Introduction
The human intervertebral disc (IVD) is an important
component of the spinal column and its dysfunction leads to lower
back pain that may reduce the patient’s quality of life (1). The degeneration of the IVD is a
complex process characterized by a series of biochemical and
structural changes in the nucleus pulposus (NP) (2). IVD degeneration often leads to the
unclear boundary between the annulus fibrosus and NP, composition
changes of the collagen fibers, as well as a reduction in the
proteoglycan content and loss of water (3). NP cells are chondrocyte-like cells
and secrete a complex extracellular matrix (ECM) that predominantly
consists of proteoglycan and fibrillar collagen. Aggrecan, one type
of proteoglycan, maintains the normal structure, metabolism and
biomechanical function of the disc (4). Glycosaminoglycan (GAG), a component
of proteoglycans, contains large quantities of water, which allows
the NP to be flexible enough to withstand loading. The considerable
loss of GAG in the process of disc degeneration results in
reductions in water content and NP elasticity, which lead to the
dysfunction of IVD biomechanics (5). A previous study revealed that the
loss of GAG predominantly results from the increased hydrolysis of
proteoglycans (6). Aggrecan
synthesis and degradation are in a dynamic equilibrium that
maintains the physiological function of the IVD. However, this
dynamic balance gradually becomes distorted under the influence of
age and stress, resulting in the degeneration of human IVDs. A
previous study has demonstrated that the expression and activity of
matrix-degrading enzymes are increased and elicit degradation of
the ECM in the degenerative disc (7).
There are two predominant degrading enzymes that are
able to hydrolyze aggrecan core proteins; these are matrix
metalloproteinases (MMPs) and aggrecanases (8). MMPs have been demonstrated to
hydrolyze aggrecan and collagen, as well as fibronectin proteins.
Aggrecanase belongs to the a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS) family and has the ability to
degrade aggrecan. In the NP matrix, aggrecan is predominantly
degraded by the ADAMTS family members ADAMTS-4 and ADAMTS-5
(8,9). Tissue inhibitor of metalloproteinase
3 (TIMP-3), inhibits aggrecanase and therefore, an increase in the
level of TIMP-3 slows disc degeneration. Studies have demonstrated
that growth factors, including transforming growth factor-β1
(TGF-β1), and cytokines, such as interleukin-1β (IL-1β), are
involved in ECM metabolism and cell proliferation (10,11).
The present study investigated the effects of TGF-β1 and IL-1β on
the expression levels of ADAMTS enzymes and their inhibitor TIMP-3
in human IVD degeneration, and aimed to identify a potential
therapeutic target for human IVD degeneration.
Materials and methods
Cell isolation and culture
This study was approved by the ethics review board
of Fudan University, affiliated to Huashan Hospital (Shanghai,
China). All patients provided consent for involvement in this
study. IVD specimens were obtained from the Spinal Surgery Center
of Huashan Hospital. The degenerated IVD was classified by
Christian MRI standard (12)and
samples were obtained from six donors (three females and three
males; average age, 34.4 years). Specimens were transported in a
sterile tube to the laboratory <30 min after surgical removal.
The annulus fibrosus and transition zone were removed using a
scalpel. The NP tissue was carefully separated from the upper and
lower vertebral cartilage under a binocular microscope (Olympus
SZH, Tokyo, Japan). NP tissue was rinsed three times in D-Hank’s
solution to remove residual debris. The tissue was then cut into
1-mm3 sections using a scalpel. The sample was then
digested for 45 min at 37°C in 0.25% tryptase, followed by 4 h in
0.2% collagenase Type II. The digested sample was then filtered
through a 75-μm cell-strainer and cultured in T25 flasks at a
density of 1×104 cells/ml in Dulbecco’s modified Eagle’s
medium with Ham’s F12 nutrient mixture (DMEM/F12), containing 10%
fetal bovine serum (FBS; HyClone, Rockford, IL, USA) in an
incubator at 37°C with 5% CO2. The medium was changed
every 72 h. When cultures demonstrated an 80% confluency, cells
were trypsinized and a split ratio of 1:2 was used for
subculturing.
Determination of cell proliferation
following treatment with TGF-β1 and IL-1β
The NP cell proliferation was analyzed using a cell
containing kit-8 (CCK-8) assay (Sigma, St. Louis, MO, USA). The
CCK-8 assay is based on the reduction of a water-soluble
tetrazolium salt (WST-8) into a yellow soluble formazan product by
metabolically active cells. The colored product accumulates in the
culture medium rather than in the cells, allowing the assessment of
cell growth at various times of culture. Upon reaching 80%
confluency, the cells (passage 3) were trypsinized and cultured in
96-well plates containing 0.1 ml DMEM/F12 with 10% FBS, per well.
The control group was cultured in DMEM/F12 containing 10% FBS,
while the treatment group was cultured in DMEM/F12 containing 10%
FBS and treated with IL-1β or TGF-β1 at varying concentrations
(0.1, 1 and 10 ng/ml) for varying times (4, 8, 16, 24 and 48 h).
The absorbance values of the culture medium of the samples were
recorded at 450 nm, using a microplate reader (FluoDia T70; Photon
Technology International, Lawrenceville, NJ, USA).
RNA extraction and qPCR
Total RNA, of the control and treatment groups, was
isolated with the TRIzol® Reagent kit (Invitrogen Life
Technologies, Carlsbad, CA, USA). cDNA was synthesized with reverse
transcription PCR using: 2 μg RNA, 1 μl 0.5 μg Oligo (dT) 15 Primer
(Promega Corporation, Madison, WI, USA), 1 μl of 10 mM dNTP mix, 5
μl of M-MLV 5X reaction buffer and 1 μl of 200 U M-MLV reverse
transcriptase in RNase free water (final volume, 25 μl). All
reactions were prepared according to the manufacturer’s
instructions and were incubated at 42°C for 60 min.
qPCR was performed for ADAMTS-4, ADAMTS-5, TIMP-3
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the
SYBR®-Green PCR Master mix kit (Applied Biosystems Inc.,
Foster City, CA, USA) in an ABI 7300 sequence detection system
(Applied Biosystems). All the primers (Table I) were designed with the Primer
Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA)
according to the cDNA sequences published in GenBank.
 | Table IPrimers used in qPCR experiments. |
Table I
Primers used in qPCR experiments.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) | Product size
(bp) |
|---|
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC | 258 |
| TIMP-3 |
AGATTCTGACTTCTTCCTCCG |
TGGGCTAGATTTCCAGCAAGTG | 215 |
| ADAMTS-4 |
ACCCAAGCATCCGCAATC |
CAGGTCCTGACGGGTAAACA | 200 |
| ADAMTS-5 |
GCAGTATGACAAGTGCGGAGT |
CAGGGCTAAATAGGCAGTGAA | 161 |
Western blot analysis
The expression levels of ADAMTS-4, ADAMTS-5 and
TIMP-3 were detected by western blot analysis. Briefly, the human
NP cells from the control and treatment groups were harvested after
48 h of treatment with TGF-β1 and IL-1β, and the protein
concentration was determined using a Bicinchoninic Acid Protein
assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Total
protein (30 μg) was denatured at 100°C for 5 min and loaded into
each well of a 10% sodium dodecyl sulfate-polyacrylamide gel.
Following electrophoresis, the protein was transferred onto a
nitrocellulose membrane using a charge of 250 mA for 60 min. The
membrane was blocked in 5% milk in Tris-buffered saline (TBS) for 1
h at room temperature. The primary antibodies including ADAMTS-4/5
(Abcam, Cambridge, UK) and TIMP-3 (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) were added and incubated with the membrane
overnight at room temperature, and then washed three times with TBS
and Tween 20 (TBST). The anti-rabbit secondary antibody (Santa Cruz
Biotechnology, Inc.) was added to the membrane, which was then
incubated for 2 h at room temperature with gentle agitation. The
membrane was washed three times with TBST for 10 min per wash. The
bands were visualized using an enhanced chemiluminescence kit
(Amersham, Arlington Heights, IL, USA) following exposure to an
X-ray film.
Statistical analysis
Data are presented as the mean ± SEM and were
analyzed using SPSS software (SPSS, Inc., Chicago, IL, USA).
Student’s t-test and analysis of variance were used for comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of TGF-β1 and IL-1β on the
proliferation of human NP cells
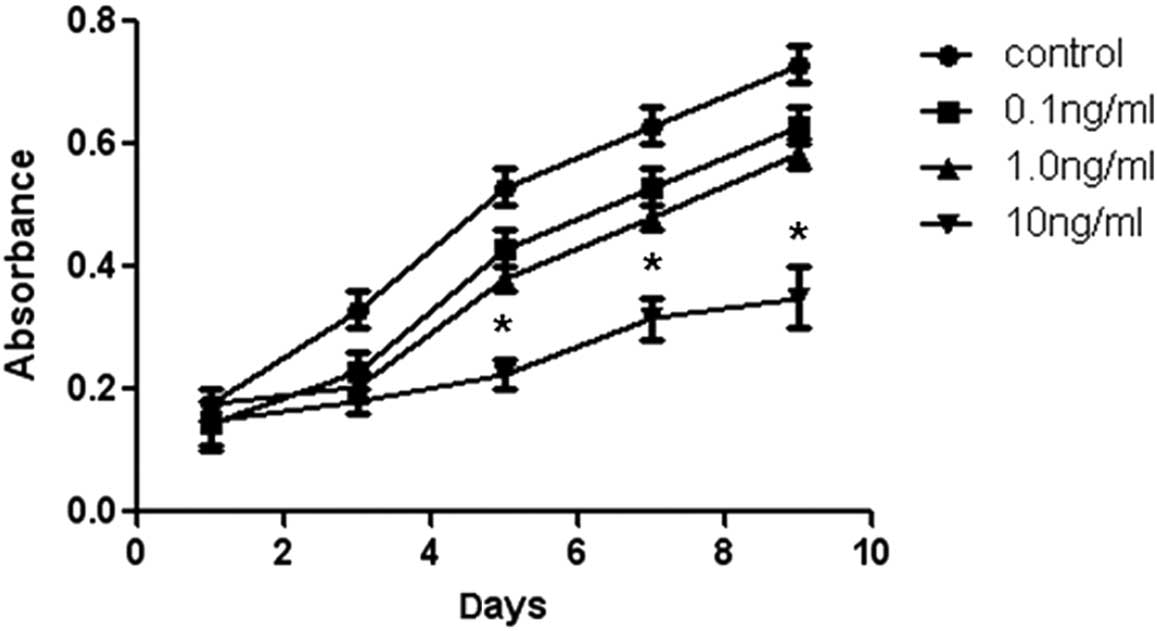
To determine the effect of TGF-β1 and IL-1β on NP
cell proliferation, a time-response experiment of human NP cell
proliferation was performed using the CCK-8 assay in 96-well
plates. Fig. 1 demonstrates that
treatment with TGF-β1 at a concentration of 0.1 ng/ml exhibited no
significant effect on the proliferation of NP cells. However, a
high concentration of TGF-β1 (1 and 10 ng/ml) treatment
significantly promoted cell proliferation. Furthermore, Fig. 2 shows that 10 ng/ml IL-1β
significantly inhibited the human NP cell proliferation, while
lower concentrations of IL-1β did not exhibit a marked effect.
These results revealed that 1 or 10 ng/ml for TGF-β1 and 10 ng/ml
for IL-1β appeared to be the optimal concentrations for the
inhibition of human NP cell proliferation and were applied in the
subsequent experiment.
Effects of TGF-β1 on the expression of
ADAMTS enzymes and TIMP-3 in human NP cells
Human IVD degeneration is accompanied by the
increased expression of degrading enzymes, including ADAMTS.
Therefore, the effect of TGF-β1 on the expression of ADAMTS-4 and
ADAMTS-5, the enzymes that are predominantly involved in the matrix
degrading process, was examined. qPCR showed that the expression of
ADAMTS-4 and -5 mRNA significantly decreased in a time-dependent
manner following treatment with TGF-β1 at a concentration of 1
ng/ml. Western blot analysis also demonstrated that the protein
expression of ADAMTS-4 and -5 significantly decreased following
treatment with TGF-β1 for 48 h (Fig.
3A and B). However, treatment with 10 ng/ml TGF-β1 appeared to
increase the expression of the ADAMTS enzymes (Fig. 3C and D), indicating that a high
concentration of TGF-β1 may produce an adverse effect.
Subsequently, the expression of the MMP inhibitor, TIMP-3, was
examined and the results revealed that TGF-β1 treatment at
concentrations of 1 and 10 ng/ml (data not shown for the latter)
significantly increased the expression of TIMP-3 at the mRNA level
in a time-dependent manner. The western blot analysis revealed that
the TIMP-3 protein levels were increased following treatment with 1
ng/ml TGF-β1 for 48 h (Fig.
3E).
Effects of IL-1β on the expression of
ADAMTS enzymes and TIMP-3 in human NP cells
The effects of IL-1β on the expression of ADAMTS-4,
ADAMTS-5 and TIMP-3 in the NP cells were examined. qPCR revealed
that the expression of ADAMTS-4 and -5 significantly increased in a
time-dependent manner following treatment with IL-1β at a
concentration of 10 ng/ml. Western blot analysis also demonstrated
that the protein expression levels of ADAMTS-4 and -5 significantly
increased with 48 h of IL-1β treatment (Fig. 4A and B). However, IL-1β appeared to
have no marked effect on the expression of TIMP-3 (Fig. 4C).
Discussion
IVD degeneration is characterized by morphological
changes and is closely associated with the degradation of the ECM,
which leads to a reduction in the NP cell population (13). The proteoglycan (predominantly
aggrecan) content is important in maintaining proper IVD function,
particularly in the NP (5). The
role of MMPs in the articular cartilage and IVD ECM has been
studied for a number of years and is relatively well understood.
Several members of the aggrecanase enzyme family that participate
in ECM breakdown have been identified, and studies have shown that
aggrecanases may be involved in the initiation and progression of
IVD degeneration (14). ADAMTS-4
and ADAMTS-5 are critical in the breakdown of ECM in the
degenerative disc (15,16). The expression of TIMP-3 has been
identified to be reduced in degenerated NP and annulus fibrosus
samples and is known to inhibit the two ADAMTS enzymes and numerous
other matrix proteinases, such as MMPs (17).
Cytokines are critical in ECM synthesis as they
regulate cell metabolism. TGF-β1 has been shown to stimulate NP
cell proliferation and has been applied in tissue engineering for
the repair of IVD. TGF-β1 treatment has been demonstrated to
increase mitogen-activated protein kinase activity and sex
determining region Y-box 9, aggrecan, and collagen type II gene
expression (18). In addition,
another study reported that in IVD cells, stimulation by TGF-β1,
epidermal growth factor and insulin-like growth factor may
significantly increase proteoglycan synthesis and promote cell
proliferation (17,19). IL-1β plays a biological role by
promoting cell metabolism and inhibiting anabolism. It stimulates
cells to produce a variety of neutral proteases, particularly MMPs,
and reduce the expression of proteoglycan in the ECM (11,13,20).
Studies have shown that IL-1β is expressed in degenerating discs
and is involved in the catabolism of the ECM. Tsuji et
al(21) showed that the
stimulation of NP cells with IL-1β for 24 h significantly increased
the expression level of ADAMTS-4 mRNA and demonstrated that IL-1β
is involved in the degradation of ECM in the IVD cells. Therefore,
it was hypothesized that the inhibition of IL-1β expression may be
a treatment option for IVD degeneration. The present study aimed to
investigate the involvement of TGF-β1 and IL-1β in the
proliferation of NP cells and expression of genes involved in ECM
synthesis. The results demonstrated that TGF-β1 promoted NP cell
proliferation. Furthermore, the downregulation of ADAMTS-4 and -5
and upregulation of TIMP-3 was demonstrated following treatment
with 1 ng/ml TGF-β1. However, treatment with a higher concentration
of TGF-β1 (10 ng/ml) appeared to increase the expression of ADAMTS
enzymes, indicating that an over-dose of TGF-β1 treatment may
produce an adverse effect. Conversely, IL-1β treatment inhibited NP
cell proliferation and significantly increased the expression of
ADAMTS-4 and -5. However, IL-1β appeared to have no marked effect
on the expression of TIMP-3.
In conclusion, this study demonstrated that the
cytokines TGF-β1 and IL-β, are involved in the synthesis and
degradation of the ECM and may be potential therapeutic targets for
the prevention or reversal of IVD degeneration.
References
|
1
|
Speed C: Low back pain. BMJ.
328:1119–1121. 2004. View Article : Google Scholar
|
|
2
|
Tolonen J, Grönblad M, Vanharanta H, Virri
J, Guyer RD, Rytömaa T and Karaharju EO: Growth factor expression
in degenerated intervertebral disc tissue. An immunohistochemical
analysis of transforming growth factor beta, fibroblast growth
factor and platelet-derived growth factor. Eur Spine J. 15:588–596.
2006.
|
|
3
|
Fraser RD, Osti OL and Vernon-Roberts B:
Intervertebral disc degeneration. Eur Spine J. 1:205–213. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007.PubMed/NCBI
|
|
5
|
Roughley PJ, Alini M and Antoniou J: The
role of proteoglycans in aging, degeneration and repair of the
intervertebral disc. Biochem Soc Trans. 30:869–874. 2002.
View Article : Google Scholar
|
|
6
|
Inkinen RI, Lammi MJ, Lehmonen S,
Puustjärvi K, Kääpä E and Tammi MI: Relative increase of biglycan
and decorin and altered chondroitin sulfate epitopes in the
degenerating human intervertebral disc. J Rheumatol. 25:506–514.
1998.PubMed/NCBI
|
|
7
|
Roberts S, Caterson B, Menage J, Evans EH,
Jaffray DC and Eisenstein SM: Matrix metalloproteinases and
aggrecanase: their role in disorders of the human intervertebral
disc. Spine (Phila Pa 1976). 25:3005–3013. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel KP, Sandy JD, Akeda K, Miyamoto K,
Chujo T, An HS and Masuda K: Aggrecanases and aggrecanase-generated
fragments in the human intervertebral disc at early and advanced
stages of disc degeneration. Spine (Phila Pa 1976). 32:2596–2603.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatano E, Fujita T, Ueda Y, Okuda T,
Katsuda S, Okada Y and Matsumoto T: Expression of ADAMTS-4
(aggrecanase-1) and possible involvement in regression of lumbar
disc herniation. Spine (Phila Pa 1976). 31:1426–1432. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu
HY, Lin TW, Lin WC, Huang TY and Deng WP: Tissue-engineered
intervertebral disc and chondrogenesis using human nucleus pulposus
regulated through TGF-beta1 in platelet-rich plasma. J Cell
Physiol. 209:744–754. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005.
|
|
12
|
Christian WA, Alexander M and Marco Z:
Magnetic resonance classification of 275 lumbar intervertebral disc
degeneration. Spine (Phila Pa 1976). 17:1873–1878. 2762001.
|
|
13
|
Kluba T, Niemeyer T, Gaissmaier C and
Gründer T: Human anulus fibrosis and nucleus pulposus cells of the
intervertebral disc: effect of degeneration and culture system on
cell phenotype. Spine (Phila Pa 1976). 30:2743–2748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majumdar MK, Askew R, Schelling S, Stedman
N, Blanchet T, Hopkins B, Morris EA and Glasson SS: Double-knockout
of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal
animals and prevents the progression of osteoarthritis. Arthritis
Rheum. 56:3670–3674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stanton H, Rogerson FM, East CJ, Golub SB,
Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et
al: ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and
in vitro. Nature. 434:648–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osada R, Ohshima H, Ishihara H, Yudoh K,
Sakai K, Matsui H and Tsuji H: Autocrine/paracrine mechanism of
insulin-like growth factor-1 secretion, and the effect of
insulin-like growth factor-1 on proteoglycan synthesis in bovine
intervertebral discs. J Orthop Res. 14:690–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Risbud MV, Albert TJ, Guttapalli A,
Vresilovic EJ, Hillibrand AS, Vaccaro AR and Shapiro IM:
Differentiation of mesenchymal stem cells towards a nucleus
pulposus-like phenotype in vitro: implications for cell-based
transplantation therapy. Spine (Phila Pa 1976). 29:2627–2632. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gruber HE, Fisher EC Jr, Desai B, Stasky
AA, Hoelscher G and Hanley EN Jr: Human intervertebral disc cells
from the annulus: three-dimensional culture in agarose or alginate
and responsiveness to TGF-beta1. Exp Cell Res. 235:13–21. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burke JG, Watson GRW, Conhyea D, McCormack
D, Dowling FE, Walsh MG and Fitzpatrick JM: Human nucleus pulposis
can respond to a pro-inflammatory stimulus. Spine (Phila Pa 1976).
28:2685–2693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji T, Chiba K, Imabayashi H, Fujita Y,
Hosogane N, Okada Y and Toyama Y: Age-related changes in expression
of tissue inhibitor of metalloproteinases-3 associated with
transition from the notochordal nucleus pulposus to the
fibrocartilaginous nucleus pulposus in rabbit intervertebral disc.
Spine (Phila Pa 1976). 32:849–856. 2007. View Article : Google Scholar
|