of cultured aortic adventitial
fibroblasts from spontaneously hypertensive rats. Sheng Li Xue Bao.
56:21–24. 2004.(In Chinese).
|
|
17.
|
Zhang DZ, Zhang TL, Hu CM, Liu QJ, Sun Y
and Hao SH: PTEN gene transfection on rabbit inhibition of
restenosis experimental research. China Health and Nutrition.
7:1–2. 2012.
|
|
18.
|
Stadius ML, Gown AM, Kernoff R and Collins
CL: Cell proliferation after balloon injury of iliac arteries in
the cholesterol-fed New Zealand White rabbit. Atheroscler Thromb.
14:727–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Branca M, Ciotti M, Giorgi C, et al:
Up-regulation of proliferating cell nuclear antigen (PCNA) is
closely associated with high-risk human papillomavirus (HPV) and
progression of cervical intraepithelial neoplasia (CIN) but does
not predict disease outcome in cervical cancer. Eur J Obstet
Gynecol Reprod Biol. 130:223–231. 2007. View Article : Google Scholar
|
|
20.
|
Gingras M, Farand P, Safar ME and Plant
GE: Adventitia: the vital wall of conduit arteries. J Am Soc
Hypertens. 3:166–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ahmad S, Hewett PW, Al-Ani B, et al:
Autocrine activity of soluble Flt-1 controls endothelial cell
function and angiogenesis. Vasc Cell. 3:152011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liu HW, Iwai M, Takeda-Matsubara Y, et al:
Effect of estrogen and ATl receptor blocker on neointima formation.
Hypertension. 40:451–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Stenmark KR, Gorasimovskaya E, Nemenoff RA
and Das M: Hypoxic activation of adventidal fibroblasts: role in
vascular remodeling. Chest. 122(Suppl 6): S326–S334. 2002.
View Article : Google Scholar
|
|
24.
|
Díez-Juan A and Andrés V: Coordinate
control of proliferation and migration by the
p27Kipl/cyclin-dependent kinase/retinoblastoma pathway in vascular
smooth muscle cells and fibroblasts. Circ Res. 92:402–410.
2003.PubMed/NCBI
|