Introduction
Fumonisins are toxic metabolites produced
predominantly by Fusarium verticillioides, one of the most
common molds present globally on maize and other agricultural
products (1–3). Areas of low and high esophageal
cancer incidence in the former Transkei region of South Africa have
been shown to correlate with low and high levels of
fumonisin-contaminated home-grown maize, respectively (4). Alizadeh et al(5) detected high levels of fumonisin
B1 (FB1) contamination in corn and rice
samples from the Golestan province of Iran, with a significant
positive correlation between the FB1 contamination in
rice and the risk of esophageal cancer (5). The high contamination rates of
FB1, the most common fumonisin, which are noted in food
from Huaian where incidences of esophageal cancer are amongst the
highest in China, suggest a possible contributing role of
FB1 in human esophageal carcinogenesis (6). It has been proposed that
FB1 may exhibit carcinogenicity in humans and
FB1 is, at present, considered to be a possible
carcinogen to humans. A number of studies have investigated the
toxicity of FB1 in human cell lines to provide more
information concerning the effects of FB1 in humans
(7–10). FB1 was shown to inhibit
clonal expansion in human keratinocytes and proliferation in human
fibroblasts (7), in addition to
causing DNA damage of an apoptotic type in human fibroblasts
(8). Furthermore, FB1
has been demonstrated to induce apoptosis in human proximal
tubule-derived cells (IHKE cells) (9) and to cause oxidative stress in the
human intestinal cell line Caco-2 (10).
Although the toxic effects of FB1 on
mammalian cells have been studied extensively, the carcinogenicity
of FB1 on normal human esophageal epithelial cells
(HEECs), and the possible mechanism underlying the effects, have
yet to be elucidated. In recent years, numerous studies have shown
that FB1 exerts significant effects on the cell cycle in
certain cells (11,12). Cell cycle progression is controlled
by cyclin-dependent kinases (CDKs) and cyclins (13,14).
Cyclins D1 and E are necessary for entry into the S phase. CDK
inhibitors, such as p16, p21 and p27, bind CDK-cyclin complexes and
inhibit CDK activity (15,16).
In this study the effects of FB1 on the
proliferation, cell cycle and apoptosis of HEECs were investigated,
in addition to the expression of molecular markers of the cell
cycle genes cyclins D1 and E, p16, p21 and p27 in HEECs.
Furthermore, the potential esophageal carcinogenicity of
FB1 in humans was examined.
Materials and methods
Materials
FB1, propidium iodide (PI),
dimethylsulfoxide (DMSO), ethidium bromide (EB) and diethyl
pyrocarbonate (DEPC) were purchased from Sigma (St. Louis, MO,
USA), while RPMI-1640 and trypsin were obtained from Gibco (Grand
Island, NY, USA). A stock solution of FB1 for cellular
assays was prepared in phosphate-buffered saline (PBS) and then
diluted in the optimal medium (≤10 μl/ml).
Ethylenediaminetetraacetic acid (EDTA) was purchased from
Calbiochem (San Diego, CA, USA) and fetal bovine serum (FBS) was
purchased from Hangzhou Sijiqing Biological Engineering Material
Co., Ltd. (Hangzhou, China). The reagents and membranes used for
the protein assays, electrophoresis and western blotting were
obtained from Bio-Rad (Hercules, CA, USA).
Cell culture
The HEECs were obtained from Wuhan PriCells
Biomedical Technology Co., Ltd. (Wuhan, China). The cell line is
derived from the esophageal tissues of a 4-month-old female aborted
fetus. Immunocytochemistry demonstrated the expression of
cytokeratin, confirming the epithelial origin of the cells. The
cells were cultured in RPMI-1640 medium supplemented with 5% FBS at
37ºC/95% humidity/5% CO2 in a humidified incubator. The
growth of the cultured cells was observed daily using an inverted
microscope (Olympus IX51; Olympus Corporation, Tokyo, Japan), and
the RPMI-1640 medium was changed according to its color every 2–3
days.
Cell viability assay
The HEECs (1×104 cells/100 μl/well) were
seeded in 96-well plates with 100 μl culture medium containing 5%
FBS, and were incubated for 24 h to allow the cells to attach to
the bottom of the plate. The cells were then incubated with 5, 10,
20 and 40 μmol/l FB1, respectively, for 24, 48, 72 and
96 h. Following the addition of 100 μl MTT (5 mg/ml in PBS) to the
culture medium, the cells were incubated for 4 h at 37ºC with 5%
CO2 in a humidified atmosphere. The medium was
subsequently aspirated and the cells were suspended in 150 μl DMSO.
The absorption was measured at 490 nm with a Mithras LB 940
Multimode Microplate Reader (Berthold Technologies GmbH, Bad
Wildbad, Germany). The cell proliferation was calculated as
follows: [optical density (OD) of the experimental sample/OD of the
control] ×100. The experiment and assay were repeated at least
three times.
Harvesting cells
HEECs in the logarithmic growth phase were plated at
a density of 1×105 cells/ml in 50-cm2 culture
flasks and allowed to grow in 4 ml culture medium. Following cell
attachment, the culture medium was poured away and the cells were
treated with FB1 (5, 10, 20 and 40 μmol/l) for 72 h. The
cells were then trypsinized and collected for cell cycle and
apoptosis analyses, or washed twice with ice-cold PBS and removed
from the surface of the flask using a rubber scraper for western
blot analysis.
Cell cycle and apoptosis analyses
The cell cycle phase and apoptosis were examined
using a Becton-Dickinson FACSCalibur Flow Cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The cells were stained with
Vindelov’s reagent (40 mM Tris, pH 7.6; 100 mM NaCl; 10 mg RNase
A/ml; 7.5% PI and 0.1% Nonidet P-40), and data from 10,000 cells
were collected for each data file. The experiment and assay were
repeated three times.
Western blotting
The cells detached by scraping were centrifuged for
10 min at 16,000 × g, 4ºC. The cell pellets were lysed in Mammalian
Cell Protein Extraction Reagent [20 mM Tris, 0.1% sodium dodecyl
sulfate (SDS), 1% Triton X-100, 1% sodium deoxycholate, pH 7.4] and
Mammalian Protease Inhibitor Mixture. The supernatant was collected
following centrifugation for 20 min at 10,000 × g, 4ºC. The protein
concentration was assessed using a Pierce® bicinchoninic
acid (BCA) protein assay kit (Thermo Fisher Scientific Inc.,
Rockford, IL, USA) and lysates (30 μl total protein) were separated
using SDS-polyacrylamide gel electrophoresis (PAGE). Following
electrophoresis, the proteins were transferred onto polyvinylidene
fluoride membranes. The membranes were then blocked in
Tris-buffered saline with 0.1% Tween-20 (TBST), containing 5%
non-fat dry milk, for 1 h at room temperature and incubated with
primary antibody at 4ºC overnight. The membranes were then
incubated at room temperature with horseradish peroxidase
(HRP)-conjugated mouse or rabbit immunoglobulin G (IgG). The
antibodies used in the western blotting and their dilutions are
shown in Table I. After washing
with TBST, incubation with West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific Inc., Waltham, MA, USA) and detection
using Kodak In-Vivo Imaging systems (Carestream Health Inc.,
Rochester, NY, USA) enabled the visualization of the proteins,
which were then quantified by strip densitometry. Actin was used as
an internal control.
 | Table IAntibodies for western blot
analysis. |
Table I
Antibodies for western blot
analysis.
| Antibody | Source | Producer | Dilution |
|---|
| Cyclin D1 | Rabbit polyclonal
Ab | Cell Signaling
Tech, USA | 1:1,000 |
| Cyclin E
(HE12) | Mouse monoclonal
Ab | Cell Signaling
Tech, USA | 1:1,000 |
| p16 INK4A | Rabbit polyclonal
Ab | Cell Signaling
Tech, USA | 1:1,000 |
| p21 Waf1/Cip1
(DCS60) | Mouse monoclonal
Ab | Cell Signaling
Tech, USA | 1:2,000 |
| p27 Kip1
(SX53G8.5) | Mouse monoclonal
Ab | Cell Signaling
Tech, USA | 1:1,000 |
| Actin (AC-15) | Mouse monoclonal
Ab | Sigma, USA | 1:2,000 |
| Mouse IgG,
HRP-conjugated | Goat anti-mouse
polyclonal Ab | KPL, UK | 1:6,000 |
| Rabbit IgG,
HRP-conjugated | Goat anti-rabbit
polyclonal Ab | Upstate, UK | 1:6,000 |
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical analysis of the data was performed by
one-way analysis of variance (ANOVA) with the SPSS statistical
software package (version 13.0; SPSS, Inc., Chicago, IL, USA).
Differences among the groups were evaluated using the parametric
Least Significant Difference (LSD) test and differences between the
experimental and the negative control groups were evaluated using a
Dunnett’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of FB1 on the
proliferation of HEECs
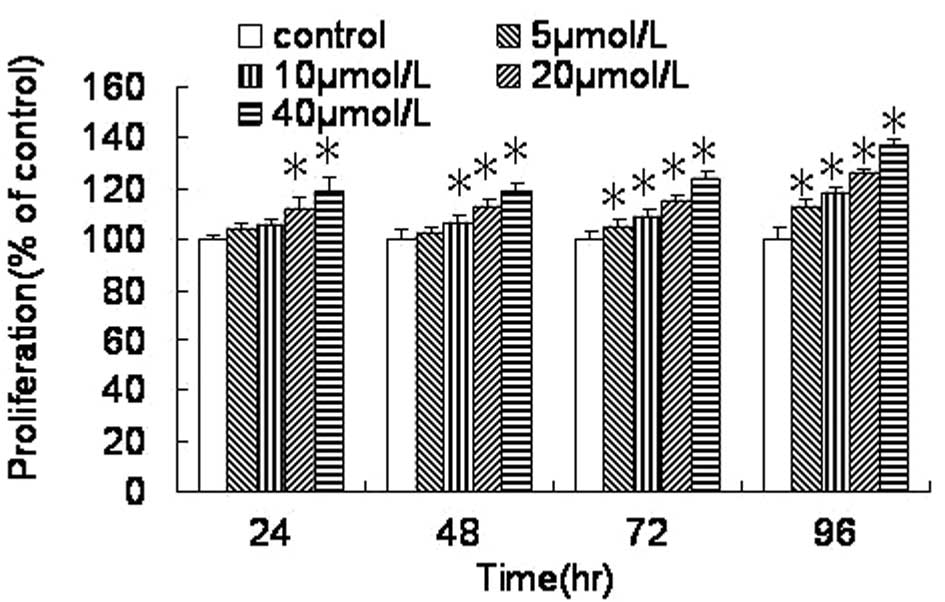
As shown in Fig. 1,
the proliferation of the HEECs was increased compared with that in
the control group following treatment with FB1 for 24,
48, 72 and 96 h. The HEEC proliferation was significantly
stimulated by treatment with FB1 (20 and 40 μmol/l) for
24 h, with FB1 (10, 20 and 40 μmol/l) for 48 h and with
FB1 (5, 10, 20 and 40 μmol/l) for 72 and 96 h. Compared
with the control group (100%), the proliferation of the HEECs was
significantly increased to ~137.3±2.0% following treatment with 40
μmol/l FB1 for 96 h. The proliferation of the HEECs
induced by treatment with 5, 10, 20 and 40 μmol/l FB1
for 72 h was increased significantly to 105.0±3.3%, 109.0±2.9%,
115.4±2.2% and 123.4±3.4%, respectively.
Effect of FB1 on the cell
cycle and apoptosis of HEECs
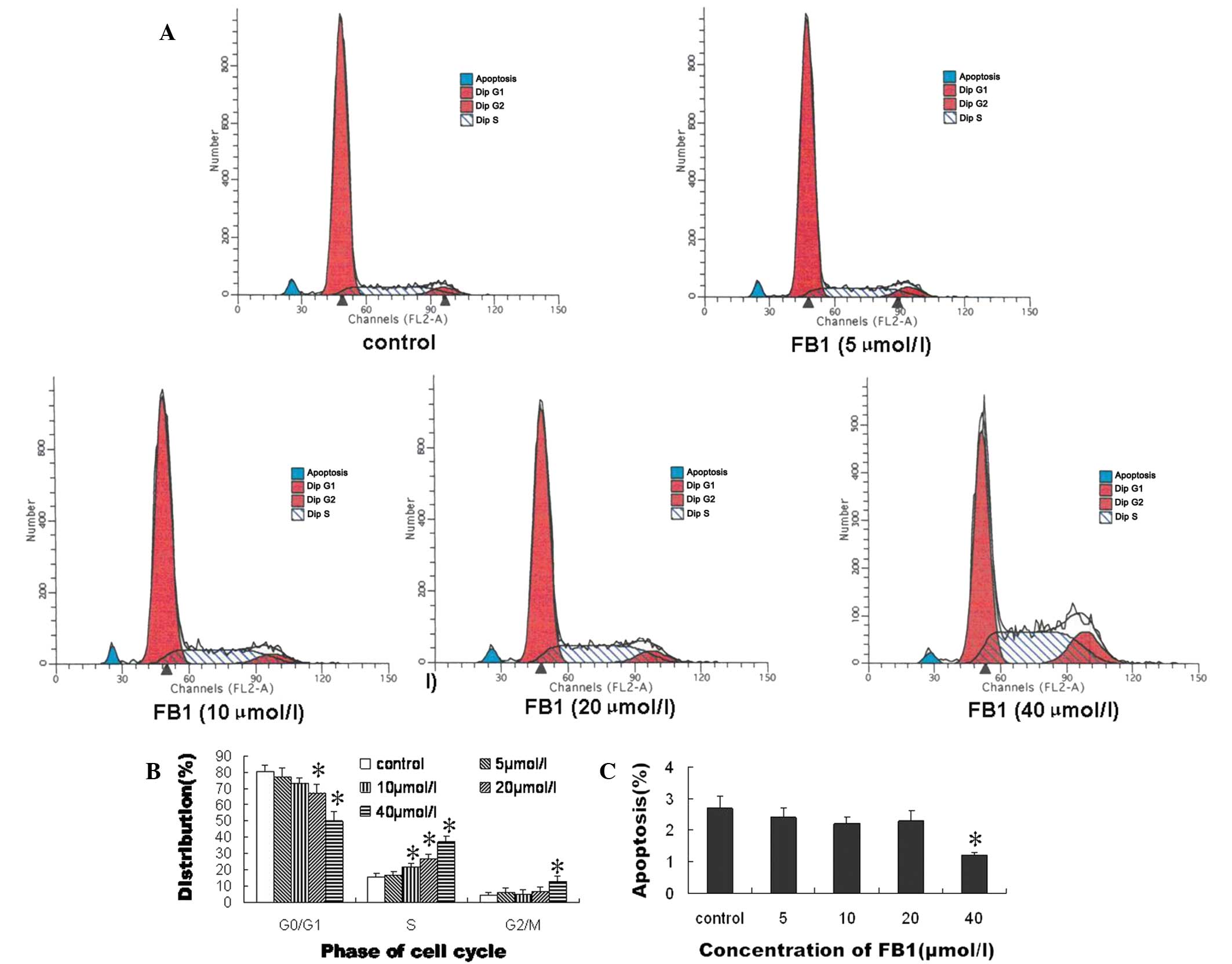
In order to explore the mechanism by which
FB1 affected the proliferation of the HEECs, the
percentages of HEECs in the different phases of the cell cycle and
cell apoptosis were assessed by flow cytometry. As shown in
Fig. 2, the cell cycle progression
of the HEECs was blocked in the S phase by treatment with
FB1 in a dose-dependent manner, and was blocked in the
G2/M phase by treatment with 40 μmol/l FB1. The
percentages of HEECs in the G0/G1 phase were 67.1±4.7 and
49.8±5.9%, respectively, following treatment with 20 and 40 μmol/l
FB1 (Fig. 2B). Compared
with the control, the percentage of HEECs undergoing apoptosis was
significantly decreased by treatment with 40 μmol/l FB1
(Fig. 2C).
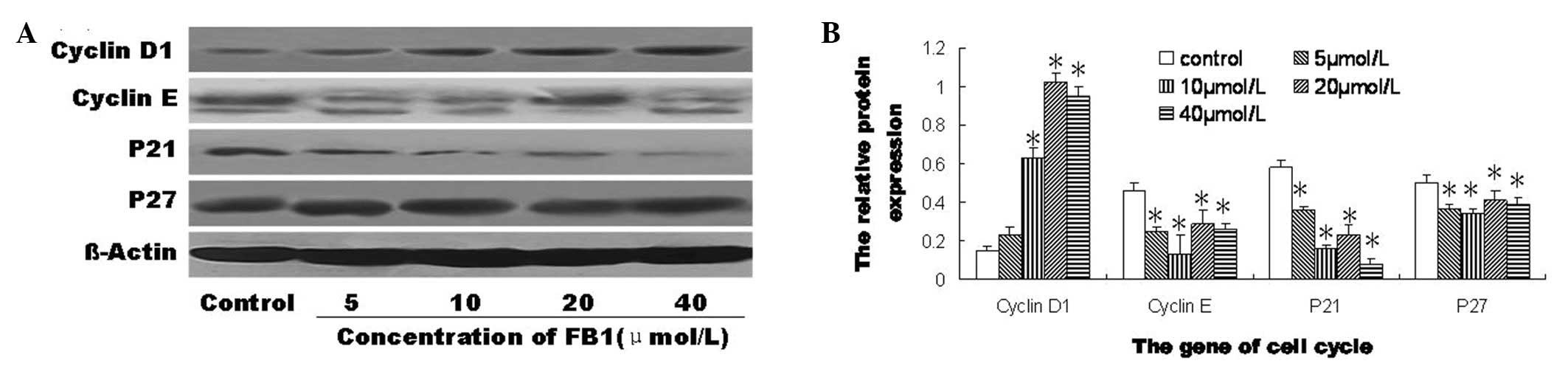
Effect of FB1 on the protein
expression of genes involved in the cell-cycle in HEECs
The protein expression levels of cyclins D1 and E,
p21 and p27 in the HEECs were significantly changed by treatment
with FB1 for 72 h compared with the levels in the
control (Fig. 3). However, the
HEECs treated with FB1 and the control cells were
negative for the protein expression of p16 (data not shown).
Compared with the control, the protein expression of cyclin D1 was
significantly increased by treatment with 10, 20 and 40 μmol/l
FB1, and the protein expression levels of cyclin E, p21
and p27 were significantly decreased by treatment with 5, 10, 20
and 40 μmol/l FB1 (Fig.
3B). These results showed that FB1 significantly
increased the protein expression level of cyclin D1 and
significantly decreased the protein expression levels of cyclin E,
p21 and p27 in the HEECs.
Discussion
Mycotoxins pose a health hazard to animals and
humans through commonly contaminated staple food grains.
FB1 is a cytotoxic and carcinogenic mycotoxin produced
by Fusarium verticillioides, which causes porcine pulmonary
edema and equine leukoencephalomalacia and has been implicated in
the etiology of esophageal cancer in the Transkei, South Africa
(17). Previous epidemiological
surveys in China have revealed that FB1 is associated
with the occurrence of human esophageal cancer (6,18).
The International Agency for Research on Cancer has classified
FB1 as a class 2B carcinogen, a probable human
carcinogen (19). In order to
investigate the effect of FB1 on the human esophagus,
HEECs derived from a normal human esophagus were tested in the
present study.
The results demonstrated that cell proliferation was
stimulated in HEECs treated with FB1. Similarly, lower
concentrations of mycotoxins (aflatoxin B1, FB1,
deoxynivalenol and nivalenol) have been shown to enhance cellular
proliferation, with the effect being more pronounced in human than
in porcine lymphocytes (20). In
another study, the stimuli for cell proliferation were introduced 7
days subsequent to the start of the administration of
FB1, by gavage with different daily doses ranging from
0.14 to 3.5 mg FB1/100 g body weight, while cancer
initiation was effected over a period of 14 days of FB1
treatment in the rat liver (21).
The results of the present study demonstrated that the maximum
proliferation of the HEECs was induced by treatment with 40 μmol/l
FB1 for 96 h (137.3±2.0%). Furthermore, the
proliferation of the HEECs induced by treatment with 5, 10, 20 and
40 μmol/l FB1 for 72 h was increased significantly to
105.0±3.3, 109.0±2.9, 115.4±2.2 and 123.4±3.4%, respectively.
However, antiproliferative effects of FB1 have been
observed in human hepatoma cells (22) and in swine peripheral blood
mononuclear cells (23). In
addition, Fornelli et al (24) showed an inhibition of
proliferation in SF-9 cells treated with FB1 of ~20%.
The varying effects of the FB1 on cell proliferation are
dependent on the dose of the mycotoxins and the types of cells.
Compared with the control, treatment with 40 μmol/l
FB1 for 72 h decreased the percentage of cells in the
G0/G1 phase and increased the percentage of cells in the S and G2/M
phases, in addition to significantly decreasing the percentage of
HEECs undergoing apoptosis. In a previous study, flow cytometric
and morphological analyses showed that FB1 lowered the
marked apoptosis induced by prostaglandins, particularly
prostaglandin A2, in esophageal carcinoma (WHCO3) cells. However,
in combination with arachidonic acid and prostaglandins E2 and A2,
FB1 increased the number of G2/M cells (25). In a different study, the results
showed that the number of C6 glioma cells in the S phase decreased
significantly compared with the control, from 18.7±2.5 to 8.1±1.1%
for 9 μmol/l FB1, and the number of cells in the G2/M
phase increased significantly compared with the control, from
45.7±0.4 to 54.8±1.1% for 9 μmol/l FB1. However, no
change occurred in the number of cells in G0/G1 phase (26). It has been demonstrated that
FB1 interferes with the G1/S phase checkpoint, which
leads to changes in the cell cycle in animal experiments (27). The percentage of cells blocked in
the G0/G1 phase of the cell cycle has been shown to be increased by
FB1 in swine peripheral blood mononuclear cells
(23). Thus, there are different
effects on the cell cycle and apoptosis in different types of cell
following treatment with FB1. The stimuli for cell
proliferation in HEECs are a common result of the
FB1-induced changes in the cell cycle and apoptosis. To
further investigate the pathogenesis of the effects of
FB1 in the present study, the protein expression of
genes involved in the cell cycle in HEECs were analyzed using
western blotting.
In eukaryotes, the cell cycle is tightly regulated
by a number of protein kinases composed of CDKs, with corresponding
regulatory cyclins and CDK inhibitors (28). The activity of the CDK/cyclin
complexes is regulated by proliferating cell nuclear antigen, which
binds to cyclin D1; cyclin E promotes the progression through G1
phase into S phase. The activity of the CDK/cyclin complexes is
negatively regulated by binding to CDK inhibitors. CDK inhibitors
are grouped into two distinct families (29): the INK4 family, including p15INK4b,
p16INK4a, p18INK4c and p19INK4d (30), and the CIP/KIP family, including
p21WAF1/CIP1, p27KIP1 and p57KIP2 (31). In the present study, the increased
expression of cyclin D1 and decreased expression of CDK inhibitors,
including p21 and p27, strongly suggest that FB1 induced the low
expression of the members of KIP/CIP family, which sequentially
stimulated the activities of the CDK/cyclin complexes.
In conclusion, the present in vitro study
demonstrated that FB1 stimulated cell proliferation in
HEECs, most likely by decreasing the percentage of cells in the
G0/G1 phase of the cell cycle, increasing the percentage of cells
in S phase, increasing the percentage of cells in G2/M phase and
arresting cell apoptosis. The changes in the cell cycle may have
been mediated by stimulation of cyclin D1 and inhibition of p21 and
p27 expression, thereby accelerating the passing of cells through
the G1-S checkpoint (32,33). The inhibition of cyclin E was
offset by the role of these genes. It has been shown that the
expression of cyclin D1, p21 and p27 is involved in the occurrence
of esophageal cancer (34), and
p21 and p27 have been proposed as candidate tumor suppressor genes
(35). In a study by Huang et
al(36), cyclin D1 was shown
to be overexpressed in esophageal cancer in southern China
(36). Furthermore, it has been
demonstrated that the overexpression of cyclin D1, rather than
cyclin E, is involved in the pathogenesis of esophageal cancer
(37). However, additional
studies, particularly in vivo experiments, are required to
further demonstrate the effect of FB1 in normal human
esophageal epithelium and to elucidate the correlation between
FB1 and human esophageal cancer.
Acknowledgements
The authors would like to thank Jia-sheng Wang
(Department of Environmental Health Science, College of Public
Health, the University of Georgia) for his help throughout the
study. This study was supported by grants from the National Natural
Science Foundation of China (no. 30800914,81372985) and the Dietary
Nutrition Research and Education Foundation of Danone (no.
DIC2011-05).
References
|
1
|
Theumer MG, Clop EM, Rubinstein HR and
Perillo MA: The lipid-mediated hypothesis of fumonisin B1
toxicodynamics tested in model membranes. Colloids Surf B
Biointerfaces. 64:22–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stockmann-Juvala H and Savolainen K: A
review of the toxic effects and mechanisms of action of fumonisin
B1. Hum Exp Toxicol. 27:799–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cano-Sancho G, Ramos AJ, Marin S and
Sanchis V: Occurrence of fumonisins in Catalonia (Spain) and an
exposure assessment of specific population groups. Food Addit
Contam Part A Chem Anal Control Expo Risk Assess. 29:799–808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Westhuizen L, Shephard GS, Rheeder
JP and Burger HM: Individual fumonisin exposure and sphingoid base
levels in rural populations consuming maize in South Africa. Food
Chem Toxicol. 48:1698–1703. 2010.PubMed/NCBI
|
|
5
|
Alizadeh AM, Rohandel G, Roudbarmohammadi
S, et al: Fumonisin B1 contamination of cereals and risk of
esophageal cancer in a high risk area in northeastern Iran. Asian
Pac J Cancer Prev. 13:2625–2628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun G, Wang S, Hu X, et al: Fumonisin B1
contamination of home-grown corn in high-risk areas for esophageal
and liver cancer in China. Food Addit Contam. 24:181–185. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwerdt G, Königs M, Holzinger H, Humpf
HU and Gekle M: Effects of the mycotoxin fumonisin B(1) on cell
death in human kidney cells and human lung fibroblasts in primary
culture. J Appl Toxicol. 29:174–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galvano F, Russo A, Cardile V, Galvano G,
Vanella A and Renis M: DNA damage in human fibroblasts exposed to
fumonisin B(1). Food Chem Toxicol. 40:25–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seefelder W, Humpf HU, Schwerdt G,
Freudinger R and Gekle M: Induction of apoptosis in cultured human
proximal tubule cells by fumonisins and fumonisin metabolites.
Toxicol Appl Pharmacol. 192:146–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kouadio JH, Mobio TA, Baudrimont I, Moukha
S, Dano SD and Creppy EE: Comparative study of cytotoxicity and
oxidative stress induced by deoxynivalenol, zearalenone or
fumonisin B1 in human intestinal cell line Caco-2. Toxicology.
213:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bondy GS, Barker MG, Lombaert GA, et al: A
comparison of clinical, histopathological and cell-cycle markers in
rats receiving the fungal toxins fumonisin B1 or fumonisin B2 by
intraperitoneal injection. Food Chem Toxicol. 38:873–886. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang SK, Liu S, Yang LG, et al: Effect of
fumonisin B1 on the cell cycle of normal human liver cells. Mol Med
Rep. 7:1970–1976. 2013.PubMed/NCBI
|
|
13
|
Czerednik A, Busscher M, Bielen BA,
Wolters-Arts M, de Maagd RA and Angenent GC: Regulation of tomato
fruit pericarp development by an interplay between CDKB and CDKA1
cell cycle genes. J Exp Bot. 63:2605–2617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto S, Kohsaka S and Nakajima K: Role
of cell cycle-associated proteins in microglial proliferation in
the axotomized rat facial nucleus. Glia. 60:570–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moreira PR, Guimarães MM, Guimarães AL, et
al: Methylation of P16, P21, P27, RB1 and P53 genes in odontogenic
keratocysts. J Oral Pathol Med. 38:99–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Andrade BA, León JE, Carlos R,
Delgado-Azañero W, Mosqueda-Taylor A and de Almeida OP:
Immunohistochemical expression of p16, p21, p27 and cyclin D1 in
oral nevi and melanoma. Head Neck Pathol. 6:297–304.
2012.PubMed/NCBI
|
|
17
|
Myburg RB, Dutton MF and Chuturgoon AA:
Cytotoxicity of fumonisin B1, diethylnitrosamine, and catechol on
the SNO esophageal cancer cell line. Environ Health Perspect.
110:813–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Wei H, Ma J and Luo X: The
fumonisin B1 content in corn from North China, a high-risk area of
esophageal cancer. J Environ Pathol Toxicol Oncol. 19:139–141.
2000.PubMed/NCBI
|
|
19
|
Severino L, Russo R, Luongo D, De Luna R,
Ciarcia R and Rossi M: Immune effects of four Fusarium-toxins (FB1,
ZEA, NIV, DON) on the proliferation of Jurkat cells and porcine
lymphocytes: in vitro study. Vet Res Commun. 32(Suppl 1):
S311–S313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taranu I, Marina DE, Burlacu R, Pinton P,
Damian V and Oswald IP: Comparative aspects of in vitro
proliferation of human and porcine lymphocytes exposed to
mycotoxins. Arch Anim Nutr. 64:383–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gelderblom WC, Galendo D, Abel S,
Swanevelder S, Marasas WF and Wild CP: Cancer initiation by
fumonisin B(1) in rat liver - role of cell proliferation. Cancer
Lett. 169:127–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McKean C, Tang L, Tang M, et al:
Comparative acute and combinative toxicity of aflatoxin B1 and
fumonisin B1 in animals and human cells. Food Chem Toxicol.
44:868–876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marin DE, Gouze ME, Taranu I and Oswald
IP: Fumonisin B1 alters cell cycle progression and interleukin-2
synthesis in swine peripheral blood mononuclear cells. Mol Nutr
Food Res. 51:1406–1412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fornelli F, Minervini F and Mulè G:
Cytotoxicity induced by nivalenol, deoxynivalenol, and fumonisin B1
in the SF-9 insect cell line. In Vitro Cell Dev Biol Anim.
40:166–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seegers JC, Joubert AM, Panzer A, et al:
Fumonisin B1 influenced the effects of arachidonic acid,
prostaglandins E2 and A2 on cell cycle progression, apoptosis
induction, tyrosine- and CDC2-kinase activity in oesophageal cancer
cells. Prostaglandins Leukot Essent Fatty Acids. 62:75–84. 2000.
View Article : Google Scholar
|
|
26
|
Mobio TA, Anane R, Baudrimont I, et al:
Epigenetic properties of fumonisin B(1): cell cycle arrest and DNA
base modification in C6 glioma cells. Toxicol Appl Pharmacol.
164:91–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramljak D, Calvert RJ, Wiesenfeld PW, et
al: A potential mechanism for fumonisin B(1)-mediated
hepatocarcinogenesis: cyclin D1 stabilization associated with
activation of Akt and inhibition of GSK-3beta activity.
Carcinogenesis. 21:1537–1546. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: Potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Douville JM, Cheung DY, Herbert KL,
Moffatt T and Wigle JT: Mechanisms of MEOX1 and MEOX2 regulation of
the cyclin dependent kinase inhibitors p21 and p16 in vascular
endothelial cells. PLoS One. 6:e290992011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Canepa ET, Scassa ME, Ceruti JM, et al:
INK4 proteins, a family of mammalian CDK inhibitors with novel
biological functions. IUBMB Life. 59:419–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ito Y, Yoshida H, Matsuzuka F, et al:
Expression of the components of the Cip/Kip family in malignant
lymphoma of the thyroid. Pathobiology. 71:164–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugimoto M, Martin N, Wilks DP, et al:
Activation of cyclin D1-kinase in murine fibroblasts lacking both
p21(Cip1) and p27(Kip1). Oncogene. 21:8067–8074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verlinden L, Verstuyf A, Convents R,
Marcelis S, Van Camp M and Bouillon R: Action of
1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and
p27 in MCF-7 cells. Mol Cell Endocrinol. 142:57–65. 1998.
|
|
34
|
Hirai T, Kuwahara M, Yoshida K, Osaki A
and Toge T: The prognostic significance of p53, p21 (Waf1/Cip1),
and cyclin D1 protein expression in esophageal cancer patients.
Anticancer Res. 19:4587–4591. 1999.PubMed/NCBI
|
|
35
|
Faria MH, Patrocinio RM, Moraes Filho MO
and Rabenhorst SH: Immunoexpression of tumor suppressor genes p53,
p21 WAF1/CIP1 and p27 KIP1 in human astrocystic tumors. Arq
Neuropsiquiatr. 65:1114–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang XP, Rong TH, Lin P, et al: Cyclin D1
overexpression in esophageal cancer from southern China and its
clinical significance. Cancer Lett. 231:94–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anayama T, Furihata M, Takeuchi T, et al:
Insufficient effect of p27(KIP1) to inhibit cyclin D1 in human
esophageal cancer in vitro. Int J Oncol. 18:151–155.
2001.PubMed/NCBI
|