Introduction
Previous studies indicate that oral squamous cell
carcinoma (OSCC) is the eighth most prevalent type of cancer
worldwide (1), in men and women
(2). Despite considerable
investigation and novel therapeutic developments, the 5-year
survival rate associated with OSCC is ~50%, which is in part due to
the lack of effective biomarkers for early diagnosis and optimal
treatment (1,3). Therefore, OSCC is a significant public
health issue worldwide (4). A
significant number of genetic single-nucleotide polymorphisms
(SNPs) have been associated with OSCC (5–8);
however, the contribution of these gene interactions in the
development of OSCC remains unclear. Therefore, the identification
of novel and effective biomarkers is necessary to improve the
prognosis of OSCC.
Apoptosis, or programmed cell death, is a crucial
mechanism against hyperproliferation and malignancy (9). Apoptosis is predominantly mediated by
enzymes known as caspases (9,10).
Caspase-8 (CASP8) is a key regulator of apoptosis in T lymphocytes
and is encoded by the CASP8 gene. However, a previous study
has suggested that CASP8 additionally serves certain non-apoptotic
functions in cells, such as promoting activation nuclear factor
(NF)-κB signaling, regulating autophagy, altering endosomal
trafficking and enhancing cellular adhesion and migration (11). The human CASP8 gene contains
at least 11 exons, spanning 30 kb on the highly polymorphic
chromosome 2q33–34 (12,13). In addition to rare mutations, a
number of common variants of the CASP8 gene disrupt
apoptosis and enhance the risk of developing various types of
cancer, including breast cancer (14,15),
colorectal cancer (16), ovarian
cancer (17), prostate cancer
(18) and others (19,20). The
ins/del polymorphism rs3834129 in the promoter region of the
CASP8 gene may block the stimulatory protein 1 (Sp1) binding
site, and has been associated with reduced susceptibility to
various types of cancer, including lung, esophageal, gastric,
colorectal, cervical and breast cancers in Chinese individuals
(21). Barrett et al
(22) conducted a genome-wide
association study (GWAS) among European populations and identified
three novel melanoma susceptibility loci, including an SNP adjacent
to CASP8 (rs13016963; P=8.6×10−10). In addition,
a GWAS study revealed that rs13016963 is a significant
susceptibility locus for esophageal squamous cell carcinoma in
Chinese individuals (23). The SNP
rs1045485 (Asp302His), which is located in the CASP8 gene,
has been investigated in previous studies, and the results suggest
that this locus is associated with breast cancer and prostate
carcinoma (18,24).
The aim of the present study was to determine
whether the CASP8 −652 6 N ins/del polymorphisms rs1045485
(Asp302His) and rs13016963 are associated with an increased risk of
OSCC. To test this hypothesis, the polymorphisms were genotyped and
their associations with OSCC risk were assessed in a
hospital-based, case-control study of Chinese patients.
Materials and methods
Patients and controls
The study population included 505 Han Chinese
patients with clinically diagnosed OSCC, that were recruited at the
West China Hospital of Stomatology, Sichuan University (Chengdu,
China) between December 2009 and January 2013, in addition to 507
healthy Han Chinese that visited the general health check-up
division of the West China Hospital of Stomatology, Sichuan
University. OSCC was diagnosed according to the 1997 World Health
Organization criteria, and clinical stage (Tumor Node Metastasis)
was determined according to the 2002 American Joint Committee on
Cancer criteria (25,26).
All subjects were informed of the detailed study
protocol, required to sign consent forms and instructed to complete
a standardized questionnaire that was conducted by two trained
interviewers. The detailed standardized questionnaire completed by
the patients was described previously (6). The ethics committee of Sichuan
University and all of the participating patients signed written
consent forms prior to the collection of samples. The
characteristics of the case group and control group are shown in
Table I and clinicopathological
characteristics of the case group are detailed in Table II.
 | Table I.Characteristics of case group
patients and control group subjects. |
Table I.
Characteristics of case group
patients and control group subjects.
| Characteristic | Case, n=505 | Control, n=507 |
P-valuea |
|---|
| Age |
|
|
|
| Mean ±
SD, years | 60.01±11.67 | 58.95±12.71 |
0.166 |
| ≤45
years, n | 77 | 56 |
0.091 |
|
45<age≤65 years, n | 264 | 292 |
|
| >65
years, n | 164 | 159 |
|
| Gender, n (%) |
|
|
|
|
Male | 330 (65.3) | 348 (68.6) |
|
|
Female | 175 (34.7) | 159 (31.4) |
0.265 |
| Smoking, n (%) |
|
|
|
|
Yes | 304 (60.2) | 165 (32.5) |
|
| No | 201 (39.8) | 342 (67.5) | <0.001 |
| Drinking, n
(%) |
|
|
|
|
Yes | 275 (54.5) | 209 (41.2) |
|
| No | 230 (45.5) | 298 (58.8) | <0.001 |
 | Table II.Clinicopathological features of the
patients in the case group (n=505). |
Table II.
Clinicopathological features of the
patients in the case group (n=505).
| Characteristic | Case, n (%) |
|---|
| Site |
|
|
Tongue | 161 (31.9) |
| Buccal
mucosa | 131 (25.9) |
|
Gingiva | 84 (6.6) |
| Floor
of mouth | 61
(12.1) |
|
Palate | 35 (6.9) |
|
Lip | 24 (4.8) |
|
Maxillary sinus | 9
(1.8) |
| Tumor size |
|
|
T1+T2 | 304 (60.2) |
|
T3+T4 | 201 (39.8) |
| Lymph node
metastases |
|
| N0 | 332 (65.7) |
| N+ | 173 (34.3) |
| Clinical stage |
|
| Stage I
+ II | 244 (48.3) |
| Stage
III + IV | 261 (51.7) |
| Pathological
stage |
|
| SCCI
(highly differentiated) | 358 (70.9) |
| SCCII
(moderately differentiated) | 128 (25.3) |
| SCCIII
(poorly differentiated) | 19 (3.8) |
DNA extraction and genotyping
Blood samples were extracted from all of the
participants by peripheral antecubital venous puncture and stored
at −20°C until required for analysis.
Genomic DNA was extracted from the samples using a
previously described salting-out method (27). The DNA concentration of each
individual sample was determined using a NanoDrop ND 1000
spectrophotometer and NanoDrop software, version 2.4.7c (NanoDrop
Technologies Inc., Wilmington, DE, USA). The details of the
genotyping method used for rs1045485 and rs3834129 are described in
previous studies (28,29). The CASP8 genotype rs13016963
was determined via polymerase chain reaction-restriction fragment
length polymorphism (PCR-RFLP). The PCR primers were designed on
the basis of the National Center for Biotechnology Information
dbSNP database reference sequence (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=13016963).
The PCR reaction mixture consisted of 2.5 µl 10X PCR buffer, 0.1
mM/l dNTPs, 1 mM/l MgCl2, 2 U Taq polymerase [Tiangen
Biotech (Beijing) Co., Ltd., Beijing China], 10 ng genomic DNA, 0.4
pM/l of each primer (forward, 5′-GTG CCG AGG CTC AGG CTA GAG GAA
GGA AAC ATC CGC-3′; and reverse, 5′-TTT CCC CAC TAT TGA GGT AA-3′)
and sufficient ddH2O to increase the volume to a total
of 25 µl per reaction.
PCR products were each digested with 3 units of the
specific endonuclease Bsh1236I for 2 h at 37°C. PCR and
digestion products were analyzed via electrophoresis in 2% agarose
gels using TAE 1X buffer in runs of 20 min at 120 V; the gels were
subsequently visualized by staining with ethidium bromide and
images were captured using Tanon 1600 Gel Imaging system (Tanon
Science & Technology Co., Ltd., Shanghai, China). To confirm
the genotyping results, Sanger sequencing was conducted by Sangon
Biotech Co., Ltd. (Shanghai, China), and the results were 100%
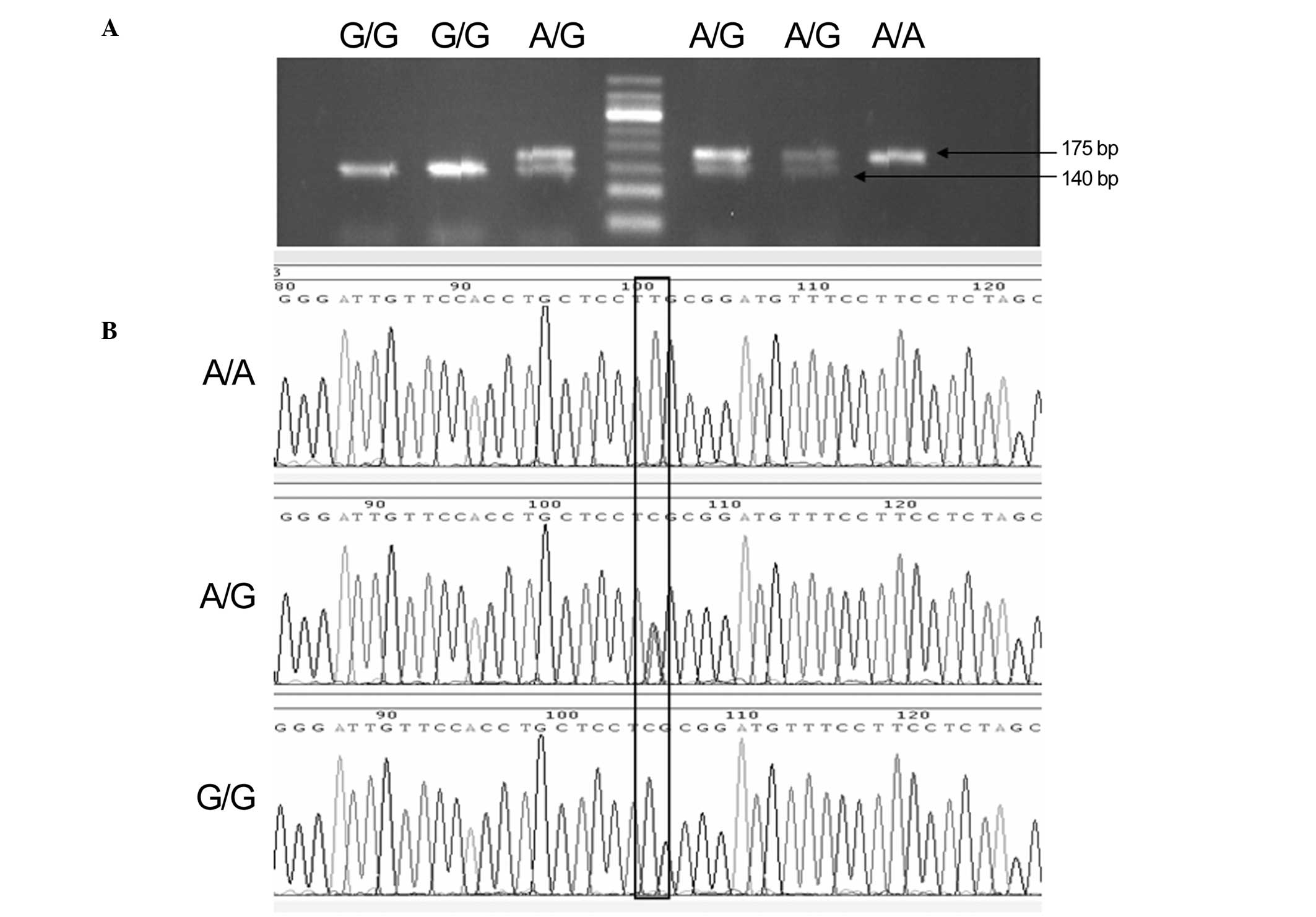
concordant. Product sizes were 175 bp for the A allele and 140/35
bp for the G allele (Fig. 1). After
each genetic polymorphism had been genotyped, 10–15% of the samples
in each genotype group were randomly selected for Sanger sequencing
to validate the results (Fig. 1).
Sanger sequencing results were consistent with those of the RFLP
analysis.
Statistical analysis
The genotype frequencies of rs3834129 and rs13016963
in the patients with OSCC and control subjects were compared using
the χ2 test. Odds ratio (OR) with a 95% confidence
interval (CI) was estimated using unconditional logistic
regression. The adjusted odds ratios (AORs) with 95% CIs of the
association between genotype frequencies and OSCC susceptibility
were estimated using multiple logistic regression models after
other covariates, such as age, gender, smoking and drinking, were
controlled. The observed genotype frequencies of CASP8
polymorphisms in the control subjects were analyzed to determine
deviation from Hardy-Weinberg equilibrium using Power-Stats
software (Promega Corporation, Madison, WI, USA). P<0.05 was
considered to indicate a statistically significant difference. SPSS
software, version 11.5 (SPSS, Inc., Chicago, IL, USA) was used to
perform all statistical analyses. SHEsis software (http://analysis.bio-x.cn/SHEsisMain.htm)
was used to analyze linkage disequilibrium (LD) (30). Lewontin's D' (31) and r2 (32) were calculated between each pair of
SNPs.
Results
Study groups
The frequency distributions of the selected
characteristics of the case and control groups are presented in
Table I. Statistical data indicated
no significant differences in gender and age between the patient
and control groups, but showed significant differences in smoking
(P<0.001) and drinking (P<0.001; Table I). Increased numbers of smokers were
present among the case group patients (60.2%) compared with the
control group subjects (32.5%; P<0.001). In addition, more
drinkers were included in the case group (54.5%) compared with the
control group (41.2%; P<0.001).
Regression analysis
Multiple logistic regression analyses were conducted
for rs3834129 (additive, ins/del vs. ins/ins and del/del vs.
ins/ins; dominant, ins/del + del/del vs. ins/ins; recessive,
del/del vs. ins/ins + ins/del) and rs13016963 (additive, AG vs. GG
and AA vs. GG; dominant, AG/AA vs. GG; recessive, AA vs. GG/GA).
rs1045485 was not detected in any of the patients with OSCC or
control subjects. Therefore, this SNP was excluded from subsequent
analyses. The AORs and 95% CIs of the genotype distributions of
rs3834129 and rs13016963 associated with the susceptibility of OSCC
between the case and control groups are shown in Table III. No significant association was
observed between genetic polymorphisms of rs1306963 and oral cancer
in additive, dominant and recessive models (Table III). However, the AA genotype
frequencies of rs1306963 were significantly associated with OSCC in
additive (AA vs. GG, 95% CI, 1.045–3.052) and recessive models (AA
vs. GG + AG, 95% CI, 1.199–3.089) in non-smokers (Table IV). In additive and recessive
models, the AA genotype was associated with a significantly higher
risk of 1.834-fold (AA vs. GG, 95% CI, 1.052–3.199) and 2.127-fold
(AA vs. GG/AG, 95% CI, 1.298–3.487) among non-drinkers (Table V).
 | Table III.Comparison of genotype frequencies of
the CASP8 gene polymorphisms between oral squamous cell
carcinoma patients and control subjects. |
Table III.
Comparison of genotype frequencies of
the CASP8 gene polymorphisms between oral squamous cell
carcinoma patients and control subjects.
| SNP | Model/allele | Genotype | Case (%) n=505 | Control (%)
n=507 |
P-valuea | OR (95%
CI)b | Pac | AOR (95%
CI)d |
|---|
| Rs3834129 | Additive | ii | 328 (65.0) | 276 (54.4) |
| 1.00 (Ref) |
|
|
|
|
| id | 159 (31.5) | 197 (38.9) | 0.04 | 0.679
(0.522–0.884) | 0.014 | 0.447
(0.235–0.850) |
|
|
| dd | 18 (3.5) | 34 (6.7) | 0.006 | 0.445
(0.246–0.806) | 0.002 | 0.640
(0.482–0.851) |
|
| Dominant | ii | 328 (65.0) | 276 (54.4) |
| 1.00 (Ref) |
|
|
|
|
| id/dd | 177 (35.0) | 231 (45.6) | 0.001 | 0.645
(0.501–0.830) | <0.001 | 0.613
(0.467–0.806) |
|
| Recessive | ii/id | 487 (96.5) | 473 (93.3) |
|
|
|
|
|
|
| dd | 18 (3.5) | 34 (6.7) | 0.024 | 0.514
(0.286–0.923) | 0.048 | 0.528
(0.281–0.993) |
| Rs13016963 | Additive | GG | 162 (32.1) | 164 (32.3) |
| 1.00 (Ref) |
|
|
|
|
| AG | 243 (48.1) | 264 (52.1) | 0.619 | 0.932
(0.705–1.231) | 0.765 | 0.955
(0.706–1.291) |
|
|
| AA | 100 (19.8) | 79
(15.6) | 0.184 | 1.281
(0.888–1.848) | 0.156 | 1.332
(0.896–1.981) |
|
| Dominant | GG | 162 (32.1) | 164 (32.3) |
| 1.00 (Ref) |
|
|
|
|
| AG/AA | 343 (67.9) | 343 (67.7) | 0.927 | 1.012
(0.778–1.318) | 0.787 | 1.040
(0.781–1.385) |
|
| Recessive | GG/AG | 405 (80.2) | 428 (84.4) |
| 1.00 (Ref) |
|
|
|
|
| AA | 100 (19.8) | 79
(15.6) | 0.079 | 1.338 (0.
967–1.851) | 0.077 | 1.1371
(0.967–1.945) |
 | Table IV.Comparison of genotype frequencies of
CASP8 gene polymorphisms in smokers and non-smokers. |
Table IV.
Comparison of genotype frequencies of
CASP8 gene polymorphisms in smokers and non-smokers.
| Smoking status | SNP | Model/allele | Genotype | Case, n (%) | Control, n (%) |
P-valuea | OR (95%
CI)b | Pac | AOR (95%
CI)d |
|---|
| Smokere | rs3834129 | Additive | ii | 194 (63.8) | 87 (52.7) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 100 (32.9) | 70 (42.4) | 0.027 | 0.641
(0.431–0.952) | 0.057 | 0.660
(0.430–1.013) |
|
|
|
| dd | 10 (3.3) | 8 (4.9) | 0.234 | 0.561
(0.214–1.469) | 0.076 | 0.395
(0.142–1.102) |
|
|
| Dominant | ii | 194 (63.8) | 87 (52.7) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 110 (36.2) | 78 (47.3) | 0.019 | 0.632
(0.430–0.929) | 0.027 | 0.627
(0.415–0.949) |
|
|
| Recessive | ii/id | 294 (96.7) | 157 (95.1) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 10 (3.3) | 8 (4.9) | 0.401 | 0.668
(0.258–1.725) | 0.132 | 0.459
(0.167–1.263) |
|
| rs13016963 | Additive | GG | 99
(32.6) | 54 (32.7) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 150 (49.3) | 83 (50.3) | 0.947 | 0.986
(0.644–1.510) | 0.916 | 1.025
(0.646–1.627) |
|
|
|
| AA | 55
(18.1) | 28 (17.0) | 0.810 | 1.071
(0.610–1.881) | 0.897 | 1.041
(0.569–1.902) |
|
|
| Dominant | GG | 99
(32.6) | 54 (32.7) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 205 (67.4) | 111 (67.3) | 0.972 | 1.007
(0.673–1.509) | 0.897 | 1.029
(0.665–1.593) |
|
|
| Recessive | GG/AG | 249 (81.9) | 137 (83.0) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 55
(18.1) | 28 (17.0) | 0.761 | 1.081
(0.655–1.783) | 0.928 | 1.025
(0.600–1.752) |
|
Non-smokerf | rs3834129 | Additive | ii | 134 (66.7) | 189 (55.3) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 59
(29.3) | 127 (37.1) | 0.029 | 0.655
(0.448–0.958) | 0.036 | 0.655
(0.440–0.973) |
|
|
|
| dd | 8
(4.0) | 26 (7.6) | 0.042 | 0.434
(0.191–0.988) | 0.032 | 0.395
(0.169–0.922) |
|
|
| Dominant | ii | 134 (66.7) | 189 (55.3) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 67
(33.3) | 153 (44.7) | 0.009 | 0.618
(0.430–0.888) | 0.010 | 0.608
(0.417–0.887) |
|
|
| Recessive | ii/id | 193 (96) | 316 (92.4) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 8 (4.0) | 26 (7.6) | 0.093 | 0.504
(0.224–1.135) | 0.069 | 0.460
(0.199–1.061) |
|
| rs13016963 | Additive | GG | 63 (31.3) | 110 (32.2) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 93 (46.3) | 181 (52.9) | 0.593 | 0.897
(0.603–1.336) | 0.563 | 0.885
(0.584–1.340) |
|
|
|
| AA | 45 (22.4) | 51 (14.9) | 0.094 | 1.541
(0.928–2.557) | 0.034 | 1.785
(1.045–3.052) |
|
|
| Dominant | GG | 63 (31.3) | 110 (32.2) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 138 (68.7) | 232 (67.8) | 0.843 | 1.039
(0.714–1.510) | 0.758 | 1.063
(0.720–1.571) |
|
|
| Recessive | GG/AG | 156 (77.6) | 291 (85.1) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 45 (22.4) | 51 (14.9) | 0.027 | 1.646
(1.054–2.570) | 0.007 | 1.924
(1.199–3.089) |
 | Table V.Comparison of genotype frequencies of
CASP8 gene polymorphisms in drinkers and non-drinkers. |
Table V.
Comparison of genotype frequencies of
CASP8 gene polymorphisms in drinkers and non-drinkers.
| Drinking
status | SNP | Model/allele | Genotype | Case, n (%) | Control, n (%) |
P-valuea | OR (95%
CI)b | Pac | AOR (95%
CI)d |
|---|
|
Drinkere | rs3834129 | Additive | ii | 175 (63.6) | 120 (57.4) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 90 (32.7) | 76 (36.4) | 0.287 | 0.812
(0.553–1.192) | 0.149 | 0.713
(0.451–1.128) |
|
|
|
| dd | 10 (3.6) | 13 (6.2) | 0.138 | 0.527
(0.224–1.242) | 0.139 | 0.471
(0.174–1.277) |
|
|
| Dominant | ii | 175 (63.6) | 120 (57.4) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 100 (36.4) | 89 (42.6) | 0.165 | 0.770
(0.533–1.113) | 0.083 | 0.678
(0.436–1.052) |
|
|
| Recessive | ii/id | 265 (96.4) | 196 (93.8) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 10 (3.6) | 13 (6.2) | 0.186 | 0.569
(0.244–1.324) | 0.210 | 0.534
(0.201–1.423) |
|
| rs13016963 | Additive | GG | 88 (32.0) | 65 (31.1) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 140 (50.9) | 100 (47.8) | 0.873 | 1.034
(0.686–1.559) | 0.768 | 1.077
(0.657–1.767) |
|
|
|
| AA | 47 (17.1) | 44 (21.1) | 0.373 | 0.789
(0.468–1.329) | 0.440 | 0.782
(0.419–1.459) |
|
|
| Dominant | GG | 88 (32.0) | 65 (31.1) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 187 (68.0) | 144 (68.9) | 0.833 | 0.959
(0.651–1.413) | 0.954 | 0.986
(0.618–1.573) |
|
|
| Recessive | GG/AG | 228 (82.9) | 165 (78.9) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 47 (17.1) | 44 (21.1) | 0.269 | 0.773
(0.489–1.221) | 0.292 | 0.747
(0.434–1.285) |
|
Non-drinkerf | rs3834129 | Additive | ii | 153 (66.5) | 156 (52.3) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 69 (30.0) | 121 (40.6) | 0.004 | 0.581
(0.401–0.842) | 0.010 | 0.596
(0.401–0.886) |
|
|
|
| dd | 8 (3.5) | 21 (7.1) | 0.024 | 0.388
(0.167–0.904) | 0.016 | 0.336
(0.138–0.817) |
|
|
| Dominant | ii | 153 (66.5) | 156 (52.3) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 77 (33.5) | 142 (47.7) | 0.001 | 0.553
(0.287–0.789) | 0.002 | 0.553
(0.378–0.809) |
|
|
| Recessive | ii/id | 222 (96.5) | 277 (92.9) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 8 (3.5) | 21 (7.1) | 0.074 | 0.475
(0.207–1.094) | 0.069 | 0.460
(0.199–1.061) |
|
| rs13016963 | Additive | GG | 74 (32.2) | 99 (33.2) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 103 (44.8) | 164 (55.0) | 0.380 | 0.840
(0.569–1.240) | 0.258 | 0.786
(0.517–1.193) |
|
|
|
| AA | 53 (23.0) | 35 (11.8) | 0.008 | 2.026
(1.201–3.416) | 0.033 | 1.834
(1.052–3.199) |
|
|
| Dominant | GG | 74 (32.2) | 99 (33.2) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 156 (67.8) | 199 (66.8) | 0.799 | 1.049
(0.727–1.514) | 0.893 | 0.973
(0.656–1.445) |
|
|
| Recessive | GG/AG | 177 (77.0) | 263 (88.2) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 53 (23.0) | 35 (11.8) | 0.001 | 2.250
(1.410–3.591) | 0.003 | 2.127
(1.298–3.487) |
The genotype frequencies of rs3834129 were found to
be significantly associated with OSCC in the additive, dominant and
recessive models (Table III). The
frequencies of the rs3834129 genotypes containing the del allele
exhibited significant differences of 0.613-fold between patients
and control subjects in the dominant model (ins/del + del/del vs.
ins/ins, 95% CI, 0.467–0.806). In the additive model, ins/del and
del/del genotypes were associated with a significantly lower risk
of 0.447-fold (ins/del vs. ins/ins, 95% CI, 0.235–0.850) and
0.640-fold (del/del vs. ins/ins, 95% CI, 0.482–0.851). In the
recessive model, del/del genotype was associated with a 0.528-fold
lower risk (del/del vs. ins/del + ins/ins, 95% CI,
0.281–0.993).
The genotype frequencies of rs3834129 were
significantly associated with OSCC in men and women in the additive
and dominant models (Table VI). In
the additive model, the del/del genotype was associated with a
significantly reduced risk of 0.687-fold (del/del vs. ins/ins, 95%
CI, 0.477–0.989) in men and 0.582-fold (ins/del vs. ins/ins, 95%
CI, 0.325–0.961) in women. In the dominant model, the frequencies
of the genotypes containing the del allele in the OSCC case group
showed significant differences of 0.652-fold (ins/del + del/del vs.
ins/ins, 95% CI, 0.458–0.928) in men and 0.549-fold (ins/del +
del/del vs. ins/ins, 95% CI, 0.341–0.883) in women compared with he
control group (Table VI). No
significant association between rs13016963 and OSCC was observed in
either gender. In addition, no significant association was
identified for rs3834129 and OSCC in the recessive model in women
(Table VI).
 | Table VI.Comparison of genotype frequencies of
CASP8 gene polymorphisms in men and women. |
Table VI.
Comparison of genotype frequencies of
CASP8 gene polymorphisms in men and women.
| Gender | SNP | Model/allele | Genotype | Case, n (%) | Control, n (%) |
P-valuea | OR (95%
CI)b | Pac | AOR (95%
CI)d |
|---|
| Malee | rs3834129 | Additive | ii | 212 (64.2) | 195 (56.0) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 109 (33.0) | 133 (38.2) | 0.082 | 0.754
(0.548–1.037) | 0.056 | 0.409
(0.164–1.022) |
|
|
|
| dd | 9 (2.8) | 20 (5.8) | 0.028 | 0.414
(0.184–0.931) | 0.044 | 0.687
(0.477–0.989) |
|
|
| Dominant | ii | 212 (64.2) | 195 (56.0) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 118 (35.8) | 153 (44) | 0.029 | 0.709
(0.521–0.966) | 0.017 | 0.652
(0.458–0.928) |
|
|
| Recessive | ii/id | 321 (97.2) | 328 (94.2) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 9 (2.8) | 20 (5.8) | 0.052 | 0.460
(0.206–1.025) | 0.100 | 0.467
(0.189–1.155) |
|
| rs13016963 | Additive | GG | 101 (30.6) | 120 (34.5) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 166 (50.3) | 176 (50.6) | 0.510 | 1.121
(0.798–1.573) | 0.651 | 1.094
(0.742–1.611) |
|
|
|
| AA | 63 (19.1) | 52 (14.9) | 0.114 | 1.439
(0.915–2.264) | 0.262 | 1.342
(0.803–2.241) |
|
|
| Dominant | GG | 101 (30.6) | 120 (34.5) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 229 (69.4) | 228 (65.5) | 0.282 | 1.193
(0.865–1.647) | 0.453 | 1.152
(0.797–1.665) |
|
|
| Recessive | GG/AG | 267 (80.9) | 296 (85.1) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 63 (19.1) | 52 (14.9) | 0.150 | 1.343
(0.898–2.009) | 0.303 | 1.269
(0.806–1.997) |
| Femalef | rs3834129 | Additive | ii | 116 (66.3) | 81 (50.9) |
| 1.00 (Ref) |
|
|
|
|
|
| id | 50 (28.6) | 64 (40.3) | 0.010 | 0.546
(0.342–0.870) | 0.034 | 0.582
(0.352–0.961) |
|
|
|
| dd | 9 (5.1) | 14 (8.8) | 0.070 | 0.449
(0.185–1.087) | 0.064 | 0.408
(0.158–1.054) |
|
|
| Dominant | ii | 116 (66.3) | 81 (50.9) |
| 1.00 (Ref) |
|
|
|
|
|
| id/dd | 59 (33.7) | 78 (49.1) | 0.004 | 0.528
(0.340–0.821) | 0.013 | 0.549
(0.341–0.883) |
|
|
| Recessive | ii/id | 166 (94.9) | 145 (91.2) |
| 1.00 (Ref) |
|
|
|
|
|
| dd | 9 (5.1) | 14 (8.8) | 0.187 | 0.562
(0.236–1.336) | 0.171 | 0.527
(0.210–1.320) |
|
| Rs13016963 | Additive | GG | 61 (34.9) | 44 (27.7) |
| 1.00 (Ref) |
|
|
|
|
|
| AG | 77 (44.0) | 88 (55.3) | 0.067 | 0.631
(0.385–1.034) | 0.082 | 0.619
(0.360–1.062) |
|
|
|
| AA | 37 (21.1) | 27 (17.0) | 0.971 | 0.988
(0.527–1.855) | 0.980 | 0.991
(0.503–1.955) |
|
|
| Dominant | GG | 61 (34.9) | 44 (27.7) |
| 1.00 (Ref) |
|
|
|
|
|
| AG/AA | 114 (65.1) | 115 (72.3) | 0.158 | 0.715
(0.449–1.140) | 0.182 | 0.706
(0.423–1.178) |
|
|
| Recessive | GG/AG | 138 (78.9) | 132 (83.0) |
| 1.00 (Ref) |
|
|
|
|
|
| AA | 37 (21.1) | 27 (17.0) | 0.334 | 1.311
(0.756–2.273) | 0.317 | 1.346
(0.752–2.411) |
The genotype frequencies of rs3834129 were
significantly associated with OSCC in smokers in the dominant model
(Table IV). The genotypes
containing the del allele were associated with a significantly
lower risk of 0.627-fold (del/del + del/ins vs. ins/ins, 95% CI,
0.415–0.949). In additive and dominant models, the genotype
frequencies of rs3834129 were significantly associated with OSCC in
non-smokers (del/ins vs. ins/ins, 95% CI, 0.440–0.973; del/del vs.
ins/ins, 95% CI, 0.169–0.922; del/del + del/ins vs. ins/ ins, 95%
CI, 0.417–0.887).
The genotype frequencies of rs3834129 were
significantly associated with OSCC in non-drinkers in the additive
and dominant models (Table V). In
the additive model, the del/del genotype was associated with a
significantly lower risk of 0.336-fold (del/del vs. ins/ins, 95%
CI, 0.138–0.817) and 0.596-fold (ins/del vs. ins/ins, 95% CI,
0.401–0.886) in non-drinkers. In the dominant model, the
frequencies of the genotypes containing the del allele were
significantly different by 0.553-fold (ins/del and del/del vs.
ins/ins, 95% CI, 0.378–0.809) in non-drinkers in the OSCC case
group, as compared with the control group in non-drinkers.
Furthermore, the genotype frequencies of rs13016963 were
significantly associated with OSCC in non-drinkers in the additive
and recessive models (Table V).
Among non-drinkers, the AA genotype was associated with a
significantly higher risk of 1.834-fold (AA vs. GG, 95% CI,
1.052–3.199) in the additive model and 2.127-fold (AA vs. GG and
AG, 95% CI, 1.298–3.487) in the recessive model (Table V).
The LD values of rs3834129 and 13016963 were
evaluated using SHEsis software. No significant LD was observed for
the two loci rs3834129 and 13016963; the r2 value was 0.003 and the
D' value was 0.107. Analysis using PowerStats software revealed no
marked deviations (P>0.05) from the Hardy-Weinberg equilibrium
for the two loci (rs3834129, P=0.9228; and rs13016963, P=0.1225).
The statistical power in the present study was >80%, with an
estimated OR of 2.0 (rs3834129, 83%; rs13016963, 87%).
Discussion
OSCC is among the most common malignancies of the
oral cavity (33). To date, no
reliable methods have been developed to indicate the OSCC risk of
an individual. However, until now, there are no solid methods to
warn against OSCC risk. Genetic diagnosis has attracted more
attention in recent years (34). The
aim of the present study was to identify new suitable biomarkers or
genes that are able to indicate the physiological state and
alterations in cells prior to or during the pathogenesis of OSCC,
in order to provide early and accurate prediction and diagnosis for
patients with OSCC, particularly in the early stages (35).
Apoptosis is an crucial physiological mechanism that
eliminates cells with unrepairable DNA damage, and thus sustains
homeostasis. Apoptosis occurs via two mechanisms: The death
receptor Fas/FasL (also known as extrinsic) pathway and the
mitochondrial (DNA damage-induced and p53-mediated, also known as
intrinsic) pathway (36). CASP8
participates in the FAS-FAS ligand mediated extrinsic
(death-receptor) pathway (37,38),
while additionally interacting with the BH3 interacting-domain
death agonist protein to influence the intrinsic (mitochondrial)
pathway, which functions through caspases (39). However, CASP8 has also been observed
to serve a number of non-apoptotic functions in cells, such as
promoting the activation of NF-κB signaling, regulating autophagy,
altering endosomal trafficking and enhancing cellular adhesion and
migration (11). Therefore,
depending on the specific cellular context, CASP8 may potentiate or
suppress tumor malignancy.
Molecular epidemiological studies have suggested
that SNPs may contribute to the susceptibility of an individual to
OSCC by affecting enzyme expression levels or enzyme activities
(40–42). However, the association between SNPs
and major apoptosis regulatory caspase genes in OSCC remains
unknown (43). The CASP8 promoter
−652 6 N del allele substantially affects the promoter activity of
the CASP8 gene, as this allele destroys a binding element
for Sp1, resulting in reduced apoptotic reactivity in T lymphocytes
following stimulation by cancer cells or phytohemagglutinin in an
ex vivo model (44).
Alternatively, reduced cell apoptosis involved in the antitumor
process may provide protection against cancer (44). A previous study reported that −652 6
N ins/del polymorphism is able to influence the risk of multiple
cancer types, including cancer of the lungs, esophagus, stomach,
colorectum, breast, and cervix in Chinese populations and cutaneous
melanoma in Caucasian populations (11). Li et al (44) reported that the six-nucleotide
deletion variant in the CASP8 promoter region is inversely
associated with the risk of squamous cell carcinoma of the head and
neck in non-Hispanic Caucasian populations. However, studies on
breast cancer in Europe and colorectal cancer in the United Kingdom
have not yet confirmed such an association with cancer risk
(45,46). Furthermore, Haiman et al were
unable to replicate the results of the CASP8 polymorphism
associated with the risk of cancer of the prostate, breast, and
colorectum in multiple US populations (47). However, the results of the present
study indicate that the CASP8 −652 6 N ins/del polymorphism is
reversely associated with OSCC risk in a Chinese population, which
is consistent with the findings reported by Sun et al
(21) and Li et al (44) in Chinese populations. rs13016963 is a
rarely studied locus adjacent to CASP8 (22). A GWAS indicated that rs13016963 was
significantly associated with the risk of melanoma in French and UK
populations (P=8.6×10−10; OR, 1.14; 95% CI, 1.09–1.19).
Using GWAS data, Abnet et al (23) identified an association between
esophageal squamous cell carcinoma and 2q33 that achieved
genome-wide significance. The strongest signal was for rs13016963,
with a combined OR (95% CI) of 1.29 (1.19–1.40) and
P=7.63×10−10 in a Chinese population. Abnet et al
further suggested that future studies of esophageal cancer and
other cancers should focus on the comprehensive sequencing of this
2q33 locus and functional analysis of rs13016963 and other strongly
correlated variants.
In the present study, common SNPs for CASP8, namely
rs3834129, rs13016963 and rs1045485, were selected to evaluate the
susceptibility of patients with OSCC and healthy control subjects.
To the best of our knowledge, the present study is the first to
investigate rs13016963 polymorphisms without available genotype
data from the SNP database of the National Institute of Health
(http://www.ncbi.nlm.nih.gov/projects/SNP/). In
addition, none of these polymorphisms was identified in the Han
population of Sichuan province that was included in this study.
In the present study, no significant difference was
observed in the genotype and allele frequencies of rs13016963 SNPs
between patient the and control groups. However, the AA genotype
frequencies of rs1306963 were significantly associated with OSCC in
non-smokers and non-drinkers. These results corresponded well with
those of previous GWAS studies (22). The del allele frequency of rs3834129
was significantly lower in OSCC patients compared with control
subjects in the dominant model (ins/del + del/del vs. ins/ins,
AOR=0.613, 95% CI, 0.467–0.806). The del allele of rs3834129 was
significantly reduced in smoking and non-smoking OSCC patients
compared with the control group subjects. Furthermore, the
genotypes containing the del allele were associated with a
significantly reduced risk of OSCC compared with the ins/ins
genotype among non-drinkers (ins/del + del/del vs. ins/ins,
AOR=0.553, 95% CI, 0.378–0.809; ins/del vs. ins/ins, AOR=0.596, 95%
CI, 0.401–0.886; del/del vs. ins/ins, AOR=0.336, 95% CI,
0.138–0.817). Stratification of OSCC patients on the basis of
gender showed that the del allele of the rs3834129 polymorphism
exhibited a protective effect in men and women.
In the present study, rs3834129 was found to be
associated with a significantly decreased risk of OSCC. This result
suggests that the CASP8 −652 6 N ins/del polymorphism may affect
patient susceptibility to OSCC and may be used as a biomarker for
this disease. Future studies involving a larger sample size and
various expression studies are required to evaluate the association
between these polymorphisms and OSCC risk.
Acknowledgements
This study was financially supported by the ‘Support
for the Recruitment of Under-Represented Faculty’, State Key
Laboratory of Oral Diseases, Sichuan University (no.
SKLODYISF2012-14 and SKLODSCU20130045).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kessler P, Grabenbauer G, Leher A,
Bloch-Birkholz A, Vairaktaris E and Neukam FW: Neoadjuvant and
adjuvant therapy in patients with oral squamous cell carcinoma
long-term survival in a prospective, non-randomized study. Br J
Oral Maxillofac Surg. 46:1–5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sankaranarayanan R, Masuyer E, Swaminathan
R, Ferlay J and Whelan S: Head and neck cancer: A global
perspective on epidemiology and prognosis. Anticancer Res.
18:4779–4786. 1998.PubMed/NCBI
|
|
5
|
de Maria S, Lo Muzio L, Braca A, et al:
Survivin promoter-31 G/C polymorphism in oral cancer cell lines.
Oncol Lett. 2:935–939. 2011.PubMed/NCBI
|
|
6
|
Liu Y, Zha L, Li B, Zhang L, Yu T and Li
L: Correlation between superoxide dismutase 1 and 2 polymorphisms
and susceptibility to oral squamous cell carcinoma. Exp Ther Med.
7:171–178. 2014.PubMed/NCBI
|
|
7
|
Wang Y, Long L, Li T, et al: Polymorphisms
of microRNA-binding sites in integrin genes are associated with
oral squamous cell carcinoma susceptibility and progression. Tohoku
J Exp Med. 233:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong F, Yang XC, Bu LX, Li NY and Chen
WT: Single nucleotide polymorphisms in the u-PA gene are related to
susceptibility to oral tongue squamous cell carcinoma in the
Northern Chinese Han population. Asian Pac J Cancer Prev.
14:781–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel RM: Caspases at the crossroads of
immune-cell life and death. Nat Rev Immunol. 6:308–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stupack DG: Caspase-8 as a therapeutic
target in cancer. Cancer Lett. 332:133–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grenet J, Teitz T, Wei T, Valentine V and
Kidd VJ: Structure and chromosome localization of the human CASP8
gene. Gene. 226:225–232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nunez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frank B, Hemminki K, Wappenschmidt B, et
al: Association of the CASP10 V410I variant with reduced familial
breast cancer risk and interaction with the CASP8 D302H variant.
Carcinogenesis. 27:606–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palanca Suela S, Esteban Cardenosa E,
Barragán González E, et al: CASP8 D302H polymorphism delays the age
of onset of breast cancer in BRCA1 and BRCA2 carriers. Breast
Cancer Res Treat. 119:87–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C, Liu HZ and Fu ZX: PEG-liposomal
oxaliplatin induces apoptosis in human colorectal cancer cells via
Fas/FasL and caspase-8. Cell Biol Int. 36:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engel C, Versmold B, Wappenschmidt B, et
al: Association of the variants CASP8 D302H and CASP10 V410I with
breast and ovarian cancer risk in BRCA1 and BRCA2 mutation
carriers. Cancer Epidemiol Biomarkers Prev. 19:2859–2868. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lubahn J, Berndt SI, Jin CH, et al:
Association of CASP8 D302H polymorphism with reduced risk of
aggressive prostate carcinoma. Prostate. 70:646–653.
2010.PubMed/NCBI
|
|
19
|
Bethke L, Sullivan K, Webb E, et al: CASP8
D302H and meningioma risk: An analysis of five case-control series.
Cancer Lett. 273:312–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bethke L, Sullivan K, Webb E, et al: The
common D302H variant of CASP8 is associated with risk of glioma.
Cancer Epidemiol Biomarkers Prev. 17:987–989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun T, Gao Y, Tan W, et al: A
six-nucleotide insertion-deletion polymorphism in the CASP8
promoter is associated with susceptibility to multiple cancers. Nat
Genet. 39:605–613. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrett JH, Iles MM, Harland M, et al:
GenoMel Consortium: Genome-wide association study identifies three
new melanoma susceptibility loci. Nat Genet. 43:1108–1113. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abnet CC, Wang Z, Song X, et al: Genotypic
variants at 2q33 and risk of esophageal squamous cell carcinoma in
China: A meta-analysis of genome-wide association studies. Hum Mol
Genet. 21:2132–2141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sergentanis TN and Economopoulos KP:
Association of two CASP8 polymorphisms with breast cancer risk: A
meta-analysis. Breast Cancer Res Treat. 120:229–234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pindborg JJ, Reichart PA, Smith CJ and van
der Waal I: Histological Typing of Cancer and Precancer of the Oral
Mucosa (2nd). Berlin: Springer-Verlag. 1997. View Article : Google Scholar
|
|
26
|
Green FL: AJCC Cancer Staging Manual
(6th). Berlin Heidelberg: Springer-Verlag. 2002. View Article : Google Scholar
|
|
27
|
Miller SA, Dykes DD and Polesky HF: A
simple salting out procedure for extracting DNA from human
nucleated cells. Nucleic Acids Res. 16:12151988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashemi M, Eskandari-Nasab E, Fazaeli A,
et al: Bi-directional PCR allele-specific amplification (bi-PASA)
for detection of caspase-8 −652 6 N ins/del promoter polymorphism
(rs3834129) in breast cancer. Gene. 505:176–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Z, Li C, Chen K, et al: Single
nucleotide polymorphisms in selected apoptotic genes and
BPDE-induced apoptotic capacity in apparently normal primary
lymphocytes: A genotype-phenotype correlation analysis. J Cancer
Epidemiol. 2008:1479052008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi YY and He L: SHEsis, a powerful
software platform for analyses of linkage disequilibrium, haplotype
construction and genetic association at polymorphism loci. Cell
Res. 15:97–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewontin R: On measures of gametic
disequilibrium. Genetics. 120:849–852. 1988.PubMed/NCBI
|
|
32
|
Hudson RR: The sampling distribution of
linkage disequilibrium under an infinite allele model without
selection. Genetics. 109:611–631. 1985.PubMed/NCBI
|
|
33
|
Jemal A: Global burden of cancer:
Opportunities for prevention. Lancet. 380:1797–1799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chawla JP, Iyer N, Soodan KS, Sharma A,
Khurana SK and Priyadarshni P: Role of miRNA in cancer diagnosis,
prognosis, therapy and regulation of its expression by Epstein-Barr
virus and human papillomaviruses: With special reference to oral
cancer. Oral Oncol. 51:731–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Srinivas PR, Kramer BS and Srivastava S:
Trends in biomarker research for cancer detection. Lancet Oncol.
2:698–704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang M and Zhang Z, Tian Y, Shao J and
Zhang Z: A six-nucleotide insertion-deletion polymorphism in the
CASP8 promoter associated with risk and progression of bladder
cancer. Clin Cancer Res. 15:2567–2572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Andersen MH, Becker JC and Straten P:
Regulators of apoptosis: suitable targets for immune therapy of
cancer. Nat Rev Drug Discov. 4:399–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsing AW, Sakoda LC, Rashid A, et al:
Variants in inflammation genes and the risk of biliary tract
cancers and stones: A population-based study in China. Cancer Res.
68:6442–6452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Srivastava A, Srivastava K, Pandey SN,
Choudhuri G and Mittal B: Single-nucleotide polymorphisms of DNA
repair genes OGG1 and XRCC1: Association with gallbladder cancer in
North Indian population. Ann Surg Oncol. 16:1695–1703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Srivastava K, Srivastava A, Pandey SN,
Kumar A and Mittal B: Functional polymorphisms of the
cyclooxygenase (PTGS2) gene and risk for gallbladder cancer in a
North Indian population. J Gastroenterol. 44:774–780. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Srivastava K, Srivastava A and Mittal B:
Caspase-8 polymorphisms and risk of gallbladder cancer in a
northern Indian population. Mol Carcinog. 49:684–692.
2010.PubMed/NCBI
|
|
44
|
Li C, Lu J, Liu Z, et al: The
six-nucleotide deletion/insertion variant in the CASP8 promoter
region is inversely associated with risk of squamous cell carcinoma
of the head and neck. Cancer Prev Res (Phila). 3:246–253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frank B, Rigas SH, Bermejo JL, et al: The
CASP8 −652 6 N del promoter polymorphism and breast cancer risk: A
multicenter study. Breast Cancer Res Treat. 111:139–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Vecchi G, Verderio P, Pizzamiglio S, et
al: Evidences for association of the CASP8 −652 6 N del promoter
polymorphism with age at diagnosis in familial breast cancer cases.
Breast Cancer Res Treat. 113:607–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haiman CA, Garcia RR, Kolonel LN,
Henderson BE, Wu AH and Le Marchand L: A promoter polymorphism in
the CASP8 gene is not associated with cancer risk. Nat Genet.
40:259–260. 2008. View Article : Google Scholar : PubMed/NCBI
|