Introduction
Human gliomas originate from neural stromal cells,
including glial, ependyma, choroid plexus epithelial and neural
parenchymal cells. The incidence rate in adults is ~6/100,000 and
the five-year survival rate is between 20 and 30%. Gliomas account
for 35.26–60.96% of central nervous system tumors (average, 44.69%)
(1). The tumors exhibit
infiltrating growth and have no evident boundary with the normal
brain tissue, thus, it is difficult to completely resect gliomas
via surgery. In addition, gliomas are not sensitive to radiotherapy
or chemotherapy, and have one of the worst prognoses for systemic
tumors (2). Previous studies have
shown that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling
transduction pathway plays a central role in the maintenance of
malignant glioma invasion (3). The
activation of this pathway can promote the proliferation of tumor
cells and inhibit the apoptosis of tumor cells. The dependence on
PI3K/Akt signaling transduction makes this pathway an attractive
target for the treatment of gliomas (4). In previous research of our group, saw
palmetto extract was shown to markedly inhibit the proliferation of
human glioma cells, and the underlying mechanism may be associated
with the inhibition of signal transducer and activator of
transcription 3 phosphorylation. Additionally, previous studies
have demonstrated that saw palmetto extract can induce the
apoptosis of prostate cancer cells (5). In previous research, the effect of
saw palmetto extract on human glioma U87 and U251 cells was
investigated in vitro. The results revealed that saw
palmetto extract markedly inhibited the proliferation of human
glioma cells and the underlying mechanism may be associated with
the inhibition of signal transducer and activator of transcription
3 (STAT3) phosphorylation. Therefore, the aim of the present study
was to investigate the effect of saw palmetto extract on the
PI3K/Akt signaling transduction pathway in human glioma U87 and
U251 cell lines.
Materials and methods
Human glioma cell lines
U87 and U251 cell lines were purchased from Beijing
Dingguochangsheng Biotech Co., Ltd. (Beijing, China).
Experimental apparatus
A T25 cell culture flask and 6-well cell culture
plates were purchased from Corning Inc. (Corning, NY, USA). A 3K30
model centrifuge (Sigma-Aldrich, St.Louis, MO, USA) and a BX51
model microscope (Olympus, Tokyo, Japan) were used, as well as a
Chemilmager 5500 vertical imager (Alpha Innotech, San Leandro, CA,
USA), Protean IIXi + PowerPac 3000, Bio-Rad, Herculaes, CA, USA)
electrophoresis device and a blood cell counting plate (model 3100;
Hausser Scientific, Horsham, PA, USA).
Reagents and drug
Saw palmetto extract was purchased from Yongyuan
Bio-technology, Co., Ltd. (Xi’an, China). Rabbit anti-B-cell
lymphoma-extra large (Bcl-xL), anti-p53, anti-PI3K and anti-β-actin
antibodies were purchased from Bioss, Inc. (Wuhan, China). An
enhanced chemiluminescence (ECL) kit was obtained from the Beyotime
Institute of Biotechnology (Shanghai, China).
Cell culture
Human brain glioma cell lines, U87 and U251, were
grown in a 25-cm2 cell culture bottle containing
Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal
bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin, at
37°C and 5% carbon dioxide. Cell growth was observed under an
inverted microscope and the medium was replaced every two days.
Cell count
A counting plate and cover glass was cleaned with
95% alcohol. Take ~1 μl of cell suspension and drip on the blood
counting chamber to count the cell concentration. Under a
microscope at ×10 magnification, the cell number was counted with
four angles in the grid on the plate. The procedure was performed
in triplicate. The cell number was calculated as follows: Cell
number (/ml) = (total cell number of the four angles/4) ×
104 × dilution.
Western blot analysis
U87 and U251 cell suspensions in a logarithmic
growth phase were seeded into six-well plates at a density of
104 cells/well. In the experimental group, 1 μl/ml saw
palmetto extract was added, while no drug was added to the control
group. The suspensions were cultured for 24 h. Next, the
supernatant was discarded and the plates were washed twice with
phosphate-buffered saline (PBS) at 4°C. Radioimmunoprecipitation
assay buffer (RIPA) cell lysate with phenylmethylsulfonyl fluoride
(PMSF) was added to each well (~80 μl), which was followed by
gentle shaking for 30 min on ice and repeated pipetting and
scraping of the bottom of the well. The lysate was fully removed
and placed into a 1.5-ml centrifuge tube. The samples were then
centrifuged for 25 min at 4°C under 14,000 × g. The supernatant was
then collected and the protein concentration was measured using the
Bradford method.
Proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, using a 6–15%
acrylamide resolving gel, and transferred to polyvinylidene
fluoride membranes. The membranes were blocked with Tris-buffered
saline-Tween-20 (0.1% Tween-20) containing 5% milk for 60 min at
room temperature, which was followed by incubation with primary
antibodies at 4°C overnight. Rabbit anti-Bcl-xL (1:200), anti-p53
(1:200), anti-PI3K (1:200) and anti-β-actin antibodies (1:5,000)
were used to detect the target proteins. The blots were then probed
with horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000;
Beyotime Institute of Biotechnology). According to the
manufacturer’s instructions of the ECL kit, the same volume of
solution A and B were mixed and reacted for 1 min.
Densitometry was performed using the Quantity One
software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data were analyzed using SPSS 15.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. Comparisons between the two groups were
conducted using the t-test, where P<0.05 was considered to
indicate a statistically significant difference.
Results
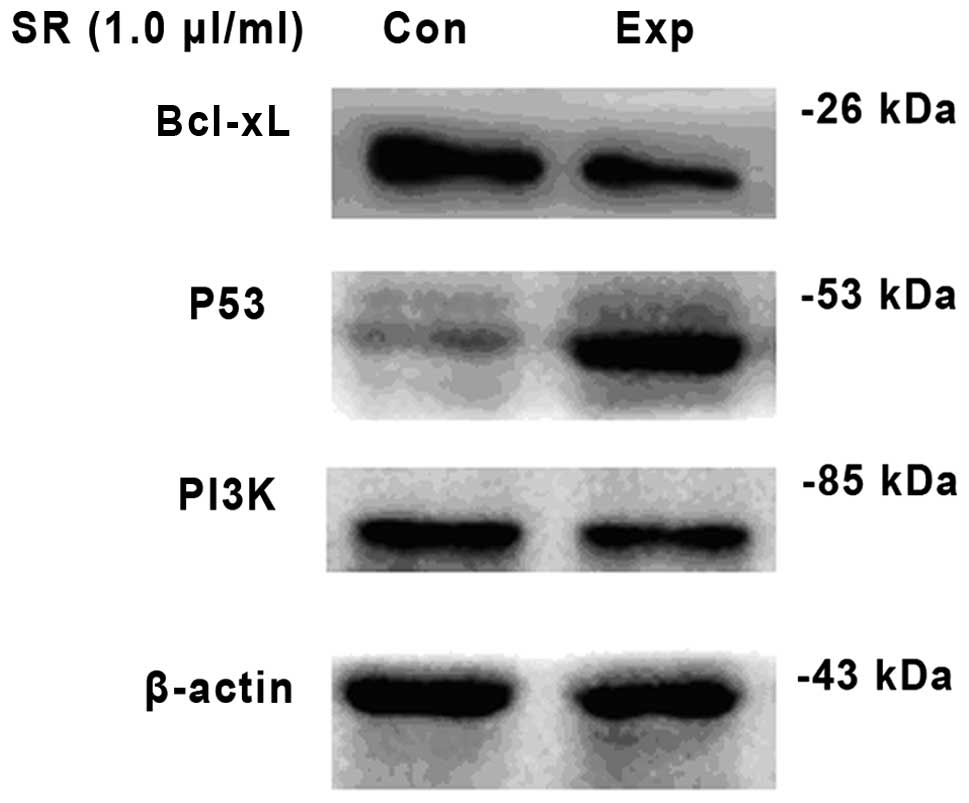
Effect of saw palmetto extract on the
protein expression levels of PI3K in U87 glioma cells
Following treatment with saw palmetto extract, the
protein expression level of PI3K had significantly decreased when
compared with the control group in U87 glioma cells (t=13.959;
P=0.000). In addition, p53 protein expression increased
significantly as compared with the untreated U87 cells (t=12.440;
P= 0.000), and U87 cells pretreated with saw palmetto extract
exhibited significantly decreased Bcl-xL protein expression as
compared with the control (t=15.107; P<0.001; Table I; Fig.
1).
 | Table IComparison in the OD of PI3K, Bcl-xL
and p53 in U87 cells between the experimental and control groups
(mean ± SD; n=6). |
Table I
Comparison in the OD of PI3K, Bcl-xL
and p53 in U87 cells between the experimental and control groups
(mean ± SD; n=6).
| Groups | PI3K | Bcl-xL | p53 |
|---|
| Control | 0.58±0.076 | 1.08±0.114 | 0.23±0.045 |
| Experimental | 0.36±0.041a | 0.34±0.034a | 1.25±0.052a |
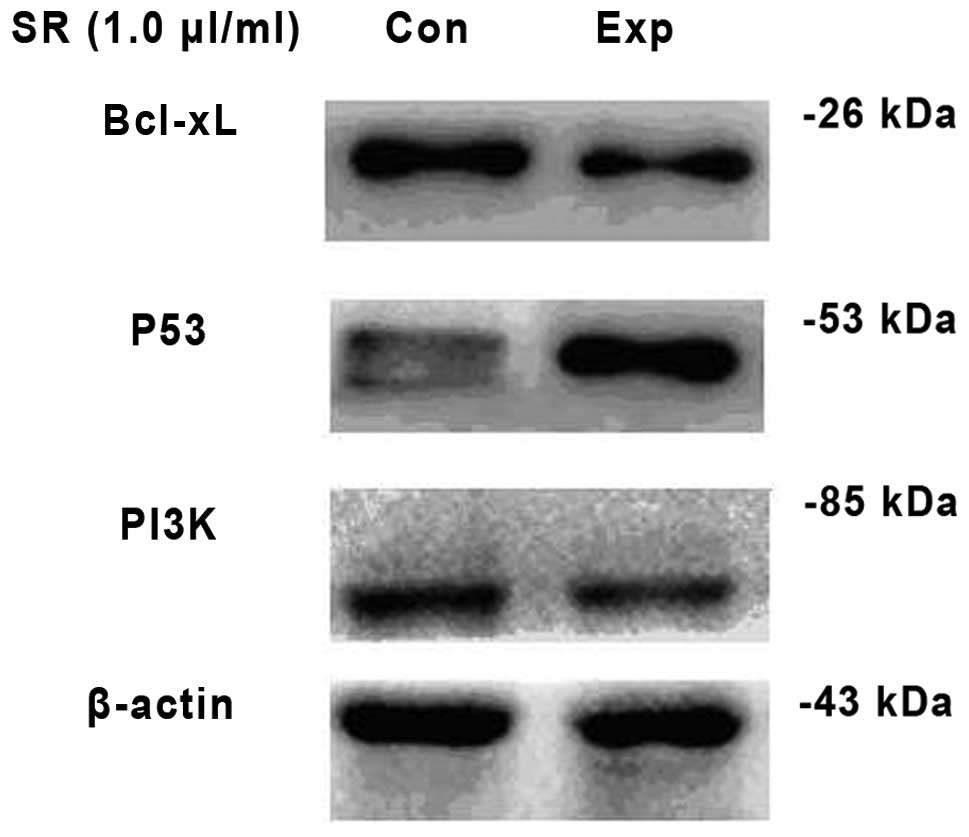
Effect of saw palmetto extract on the
protein expression levels of PI3K in U251 glioma cells
Following treatment with saw palmetto extract, U251
cells exhibited a lower expression level of PI3K protein as
compared with the control group (t=6.849; P<0.001). However, the
U251 cells exhibited a higher expression level of p53 protein as
compared with the untreated group (t=40.810; P<0.001). In
addition, the Bcl-xL protein expression decreased significantly in
the experimental group as compared with the untreated control
(t=19.640; P<0.001; Table II;
Fig. 2).
 | Table IIComparison in the OD of PI3K, Bcl-xL
and p53 in U251 cells between the experimental and control groups
(mean ± SD; n=6). |
Table II
Comparison in the OD of PI3K, Bcl-xL
and p53 in U251 cells between the experimental and control groups
(mean ± SD; n=6).
| Groups | PI3K | Bcl-xL | p53 |
|---|
| Control | 0.96±0.166 | 0.92±0.059 | 0.25±0.039 |
| Experimental | 0.39±0.119a | 0.33±0.043a | 1.30±0.049a |
Discussion
Gliomas are the most common primary tumors,
accounting for ~46% of intracranial tumors and ~2% of adult tumors.
The majority of gliomas are characterized by invasive growth and
are difficult to completely resect via surgery. In addition,
chemotherapy and radiotherapy exhibit poor therapeutic effects. In
previous years, studies have focused on chemical compounds derived
from plants that possess pharmacological activity in antitumor
therapy (6,7). A previous study demonstrated that a
number of chemical compounds in herbaceous plant sources can
inhibit the proliferation of tumor cells and induce apoptosis
through altering the tumor metabolism, inhibiting the cell cycle
and activating the immune response (8).
The effective components of saw palmetto extract, as
matured dry fruit of saw palm, are fatty acids. Previously, a
foreign study reported that saw palmetto extract had inhibitory
effects on the proliferation and induction of apoptosis in prostate
cancer cells (9). The main
pharmacological effects of saw palmetto extract are as follows: i)
Non-competitive inhibition of 5-α reductase activity and inhibition
of the conversion of testosterone to dihydrotestosterone (DHT); ii)
inhibition of the binding of DHT to the androgen receptor; and iii)
anti-inflammatory effects. However, the effect on brain gliomas
remains to be fully understood. In early in vitro
experiments, the effect of saw palmetto extract on human glioma U87
and U251 cells was studied. The results revealed that saw palmetto
extract markedly inhibited the proliferation of human glioma cells.
The mechanism may be associated with the inhibition of STAT3
phosphorylation. Previous studies have hypothesized that the STAT3
and PI3K/Akt signaling transduction pathway has an important role
in tumor cells (10,11,12).
In the present study, the influence of saw palmetto extract on
human glioma U87 and U251 cell lines was further discussed with
regard to PI3K/Akt signaling transduction.
An increasing number of studies have demonstrated
that the PI3K/Akt signaling transduction pathway plays an important
role in the occurrence and development of malignant gliomas
(13–15). The PI3K/Akt signaling pathway is
not only important in cell proliferation and apoptosis, but also in
tumor growth and the response to chemotherapy (16). Activation of Akt can directly
phosphorylate a number of transcription factors, which regulates
the transcription factor, inhibits the expression of
apoptosis-related genes and enhances the expression of
antiapoptotic genes, including the Bcl-2 family, p53 and the FKHR
forkhead transcription factor. A previous study demonstrated that
proto-oncogenes and anti-oncogenes are involved in the regulation
of apoptosis (17). Bcl-2 is one
of the original cancer genes that was identified to be associated
with apoptosis (18). Bcl-2 is
capable of encoding 1G5M (26 kDa) and 1GO/JH (22 kDa) proteins that
exist in the outer mitochondrial membrane, nuclear membrane and
endoplasmic reticulum, and the Bcl-2 encoded protein is involved in
maintaining the integrity of the mitochondrial membrane. According
to the variety of structures and functions, the Bcl-2 family can be
divided into two categories: Inhibition of apoptosis family members
(including Bcl-2 and Bcl-xL) and promotion of apoptosis family
members (including Bcl, XS, BAX, BAK, BID and BAD). Previously,
Bcl-xL, as a member of the Bcl-2 family, was shown to be widely
expressed in human tissues and could inhibit cell apoptosis. Bcl-xL
can combine with proapoptotic proteins (primarily BAX and BAK) to
form a heterologous dipolymer, which improves the survival rate of
cells via stabilizing the mitochondrial membrane potential,
maintaining the mitochondrial outer membrane integrity and
preventing the release of cytochrome c and apoptosis
inhibition factor. Under an apoptosis signal, Bcl-xL can release
from BAX, resulting in cell apoptosis through altering the
permeability of the mitochondrial outer membrane and releasing
cytochrome c and other proapoptotic material. Lower protein
expression of Bcl-xL has been identified in normal brain tissue,
while higher levels have been identified in gastric, esophageal and
gallbladder cancers, among other tumor tissues. Bcl-xL is
hypothesized to be closely associated with the occurrence and
development of malignant tumors (18). According to the results of the
present study, the expression level of PI3K and Bcl-xL decreased
significantly following treatment with saw palmetto extract in
glioma cells. Thus, the results indicate that saw palmetto extract
downregulates the PI3K/Akt signaling transduction pathway and
inhibits the expression of Bcl-xL, known as the downstream
signaling factor of the PI3K/Akt signaling transduction pathway
(19).
p53 is an important protein that mediates DNA
damage-associated apoptosis. The levels and functions of p53 can be
decreased by MDM2 ubiquitin ligase (20). As the downstream signaling molecule
of the PI3K/Akt pathway, MDM2 ubiquitin ligase is a negatively
regulated protein of p53. Akt is able to combine with MDM2 and
phosphorylate the Ser 66 and Ser 88 sites in MDM2. Upregulated
activity of ubiquitin ligase promotes p53 inactivation or
degradation. The normal p53 gene consists of 11 exons and encodes a
53 kDa nuclear phosphoprotein, which is a tumor suppressor gene
located on the short arm of chromosome 17 (21). p53 is associated with multiple
cellular processes, including gene transcription, DNA repair, cell
cycle, genome stability, chromosome separation, cell senescence and
programmed cell death (22). The
p53 gene is one of the most frequently mutated genes in human
cancers, accounting for one third of gliomas. The mutant p53 gene
not only loses its tumor suppressor capability, but also promotes
malignant transformation. There a are a number of mechanisms
underlying p53 gene transfer between tumor suppressor and oncogene
and the regulation of cell proliferation. Firstly, wild type p53 is
a negative regulator of cell growth, however, the inactivation of
the p53 gene induced by gene loss or mutation results in the growth
advantage of transformed cells and tumor cells. Secondly, mutated
p53 protein can bind to the subunit of wild-type p53 to form
oligomeric complexes, which interfere with the function of the
wild-type subunits. Finally, N-terminal transcriptional activators
of mutated p53 protein can regulate the expression of c-fos, c-myc
and other cancer genes associated with the proliferation and
differentiation of cells (23).
The results of the present study indicate that the protein
expression level of p53 in glioma cells treated with saw palmetto
extract was significantly higher than in the control group. Thus,
the expression of p53 increased due to the lower expression levels
of the p53 inhibitor, MDM2, following the inhibition of the
PI3K/Akt signaling transduction pathway (24).
The etiology of gliomas is yet to be fully
understood. Investigating the occurrence and development of tumor
cells that have resulted from the abnormal activation of PI3K
signaling transduction is important. Saw palmetto extract can
induce glioma cell growth arrest and apoptosis through decreasing
PI3K/Akt signaling transduction. Therefore, the PI3K signaling
transduction pathway takes part in the proliferation of glioma. We
speculate that interferance with the PI3K signaling pathway maybe
beneficial to the treatment of glioma in the future.
Acknowledgements
The study was supported by a grant from the Liaoning
Provincial Natural Science Foundation of China (no. 201202257).
References
|
1
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jane EP, Premkumar DR, Morales A, Foster
KA and Pollack IF: Inhibition of phosphatidylinositol 3-kinase/AKT
signaling by NVP-BKM120 promotes ABT-737-induced toxicity in a
caspase-dependent manner through mitochondrial dysfunction and DNA
damage response in established and primary cultured glioblastoma
cells. J Pharmacol Exp Ther. 349:1–60. 2014.
|
|
4
|
Castellino RC and Durden DL: Mechanisms of
disease: the P13K-Akt-PTEN signaling node - an intercept point for
the control of angiogenesis in brain tumors. Nat Clin Pract Neurol.
3:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roell D, Rösler TW, Degen S, Matusch R and
Baniahmad A: Antiandrogenic activity of anthranilic acid ester
derivatives as novel lead structures to inhibit prostate cancer
cell proliferation. Chem Biol Drug Des. 77:450–459. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smirnova ZS, Ermakova KV, Kubasova IY,
Borisova LM, Kiselyova MP, Oborotova NA, Meerovich GA and
Luk’yanets EA: Experimental study of combined therapy for malignant
glioma. Bull Exp Biol Med. 156:480–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagane M: Anti-angiogenic therapy for
malignant glioma. Gan To Kagaku Ryoho. 41:141–147. 2014.(In
Japanese).
|
|
8
|
Taraphdar AK, Madhumita R and Bhattacharya
RK: Natural products as inducers of apoptosis: Implication for
cancer therapy and prevention. Curr Sci. 80:1387–1396. 2001.
|
|
9
|
Petrangeli E, Lenti L, Buchetti B, et al:
Lipido-sterolic extract of Serenoa repens (LSESr, Permixon)
treatment affects human prostate cancer cell membrane organization.
J Cell Physiol. 219:69–76. 2009.
|
|
10
|
Chua T, Eise NT, Simpson JS and Ventura S:
Pharmacological characterization and chemical fractionation of a
liposterolic extract of saw palmetto (Serenoa repens):
effects on rat prostate contractility. J Ethnopharmacol.
152:283–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Penugonda K and Lindshield BL: Fatty acid
and phytosterol content of commercial saw palmetto supplements.
Nutrients. 5:3617–3633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Ikezoe T, Zheng Z, Taguchi H,
Koeffler HP and Zhu WG: Saw palmetto induces growth arrest and
apoptosis of androgen-dependent prostate cancer LNCaP cells via
inactivation of STAT 3 and androgen receptor signaling. Int J
Oncol. 31:593–600. 2007.PubMed/NCBI
|
|
13
|
Ding D, Wei S, Song Y, Li L, Du G, Zhan H
and Cao Y: Osthole exhibits anti-cancer property in rat glioma
cells through inhibiting PI3K/Akt and MAPK signaling pathways. Cell
Physiol Biochem. 32:1751–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vitucci M, Karpinich NO, Bash RE, et al:
Cooperativity between MAPK and PI3K signaling activation is
required for glioblastoma pathogenesis. Neuro Oncol. 15:1317–1329.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Jiang Y, Zou F, et al: Activation
of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic
capacity of glioma stem cells. Proc Natl Acad Sci USA.
110:6829–6834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barnholtz-Sloan JS, Sloan AE and Schwartz
AG: Relative survival rates and patterns of diagnosis analyzed by
time period for individuals with primary malignant brain tumor,
1973–1997. J Neurosurg. 99:458–466. 2003.PubMed/NCBI
|
|
17
|
Silvestri I, Cattarino S, Aglianò A, et
al: Effect of Serenoa repens (Permixon(R)) on the expression
of inflammation-related genes: analysis in primary cell cultures of
human prostate carcinoma. J Inflamm (Lond). 10:112013.
|
|
18
|
Akl H, Vervloessem T, Kiviluoto S,
Bittremieux M, Parys JB, De Smedt H and Bultynck G: A dual role for
the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus
endoplasmic reticulum. Biochim Biophys Acta. 14:S0167–S4889.
2014.PubMed/NCBI
|
|
19
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Los M, Burek CJ, Stroh C, et al:
Anticancer drugs of tomorrow: apoptotic pathways as targets for
drug design. Drug Discov Today. 8:67–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Q, Li Y, Mu K, Li Z, Meng Q, Wu X, Wang
Y and Li L: Amplification of Mdmx and overexpression of MDM2
contribute to mammary carcinogenesis by substituting for p53
mutations. Diagn Pathol. 9:1–8. 2014.PubMed/NCBI
|
|
22
|
Han L, Zhang AL, Xu P, et al: Combination
gene therapy with PTEN and EGFR siRNA suppresses U251 malignant
glioma cell growth in vitro and in vivo. Med Oncol. 27:843–852.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JW, Schroeder MA, Sarkaria JN and
Bram RJ: Cyclophilin B supports Myc and mutant p53-dependent
survival of glioblastoma multiforme cells. Cancer Res. 74:484–496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez J and de Groot J: Combination
therapy for malignant glioma based on PTEN status. Expert Rev
Anticancer Ther. 8:1767–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|