Introduction
Infant pneumonia, which is caused by infection with
viruses or bacteria, has aroused public concern due to the
substantial mortality and incidence in children worldwide (1,2). There
are ~750,000 neonatal mortalities associated with pneumonia each
year, which constitute >10% of child mortalities worldwide
(3). The majority of these
mortalities occur in low income areas; in proportion of cases
occurring in developing countries is ~96%, and >90% of the
estimated 1.6 million annual pneumonia mortalities in children
<5 years of age (1,4,5).
Mortality rates are >70% in south Asia and sub-Saharan Africa,
where the incidence of pneumonia is estimated to be 0.36
(interquartile range, 0.32–0.40) episodes per child-year (2). Worldwide, the neonatal period is the
most significant for pneumonia; at least one-third of the annual
10.8 million mortalities in children occur in the first month of
life (6). Moreover, infant pneumonia
may be early or late onset, where early-onset neonatal pneumonia is
generally considered to be a clinical manifestation in the first
week of life, while the latter occurs in the following 3 weeks
(7). Clinically, infant pneumonia is
considered as a multifactorial disease affected by quality of life,
poor immunization, air pollution, nutritional status, and other
factors (2). Large quantities of
bacteria and viruses may to be associated with infant pneumonia,
with viral neonatal pneumonias including intrauterine, early-onset
or late-onset pneumonias (6). It has
been suggested that C-reactive protein (CRP) may be one of the best
single tests for the early detection of pneumonia in children, and
could be widely used as marker of infection (8).
C-reactive protein (CRP), an acute phase plasma
protein of the pentraxin family, is produced and released by
hepatocytes and adipocytes, playing distinct roles in innate and
adaptive immunity with inflammatory effects (9,10).
Moreover, it has been identified to be a sensitive and reliable
factor of acute inflammation in infectious as well as in
non-infectious inflammatory disorders (11). In previous studies, CRP has been
reported to function by binding to the phosphocholine moieties that
are located on some damaged cells and certain bacteria, and to have
a crucial part in complement activation through strengthening
phagocytosis, which may clear dead cells and bacteria (12,13). In
the acute phase, an extensive inflammatory environment, such as
bacterial, viral and fungal infection, rheumatic conditions,
malignancy and tissue damage, may induce the expression of
interleukin (IL)-6 as well as other factors, such as IL-1β and
tumor necrosis factor (TNF)-α, which may be associated with an
enhancement of CRP expression (14–16). In
clinical practice, systemic inflammation may cause a significant
increase in the serum CRP level that is considered to be a main
risk factor for inflammatory disease, myocardial infarction,
peripheral vascular disease and ischemic stroke (17,18). It
has been demonstrated that CRP may take part in the pathogenesis of
atherosclerosis through the promotion of endothelial cell
activation and foam cell generation within the arterial wall
(19,20). Previous studies have identified the
significant role of the serum CRP level in the development and
prognosis of pneumonia, and have reported that an increased serum
level of CRP may be present in patients with pneumonia (21,22). In
particular, it has been found that the serum CRP level may be
useful for distinguishing bacterial pneumonia from nonbacterial
pneumonia in children, as higher serum CRP concentrations were
observed in cases with a bacterial etiology (23,24). On
the basis of this evidence, it may be hypothesized that the serum
CRP level is closely correlated with the progress and severity of
infant pneumonia (25,26). By contrast, a small number of studies
have found that an elevated serum level of CRP is relatively
non-specific and cannot predict the prognosis of infant pneumonia
(27,28). Accordingly, the purpose of the
present meta-analysis was to provide clarify whether an increased
serum level of CRP accelerates the development of infant pneumonia
and may be regarded as a poor prognostic marker.
Materials and methods
Search strategy
Related articles were identified by searching
PubMed, Embase, Cochrane Library, CINAHL, CBM and CNKI databases
comprehensively for all pertinent papers, which assessed the
correlations between CRP serum levels and infant pneumonia and were
published up to May 31, 2014. The search terms used were
(‘C-reactive protein’), (‘infant, newborn’ or ‘newborn infants’ or
‘newborns’ or ‘neonates’), and (‘pneumonia’ or ‘pneumonia,
mycoplasma’ or ‘primary atypical pneumonia’ or ‘mycoplasma
pneumonia’ or ‘Mycoplasma pneumoniae pneumonia’) for the
initial search. No limitation was set on the language of the
article. Additional potentially relevant papers were further
retrieved by a manual search of references from the original
articles.
Selection criteria
Any randomized human-associated intervention
case-control studies that involved the association of CRP serum
levels with infant pneumonia as a primary outcome were initially
taken into consideration. With the exception of healthy
participants, studies concerning patients whose ages ranged from 7
days to 36 months and who were diagnosed with infant pneumonia
confirmed by histopathologic examinations according to the World
Health Organization (WHO) guidelines for the interpretation of
chest radiographs, were also included for the initial review of
articles (29). Studies that did not
provide the number of infant pneumonia cases, or sufficient
information about serum CRP expression levels, were not included in
the meta-analysis. Extracted studies in which the minimum number of
cases was not >20 were not excluded. However, extracted studies
that had a considerable overlap (>50%) of study subjects or lack
of complete data or unavailable data were excluded. If the same
population was investigated in more than one study, only the most
recent or complete study was included following careful
reexamination.
Data extraction
In order to reduce bias and enhance credibility, two
investigators extracted information according to the selection
criteria separately, and arrived at a consensus on all the items
through discussion and reexamination. The following relevant data
were extracted from eligible studies prospectively: Surname of
first author, year of publication, source of publication, study
type, study design, sample size, age, gender, ethnicity and country
of origin, detection method of CRP serum levels, and CRP expression
levels. As there were subjects of different ethnicities,
information was extracted separately into classes for Asian,
African and Caucasian populations. All authors approved the final
decision concerning the studies to be included.
Quality assessment
To decide whether a study was of high quality, the
two authors used a set of predefined criteria based on the
Newcastle-Ottawa Scale (NOS) criteria to assess the studies
independently (30). The NOS
criteria were scored based on three aspects: i) subject selection,
0–4; ii) comparability of subject, 0–2; and iii) clinical outcome,
0–3. Total NOS scores range from 0 (lowest) to 9 (highest).
According to the NOS scores, the included studies were classified
into two levels: low quality (0–6), and high quality (7–9).
Discrepancies in the NOS scores of the included articles were
resolved by an additional reviewer through discussion and
consultation.
Statistical analysis
In order to supply quantitative evidence from all
selected studies and minimize the variance of the summary, the
current statistical meta-analyses was conducted utilizing a
random-effects model (DerSimonian and Laird method) or a
fixed-effects model (Mantel-Haenszel method) of individual study
results under the situation where data from independent studies
could be combined. A random-effect model was applied when
heterogeneity existed among studies, while a fixed-effect model was
applied when there was no statistical heterogeneity. The summary
standardized mean difference (SMD) with 95% confidence intervals
(CIs) was calculated for case versus control categories of serum
CRP levels, with utilization of the Z-test. Subgroup meta-analyses
were also conducted by ethnicity and sample size to explore
potential modification effects, and heterogeneity across the
enrolled studies was evaluated by the Cochran's Q-statistic;
P<0.05 was regarded as statistically significant (31). As a result of the low statistical
power of the Cochran's Q-statistic, an I2 test was also
used to reflect the possibility of heterogeneity between studies
(32). Sensitivity analysis was
performed to reflect the effect of an individual data set on the
pooled SMDs. A funnel plot was constructed to assess publication
bias which might affect the validity of the estimates. The symmetry
of the funnel plot was further evaluated by Egger's linear
regression test (33). All tests
were two-sided and a P-value of <0.05 was regarded as
statistically significant. To ensure that the results were credible
and accurate, Stata software, version 12.0 (Stata Corp, College
Station, TX, USA) was used for statistical analysis.
Results
Baseline characteristics of the
included studies
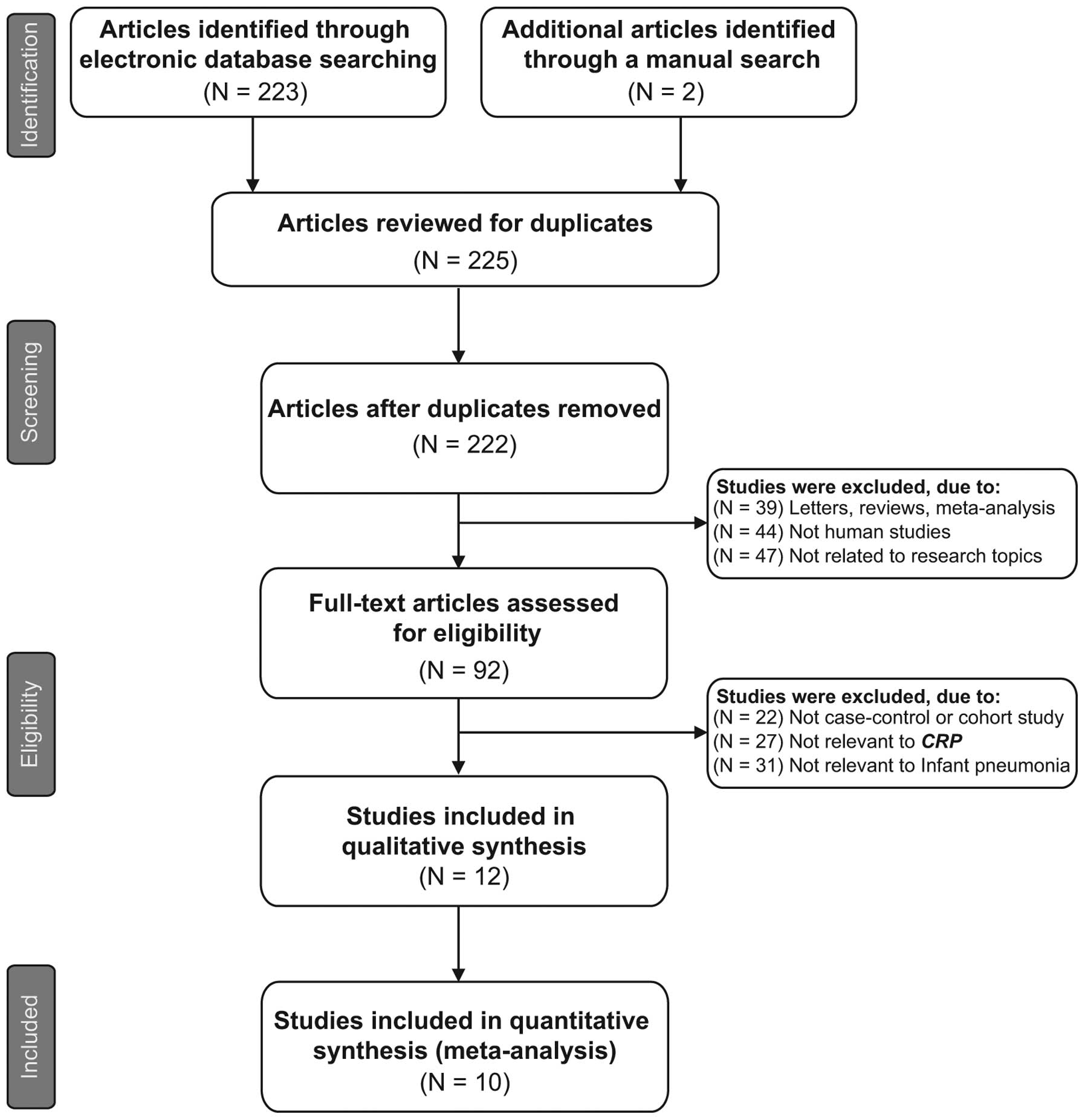
The original search yielded a total of 225 papers
associated with the searched keywords and a flow chart of the study
selection process is presented in Fig.
1. Through the step of screening the title and key words, 133
of these articles were excluded (3 were duplicates, 39 were
letters, reviews or meta-analyses, 44 were not human studies, and
47 were not related to the research topics). The full-texts from 92
articles were reviewed and an additional 80 trials were excluded
(22 were not case-control or cohort studies, 27 were not relevant
to CRP and 31 were not relevant to infant pneumonia), leaving 12
studies for further review. Of these, 2 were abandoned as they did
not supply enough information; therefore, 10 papers (25–28,34–39),
which included 652 patients with infant pneumonia and 845 healthy
controls, were finally found to conform to the inclusion criteria.
Publication years ranged from 1993 to 2014. The articles were
case-control studies that evaluated the correlation between CRP
serum levels and infant pneumonia in Asian populations (6 studies),
African populations (1 study) and Caucasian populations (3
studies). The detection methods applied were enzyme-linked
immunosorbent assay (ELISA) and immunonephelometry.
Immunonephelometry is the most commonly method utilized in the
studies included in the present meta-analysis. All quality scores
of the enrolled papers were >7 (high quality). Table I summarizes the characteristics and
methodological quality of the extracted studies. The number of
articles selected from the electronic databases over the years 2001
to 2014 are shown in Fig. 2.
 | Table I.Characteristics of included studies
focused on protein expression of CRP. |
Table I.
Characteristics of included studies
focused on protein expression of CRP.
|
|
|
|
|
| Sample size | Gender (M/F) | Age (days) |
|
|
|---|
|
|
|
|---|
| First author
(ref.) | Year | Country | Ethnicity | Sample-size | Case | Control | Case | Control | Case | Control | Method | NOS score |
|---|
| Zhou H (37) | 2014 | China | Asian | Small | 30 | 30 | 17/13 | 16/14 | – | – |
Immunonephelometry | 6 |
| Xu XY (25) | 2013 | China | Asian | Large | 70 | 236 | – | 120/116 | – | – |
Immunonephelometry | 8 |
| | | | Large | 45 | 236 | – | 120/116 | – | – |
Immunonephelometry |
|
|
|
|
|
| Large | 121 | 236 | – | 120/116 | – | – |
Immunonephelometry |
|
| Wu HM (35) | 2013 | China | Asian | Small | 34 | 40 | – | – | 2–27 | – |
Immunonephelometry | 7 |
|
|
|
|
| Large | 61 | 40 | – | – | 2–27 | – |
Immunonephelometry |
|
| Zhang GH (26) | 2012 | China | Asian | Small | 37 | 37 | 21/16 | 20/17 | 8.2±2.4 | 8.2±2.4 | ELISA | 7 |
|
|
|
|
| Small | 37 | 12 | – | 20/17 | – | 8.2±2.4 | ELISA |
|
|
|
|
|
| Small | 37 | 25 | – | 20/17 | – | 8.2±2.4 | ELISA |
|
|
|
|
|
| Small | 37 | 29 | – | 20/17 | – | 8.2±2.4 | ELISA |
|
|
|
|
|
| Small | 37 | 8 | – | 20/17 | – | 8.2±2.4 | ELISA |
|
| Feng ZM (34) | 2011 | China | Asian | Small | 20 | 30 | 12/8 | 18/12 | 13.8 | 12.4 |
Immunonephelometry | 6 |
|
|
|
|
| Small | 25 | 30 | 15/10 | 18/12 | 12.9 | 12.4 |
Immunonephelometry |
|
| Badr MA (27) | 2011 | Egypt | African | Small | 32 | 24 | 18/14 | 11/13 | 10.9±5.2 | 8.4±2.0 |
Immunonephelometry | 6 |
| Yang JH (36) | 2007 | China | Asian | Small | 13 | 35 | – | – | 2–27 | – |
Immunonephelometry | 7 |
|
|
|
|
| Large | 72 | 35 | – | – | 2–27 | – |
Immunonephelometry |
|
| Dollner H (38) | 2001 | Norway | Caucasian | Large | 8 | 124 | – | 67/57 | 1–7 | 1–7 |
Immunonephelometry | 5 |
| Romagnoli C
(39) | 2001 | Italy | Caucasian | Small | 7 | 20 | – | – | – | – |
Immunonephelometry | 5 |
| Salzer HR (28) | 1993 | Austria | Caucasian | Large | 50 | 94 | – | – | 1 | 6 |
Immunonephelometry | 6 |
|
|
|
|
| Large | 27 | 101 | – | – | 1 | 6 |
Immunonephelometry |
|
CRP serum levels in infant
pneumonia
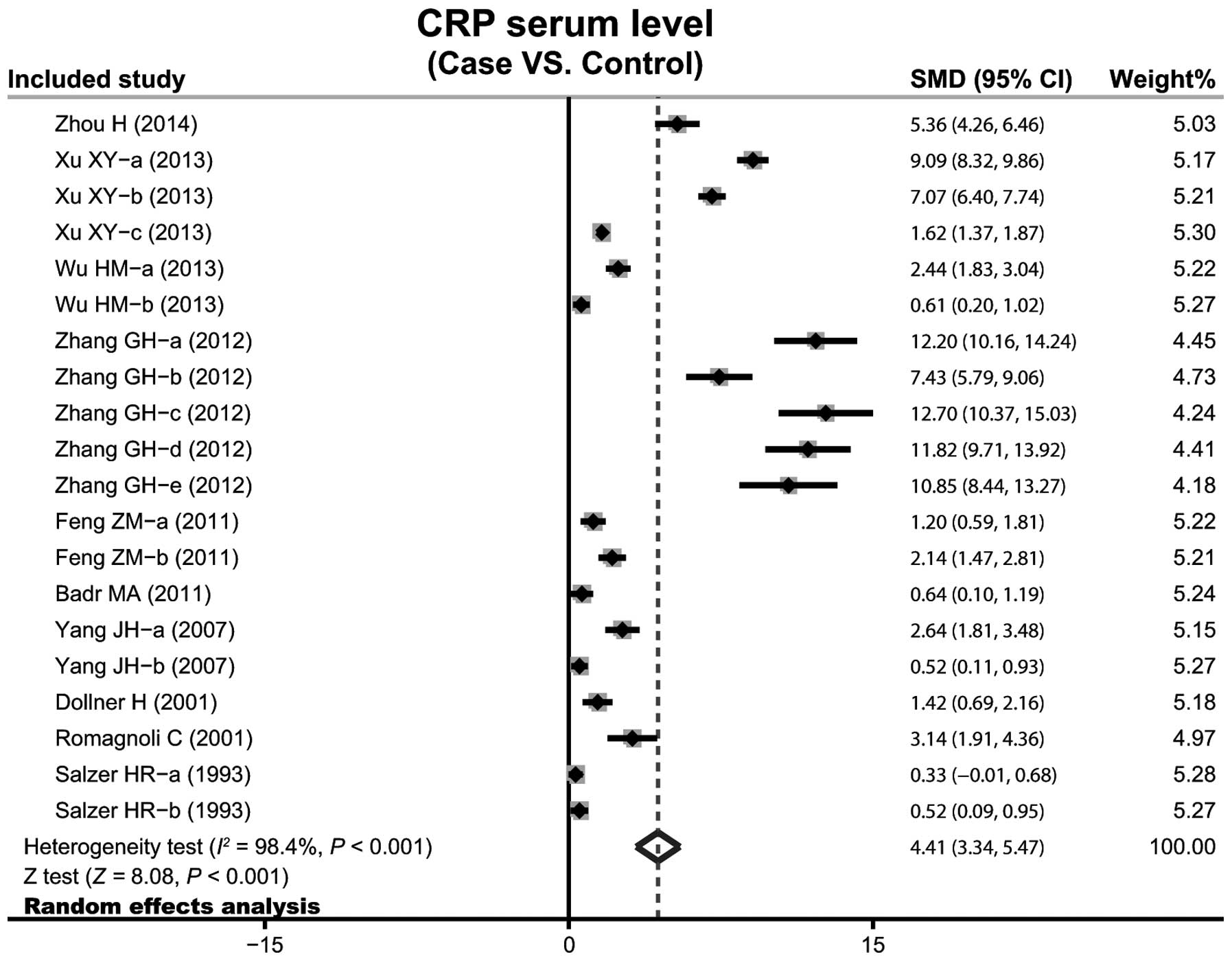
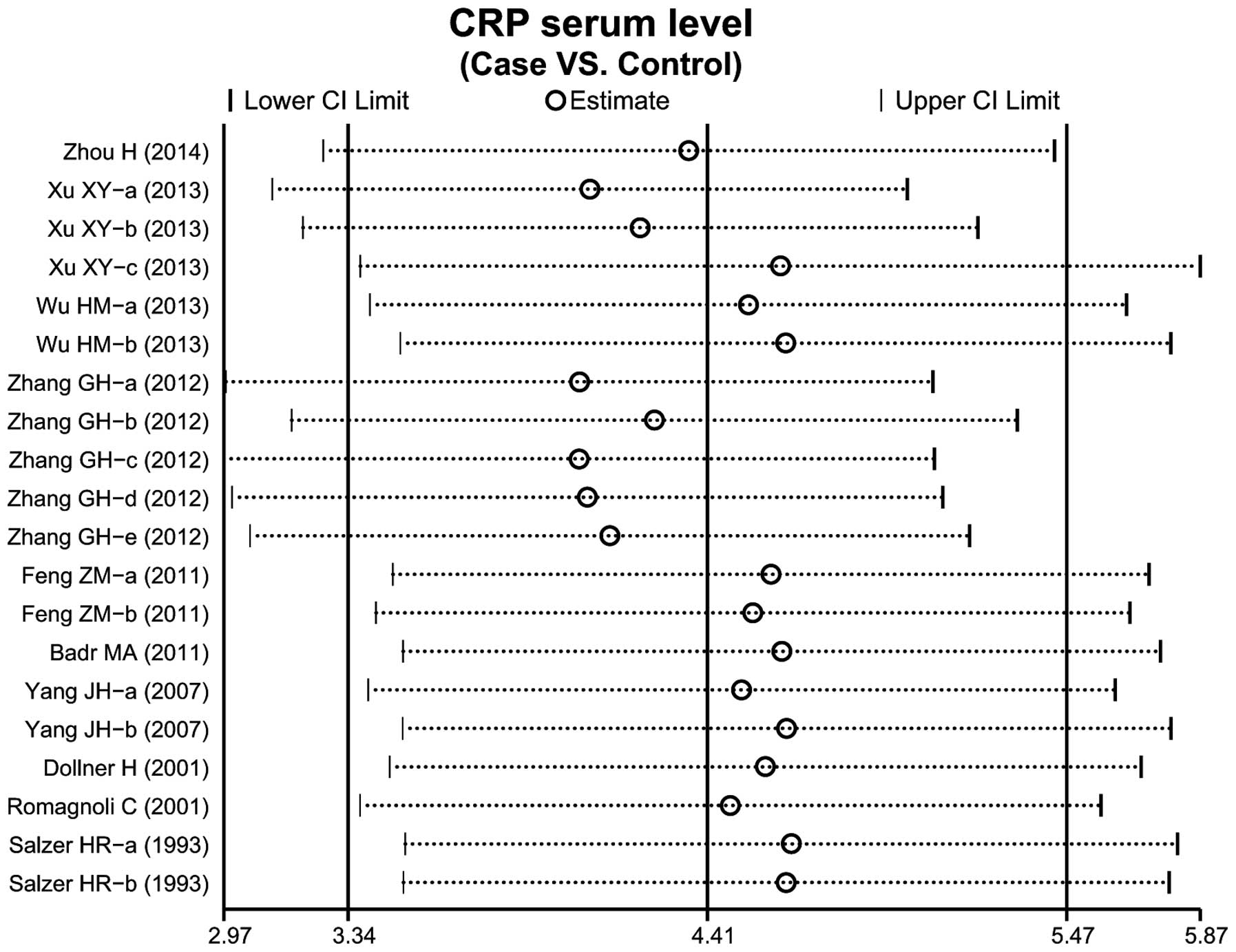
A total of 10 case-control studies considered the
CRP serum levels in infant pneumonia. The results concerning the
correlation between the levels of CRP and infant pneumonia are
presented in Fig. 3. The
random-effects model was used as heterogeneity existed
(P<0.001). The meta-analysis results identified a positive
association between CRP serum levels and infant pneumonia
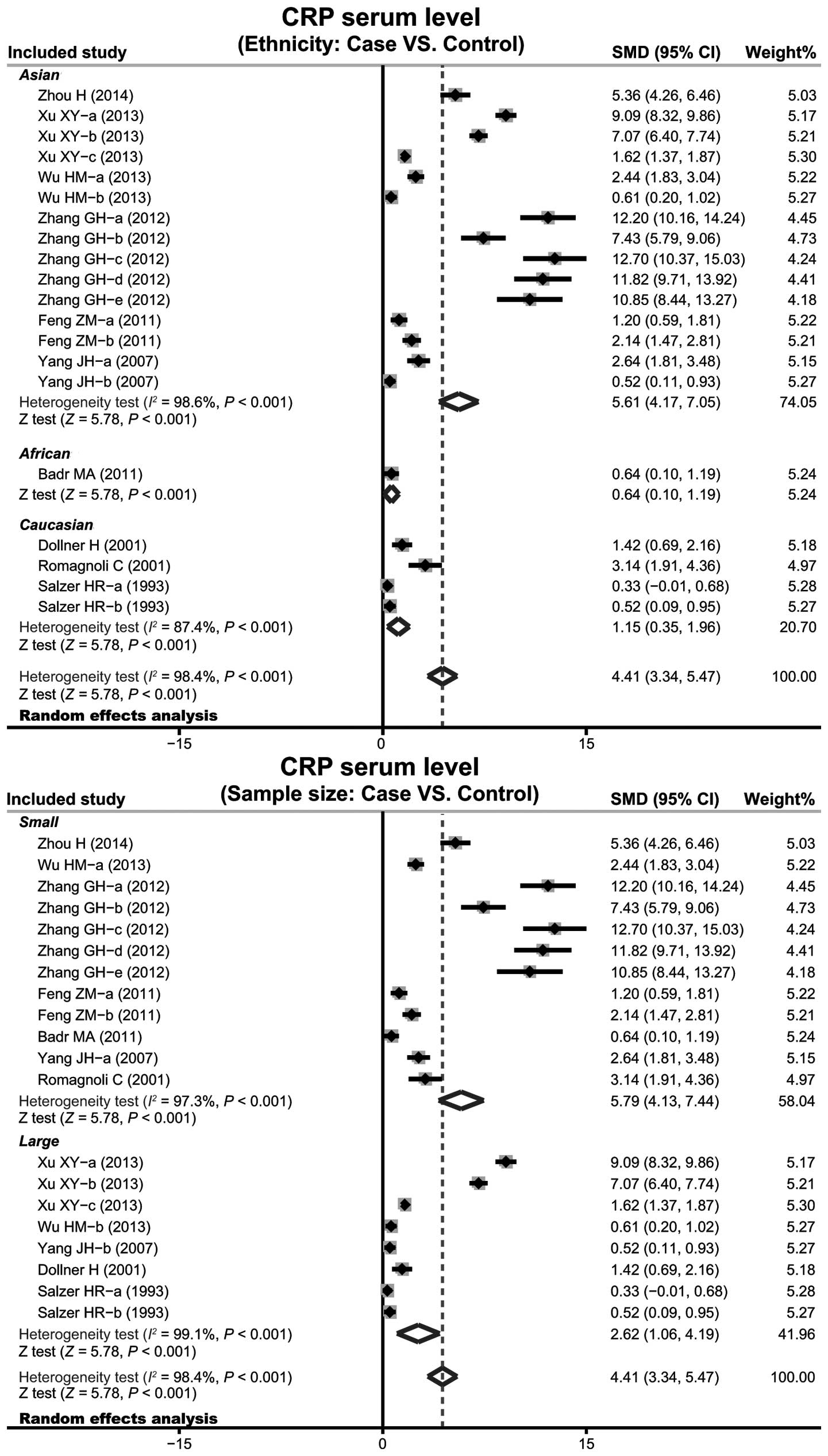
(SMD=4.41, 95% CI: 3.34–5.47, P<0.001). Subgroup analysis based
on ethnicity implied that high levels of serum CRP may be the main
risk factor of infant pneumonia in Asian (SMD=5.61, 95% CI:
4.17–7.05, P<0.001), African (SMD=0.64, 95% CI: 0.10–1.19,
P<0.001) and Caucasian (SMD=1.15, 95% CI: 0.35–1.96, P<0.001)
populations (Fig. 4). Further
subgroup analyses by sample size showed an clear association
between the levels of CRP and infant pneumonia in large sample and
small sample subgroups (small sample size: SMD=5.79, 95% CI:
4.13–7.44, P<0.001; large sample size: SMD=2.62, 95% CI:
1.06–4.19, P<0.001).
Sensitivity analysis and publication
bias
Each study enrolled in the meta-analysis was
evaluated one by one to reflect the effect on the significance of
the pooled SMDs. The results of the sensitivity analysis indicated
that the overall statistical level of significance did not change
when any single study was omitted, showing that the current
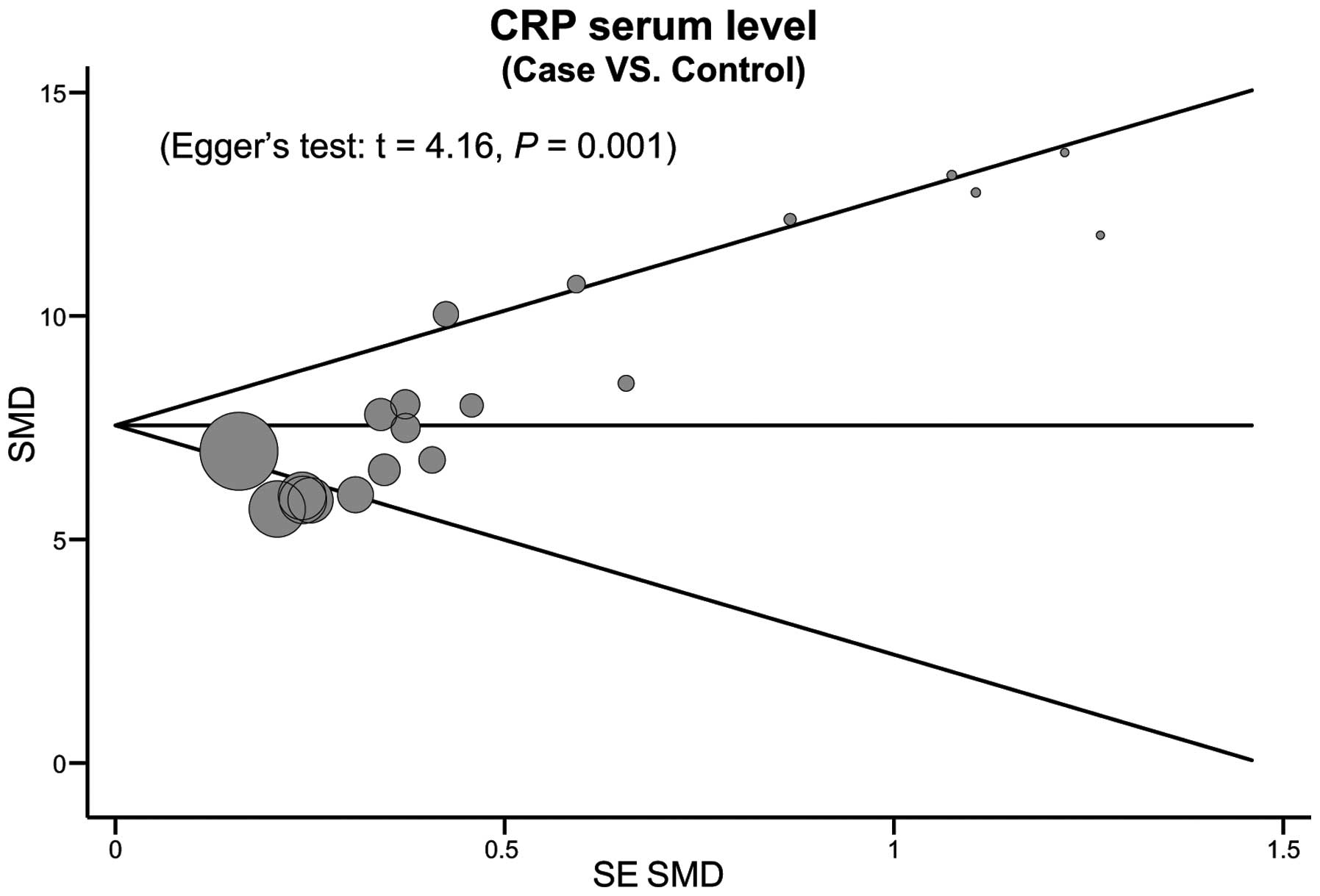
meta-analysis data is relatively stable and credible (Fig. 5). The funnel plots of the 10 included
studies exhibit a slight asymmetry, and Egger's test showed a
publication bias in this meta-analysis (t=4.16, P=0.001; Fig. 6).
Discussion
The current meta-analysis considered previous
findings from relevant studies in an attempt to determine the
correlation between serum levels of CRP and the pathogenesis of
infant pneumonia. The principal results of this meta-analysis
indicate that the serum CRP levels of newborn infants with
pneumonia were higher than those of healthy subjects, indicating
that increased serum levels of CRP might be an important risk
factor for the occurrence of pneumonia in newborn infants. CRP, a
major acute phase protein, is a member of the pentraxin family and
plays a central role in innate and adaptive immunity (40). The production of CRP is stimulated to
a large extent by TNF-α, IL-6 and IL-1β in response to infection or
inflammatory conditions (41). To be
specific, a reduction in serum CRP levels may present a relief or
alleviation of the inflammatory process, whereas persistently
upregulated levels of CRP or an initial reduction followed by a
further elevation might indicate persistent inflammation and poor
prognosis (42). Serum levels of CRP
are usually very low and difficult to detect in normal blood, but
may increase rapidly to significantly high levels at a very early
stage of the infection process (43). More importantly, CRP has been
indicated to be capable of protecting against bacterial infection
by binding to the pneumococcal C-polysaccharide and opsonization of
the bacteria for phagocytosis and killing (41,43).
Bacterial infection is a crucial risk factor leading to the
development of neonatal pneumonia (44). In this regard, it is reasonable to
conclude that serum CRP levels may be implicated in the etiology of
pneumonia in newborn infants. In accordance with the results of the
present meta-analysis, Koster et al measured the serum
levels of CRP in children and found that serum CRP levels were
independently correlated with pneumonia, indicating that the
detection of serum CRP levels may have independent value in the
diagnosis of pneumonia in children in an emergency department with
potential pneumonia (24).
Furthermore, Mintegi et al conducted a prospective
multicenter study based on 188 children younger than 36 months
cared for in pediatric emergency departments, and suggested that
serum levels of CRP might be helpful in predicting the risk of
occult pneumonia in infants with high fever without source
(45).
Stratified analysis on the basis of ethnicity and
sample size was rigorously implemented for the purpose of acquiring
a more accurate and profound understanding of serum CRP levels in
the etiology of pneumonia in newborn infants. The findings of the
ethnicity-stratified analysis revealed that newborn infants with
pneumonia had higher serum levels of CRP compared with healthy
controls among Asian, African and Caucasian populations, implying
that ethnicity is not a dominant factor impacting on the overall
results of this meta-analysis. The major discovery of the present
meta-analysis, which is partially in conformity with previous
studies, is that serum CRP levels may participate in the
development and progression of pneumonia in newborn infants,
implying that serum levels of CRP may be utilized as a valuable
biomarker in the early prediction of the occurrence and progression
of pneumonia in newborn infants.
However, the current meta-analysis has certain
limitations. Firstly, retrospective studies have innate limitations
by nature. This study was conducted with retrospective data
collection, where tests were prone to rely on previous information,
and baseline characteristics in the cases and controls varied
considerably, all of which may contribute to a possible selection
bias. Secondly, although a methodological assessment of the studies
was performed to avoid selection bias, there was a highly
significant heterogeneity among the 10 evaluable articles, which
may be attributed to the fact that the technique used to detect CRP
and the different backgrounds of the patients may be not comparable
among studies. Thirdly, the potential publication bias in this
study; Egger's test showed a publication bias in the current
meta-analysis. In addition, language may also introduce bias,
particularly since only eligible English or Chinese studies were
selected while certain potentially qualified studies were excluded
based on language criteria. A fourth potential limitation is the
relatively small sample size; the results of this study require
confirmation in prospective randomized controlled trials with
larger study populations. The above limitations may make the data
unsuitable for analysis by multiple logistic regression and
calculation of SMDs to some extent. Regardless of the limitations,
to the best of our knowledge, this is the first example of a
meta-analysis concerning the association of serum CRP levels with
the development of infant pneumonia. With the application of a
statistical approach to combine the results from multiple studies
in this meta-analysis and to achieve strong objectivity, all
research methods were carried out with strict inclusion and
exclusion criteria, suggesting that obtained information may
approximate the actual situation.
In conclusion, the results of this meta-analysis
showed that a higher serum CRP level is closely correlated with the
progression of infant pneumonia. CRP as a general systemic
inflammation biomarker may help clinicians to make difficult
therapeutic decisions for infant patients with pneumonia, according
to the present data. If the current results are confirmed by
further studies with larger sample sizes, the use of CRP may
improve the present diagnostic strategies for pneumonia in children
in pediatric clinical practice.
Acknowledgements
The authors would like to acknowledge the reviewers
for their helpful comments on this paper.
References
|
1
|
Diez-Padrisa N, Bassat Q, Machevo S, et
al: Procalcitonin and C-reactive protein for invasive bacterial
pneumonia diagnosis among children in Mozambique, a malaria-endemic
area. PLoS One. 5:e132262010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banstola A and Banstola A: The
epidemiology of hospitalization for pneumonia in children under
five in the rural western region of Nepal: a descriptive study.
PLoS One. 8:e713112013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seif El Dien HM and Abd ElLatif DAK: The
value of bedside Lung Ultrasonography in diagnosis of neonatal
pneumonia. Egypt J Radiol Nucl Med. 44:339–347. 2013. View Article : Google Scholar
|
|
4
|
Zaidi AKM, Ganatra HA, Syed S, et al:
Effect of case management on neonatal mortality due to sepsis and
pneumonia. BMC Public Health. 11 (Suppl 3):132011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falade AG and Ayede AI: Epidemiology,
aetiology and management of childhood acute community-acquired
pneumonia in developing countries - a review. Afr J Med Med Sci.
40:293–308. 2011.PubMed/NCBI
|
|
6
|
Nissen MD: Congenital and neonatal
pneumonia. Paediatr Respir Rev. 8:195–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang AT, Bang RA, Morankar VP, Sontakke PG
and Solanki JM: Pneumonia in neonates: can it be managed in the
community? Arch Dis Child. 68:550–556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansen AB, Verder H and Staun-Olsen P:
Soluble intercellular adhesion molecule and C-reactive protein as
early markers of infection in newborns. J Perinat Med. 28:97–103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swede H, Hajduk AM, Sharma J, et al:
Baseline serum C-reactive protein and death from colorectal cancer
in the NHANES III cohort. Int J Cancer. 134:1862–1870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDade TW, Rutherford J, Adair L and
Kuzawa CW: Early origins of inflammation: microbial exposures in
infancy predict lower levels of C-reactive protein in adulthood.
Proc Biol Sci. 277:1129–1137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jordan KK, Christensen IJ, Heilmann C,
Sengelov H and Muller KG: Pretransplant C-reactive protein as a
prognostic marker in allogeneic stem cell transplantation. Scand J
Immunol. 79:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukerji R, Mirza S, Roche AM, et al:
Pneumococcal surface protein A inhibits complement deposition on
the pneumococcal surface by competing with the binding of
C-reactive protein to cell-surface phosphocholine. J Immunol.
189:5327–5335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Marjon KD, Marnell LL, et al:
Recognition and functional activation of the human IgA receptor
(FcalphaRI) by C-reactive protein. Proc Natl Acad Sci USA.
108:4974–4979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martins OM, Fonseca VF, Borges I, Martins
V, Portal VL and Pellanda LC: C-Reactive protein predicts acute
myocardial infarction during high-risk noncardiac and vascular
surgery. Clinics (Sao Paulo). 66:773–776. 2011.PubMed/NCBI
|
|
15
|
Jones G, Sebba A, Gu J, et al: Comparison
of tocilizumab monotherapy versus methotrexate monotherapy in
patients with moderate to severe rheumatoid arthritis: the AMBITION
study. Ann Rheum Dis. 69:88–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beisswenger PJ, Brown WV, Ceriello A, et
al: Meal-induced increases in C-reactive protein, interleukin-6 and
tumour necrosis factor α are attenuated by prandial + basal insulin
in patients with Type 2 diabetes. Diabet Med. 28:1088–1095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Wijk DF, Boekholdt SM, Wareham NJ, et
al: C-reactive protein, fatal and nonfatal coronary artery disease,
stroke and peripheral artery disease in the prospective
EPIC-Norfolk cohort study. Arterioscler Thromb Vasc Biol.
33:2888–2894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Emerging Risk Factors Collaboration, .
Kaptoge S, Di Angelantonio E, Lowe G, et al: C-reactive protein
concentration and risk of coronary heart disease, stroke and
mortality: an individual participant meta-analysis. Lancet.
375:132–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang X, Zhang J, Liu J, et al: C-reactive
protein promotes adhesion of monocytes to endothelial cells via
NADPH oxidase-mediated oxidative stress. J Cell Biochem.
113:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mogelvang R, Pedersen SH, Flyvbjerg A, et
al: Comparison of osteoprotegerin to traditional atherosclerotic
risk factors and high-sensitivity C-reactive protein for diagnosis
of atherosclerosis. Am J Cardiol. 109:515–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Vugt SF, Broekhuizen BD, Lammens C, et
al: Grace consortium: Use of serum C reactive protein and
procalcitonin concentrations in addition to symptoms and signs to
predict pneumonia in patients presenting to primary care with acute
cough: diagnostic study. BMJ. 346:F24502013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hillas G, Vassilakopoulos T, Plantza P, et
al: C-reactive protein and procalcitonin as predictors of survival
and septic shock in ventilator-associated pneumonia. Eur Respir J.
35:805–811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diez-Padrisa N, Bassat Q, Morais L, et al:
Procalcitonin and C-reactive protein as predictors of blood culture
positivity among hospitalised children with severe pneumonia in
Mozambique. Trop Med Int Health. 17:1100–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koster MJ, Broekhuizen BD, Minnaard MC, et
al: Diagnostic properties of C-reactive protein for detecting
pneumonia in children. Respir Med. 107:1087–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu XY and Li ZF: Value of detection of
procalcitonin and C-reactive protein in diagnosis of infectious
pneumonia in neonates. Zhonghua Yi Yuan Gan Ran Xue Za Zhi.
23:2515–2517. 2013.(In Chinese).
|
|
26
|
Zhang GH, Chen CX and He JQ: Study on the
correction between the serum SIL-2R, CRP, myocardial enzyme and
neonatal pneumonia. Zhongguo Yi Yao Dao Bao. 9:75–76. 2012.(In
Chinese).
|
|
27
|
Badr MA, Ali YF, Albanna EA, Beshir MR and
Amr GE: Ventilator associated pneumonia in critically-ill neonates
admitted to neonatal intensive care unit, Zagazig University
Hospitals. Iran J Pediatr. 21:418–424. 2011.PubMed/NCBI
|
|
28
|
Salzer HR, Pollak A, Herkner K, Weninger M
and Schemper W: Value of measurement of neutrophil elastase-alpha 1
proteinase inhibitor levels in the early diagnosis of neonatal
infection. Eur J Clin Microbiol Infect Dis. 12:553–556. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ben Shimol S, Dagan R, Givon-Lavi N, et
al: Evaluation of the World Health Organization criteria for chest
radiographs for pneumonia diagnosis in children. Eur J Pediatr.
171:369–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jackson D, White IR and Riley RD:
Quantifying the impact of between-study heterogeneity in
multivariate meta-analyses. Stat Med. 31:3805–3820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zintzaras E and Ioannidis JP: HEGESMA:
genome search meta-analysis and heterogeneity testing.
Bioinformatics. 21:3672–3673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng ZM, Li X and Yang RG: Analysis on
detection results of blood lactic acid and C-reactive protein in
pneumonic neonates. Zhongguo Yi Yao Dao Bao. 8:85–86. 2011.(In
Chinese).
|
|
35
|
Wu HM: Value of detection of PCT, hs-CRP
and WBC in diagnosis of early neonatal pneumonia. Haixia Yufang
Yixue Zazhi. 19:83–84. 2013.(In Chinese).
|
|
36
|
Yang JH, Wei XS and Liao B: Significance
of the detection of PCT, hs-CRP and IL-6 in diagnosis of neonatal
pneumonia. Chongqing Yixue. 36:1194–1195. 2007.(In Chinese).
|
|
37
|
Zhou H: Significance of joint detection
serum cystatin cand CRP in neonatal pneumonia. Chengde Yixueyuan
Xuebao. 31:31–32. 2014.(In Chinese).
|
|
38
|
Døllner H, Vatten L and Austgulen R: Early
diagnostic markers for neonatal sepsis: comparing C-reactive
protein, interleukin-6, soluble tumour necrosis factor receptors
and soluble adhesion molecules. J Clin Epidemiol. 54:1251–1257.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Romagnoli C, Frezza S, Cingolani A, et al:
Plasma levels of interleukin-6 and interleukin-10 in preterm
neonates evaluated for sepsis. Eur J Pediatr. 160:345–350. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Volanakis JE: Human C-reactive protein:
expression, structure and function. Mol Immunol. 38:189–197. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arinzon Z, Peisakh A, Schrire S and Berner
Y: C-reactive protein (CRP): an important diagnostic and prognostic
tool in nursing-home-associated pneumonia. Arch Gerontol Geriatr.
53:364–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moreno MS, Nietmann H, Matias CM and Lobo
SM: C-reactive protein: a tool in the follow-up of nosocomial
pneumonia. J Infect. 61:205–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marnell L, Mold C and Du Clos TW:
C-reactive protein: ligands, receptors and role in inflammation.
Clin Immunol. 117:104–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rudan I, Boschi-Pinto C, Biloglav Z,
Mulholland K and Campbell H: Epidemiology and etiology of childhood
pneumonia. Bull World Health Organ. 86:408–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mintegi S, Benito J, Pijoan JI, et al:
Occult pneumonia in infants with high fever without source: a
prospective multicenter study. Pediatr Emerg Care. 26:470–474.
2010. View Article : Google Scholar : PubMed/NCBI
|