Spandidos Publications style
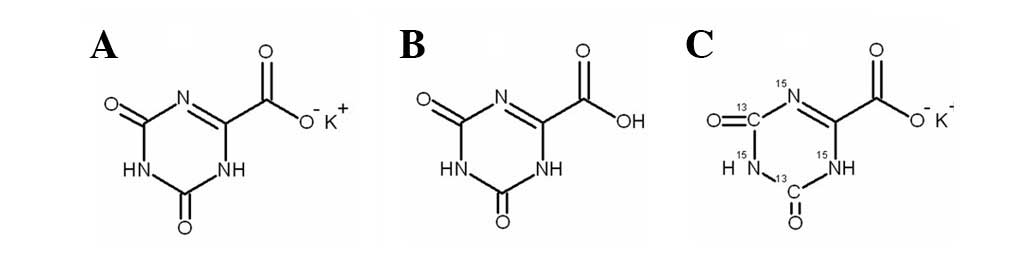
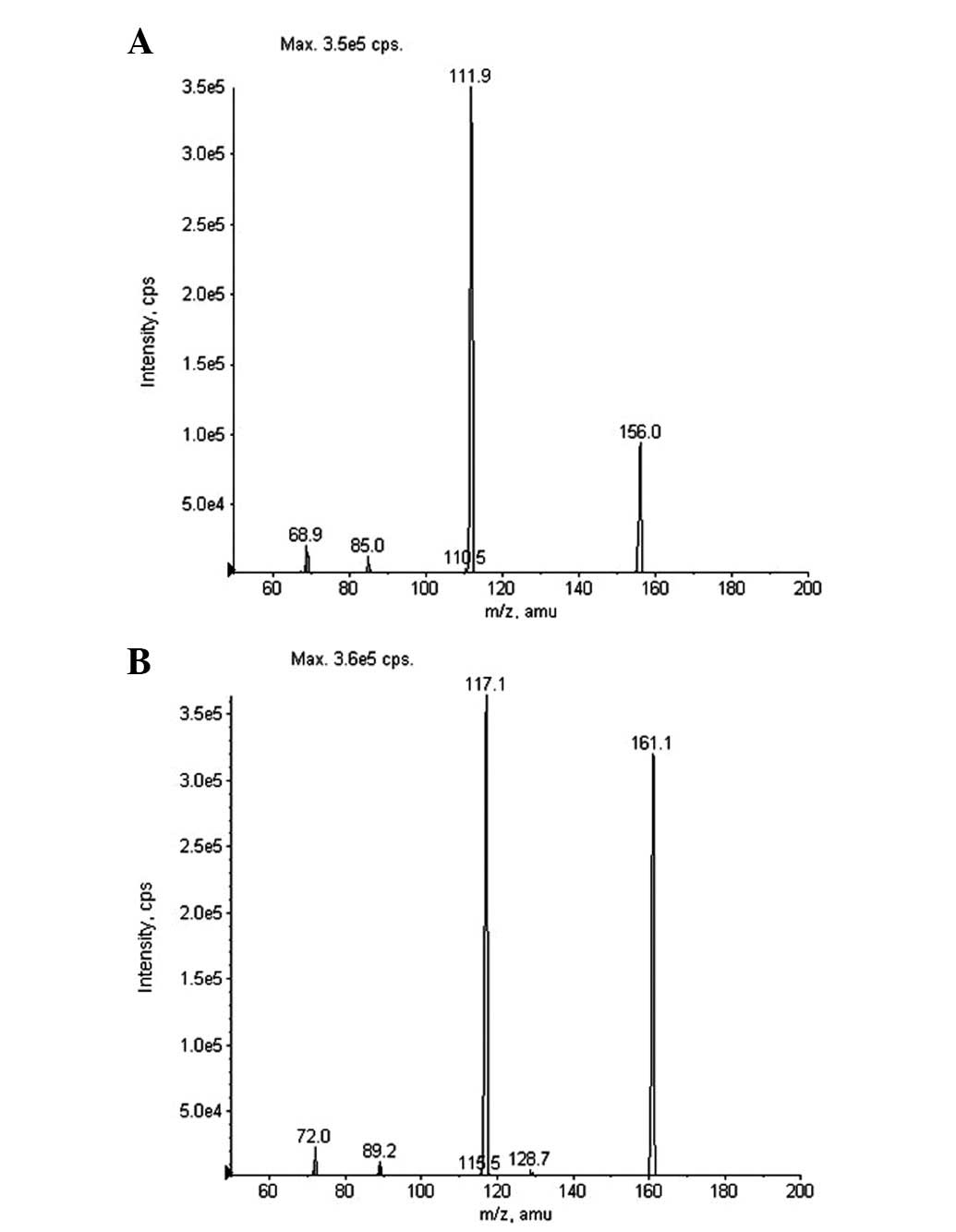
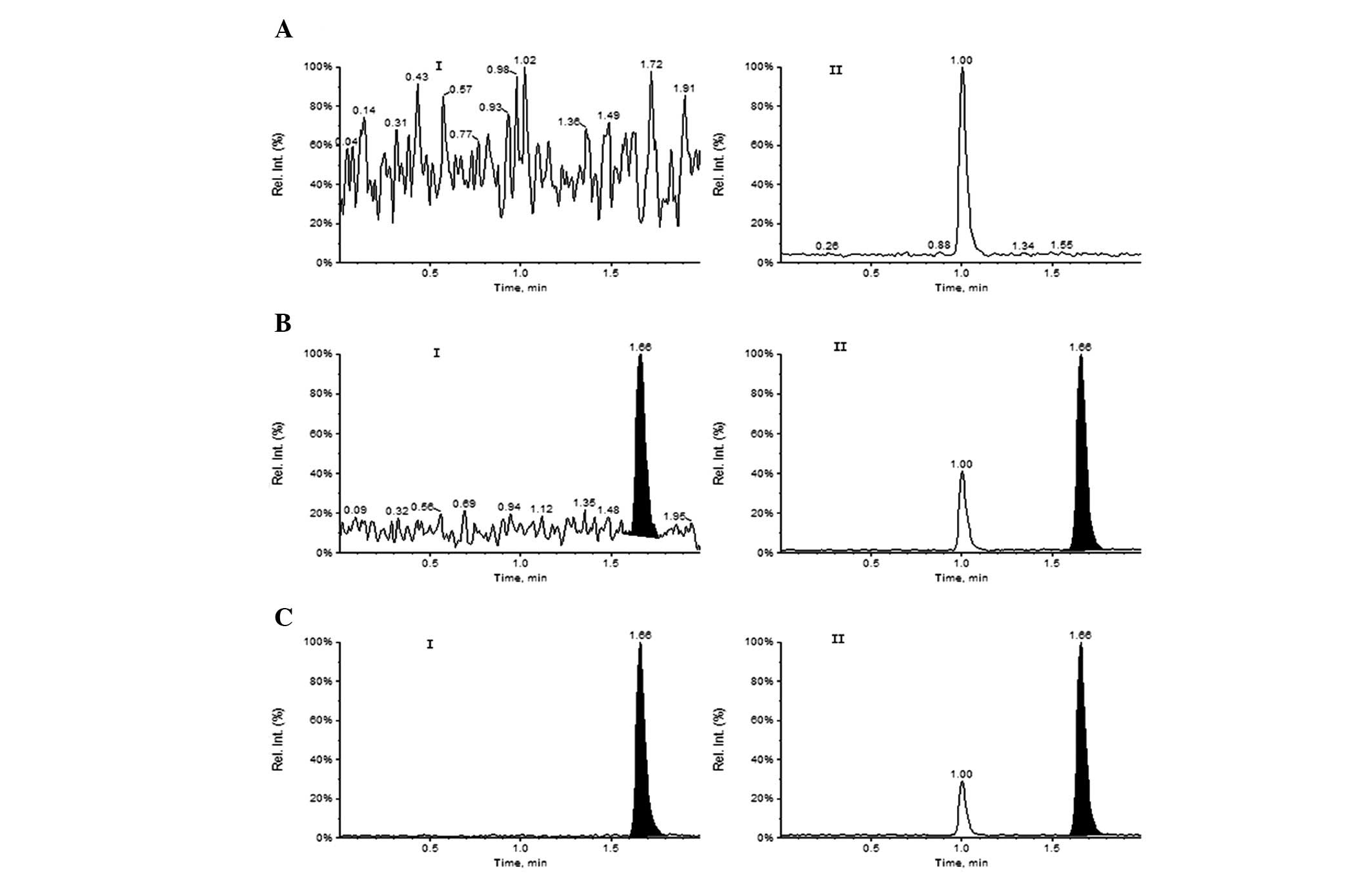
Hao G, Bai S, Liang H, Liang Y, Qu H, Gao H, Li Y, Zheng Z, Wang X, Liu Z, Liu Z, et al: Development and validation of a HPLC-MS/MS method with electrospray ionization for quantitation of potassium oxonate in human plasma: Application to a pharmacokinetic study. Exp Ther Med 5: 932-936, 2013.
APA
Hao, G., Bai, S., Liang, H., Liang, Y., Qu, H., Gao, H. ... Liu, Z. (2013). Development and validation of a HPLC-MS/MS method with electrospray ionization for quantitation of potassium oxonate in human plasma: Application to a pharmacokinetic study. Experimental and Therapeutic Medicine, 5, 932-936. https://doi.org/10.3892/etm.2013.908
MLA
Hao, G., Bai, S., Liang, H., Liang, Y., Qu, H., Gao, H., Li, Y., Zheng, Z., Wang, X., Liu, Z."Development and validation of a HPLC-MS/MS method with electrospray ionization for quantitation of potassium oxonate in human plasma: Application to a pharmacokinetic study". Experimental and Therapeutic Medicine 5.3 (2013): 932-936.
Chicago
Hao, G., Bai, S., Liang, H., Liang, Y., Qu, H., Gao, H., Li, Y., Zheng, Z., Wang, X., Liu, Z."Development and validation of a HPLC-MS/MS method with electrospray ionization for quantitation of potassium oxonate in human plasma: Application to a pharmacokinetic study". Experimental and Therapeutic Medicine 5, no. 3 (2013): 932-936. https://doi.org/10.3892/etm.2013.908