Introduction
Breast cancer is one of the most common cancer types
in females. The incidence of breast cancer has increased in
previous years, with the annual rate of increase reaching 3.1%
(1). Therefore, an in-depth
understanding of the molecular mechanisms underlying breast cancer,
and the identification of novel anticancer drug targets are of
great significance.
At present, several clinical features, including the
estrogen receptor α (ER) status, progesterone receptor (PR) status,
human epidermal growth factor receptor-2 (HER-2) status, lymph node
metastases and histological grade, have major prognostic value in
breast cancer (2). These parameters
reflect the biological features of the tumor; however, they are not
useful as outcome predictors for the individual patient. Thus, the
identification of novel prognostic markers, which are associated
with the clinical features, is required to provide a more accurate
prediction of clinical outcome, in addition to the identification
of new therapeutic targets.
Forkhead box protein A1 (FOXA1) plays an important
role in the occurrence and development of various human tumors, and
FOXA1 expression has been shown to be closely associated with
breast cancer (3,4). Therefore, FOXA1 may be a new target for
breast cancer prevention and control. Increasingly, studies have
investigated the associations between FOXA1 expression and the
clinical pathological features of breast cancer; however, the
conclusion of each study has not always been consistent (5–12).
Therefore, a comprehensive analysis was required to clarify the
inconsistencies. In the present study, a meta-analysis was
performed based on the collected literature, and the association
between FOXA1 expression and the clinical pathological features of
breast cancer was investigated comprehensively. Subsequently, the
use of FOXA1 as a biomarker for the diagnosis, treatment and
prognosis of breast cancer was assessed.
Materials and methods
Search strategy
A systematic search for eligible studies that
reported on the expression of FOXA1 and the associations in
patients with breast cancer was conducted in PubMed, EMBASE, the
Chinese National Knowledge Infrastructure and the VIP databases.
Publications were first identified by using combinations of the
following words: FOXA1 and breast cancer. Subsequently, the
references in the identified publications were screened and
examined for any other potentially relevant studies. Retrieval time
range from the time when the databases were established to May
2014. If certain studies lacked information, the authors were
contacted to obtain as much available information as possible.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) Texts were
published and available on line; ii) studies investigated the
association between FOXA1 and clinical features of breast cancer;
iii) sufficient information was provided to estimate the odds ratio
(OR) and 95% confidence interval (CI); iv) measurement methods and
experimental groups were similar among the studies. Exclusion
criteria were as follows: i) Review articles or letters; ii)
included investigation of non-human samples; iii) articles with
duplicated data.
Data extraction
Eligible articles were reviewed independently by two
investigators and disagreements were resolved by consensus. The
following data were retrieved from the studies: Name of the first
author, year of publication, country of origin, methods of testing
and definition of positivity (cut-off value).
Statistical analysis
The strength of an association was expressed as
pooled OR values along with the corresponding 95% CI. Heterogeneity
between studies was assessed by χ2-based Q-tests and
I2 tests, where I2 >50% or P<0.10 was
considered to indicate significant heterogeneity (13). A random-effects model
(DerSimonian-Laird) was used to assess the pooled ORs when
significant heterogeneity was observed (14). In cases without significant
heterogeneity, a fixed-effects model (Mantel-Haenszel) was used.
Rev-Man 4.2 software (The Cochrane Collaboration, Oxford, UK) was
used to conduct the meta-analysis.
Results
Study selection
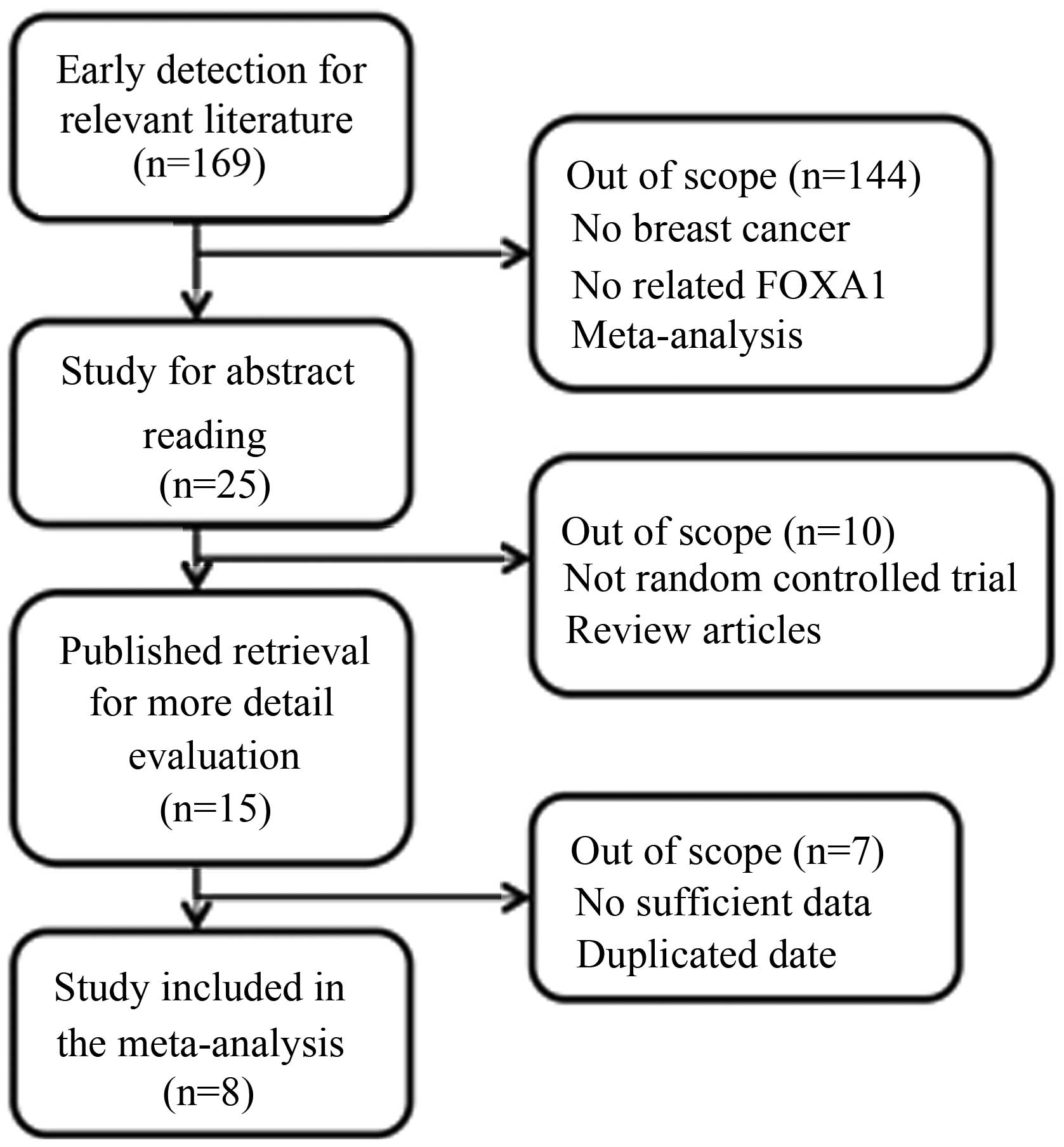
A selection flow chart is shown in Fig. 1. In total, 15 articles were eligible
for detailed reading. The studies by Ademuyiwa et al
(15), Kawase et al (16), Imamura et al (17) and Badve et al (18) were excluded due to insufficient data.
In addition, the studies by McCune et al (19) and Bernardo et al (20) were excluded due to the inclusion of
non-human-sample-based trials. There were two studies (5,21) that
used the same samples; thus, the study with the smaller sample size
was excluded. Finally, there were eight studies included in the
meta-analysis (5–12).
Study characteristics
Major characteristics of the eight eligible
publications are reported in Table
I. The studies were conducted in four countries (USA, UK, China
and Japan). Six studies were published in English, and the
remaining two were published in Chinese. The eight articles used an
immunohistochemistry method to determine the FOXA1 status.
 | Table I.Main characteristics of the studies
included in the meta-analysis. |
Table I.
Main characteristics of the studies
included in the meta-analysis.
| First author
(ref) | Year | Country | Cases (n) | Method | Cut-off |
|---|
| Wolf (15) | 2007 | USA | 100 | IHC | Not mentioned |
| Habashy (16) | 2008 | UK | 880 | IHC | Median |
| Thorat (17) | 2008 | UK | 245 | IHC | 3 |
| Albergaria (18) | 2009 | UK | 249 | IHC | 3 |
| Liu (19) | 2010 | China | 213 | IHC | 3 |
| Hisamatsu (14) | 2011 | Japan | 239 | IHC | Median |
| Ijichi (20) | 2012 | Japan | 113 | IHC | 2 |
| Jiang (21) | 2012 | China | 113 | IHC | 3 |
Meta-analysis results
No significant heterogeneity was encountered across
the studies with regard to the association between FOXA1 and any of
the prognostic factors, with the exception of the ER status and
HER-2 status. Thus, the random-effects model was selected for the
ER status and HER-2 status, while the fixed-effects model was
selected for each of the remaining three clinical features.
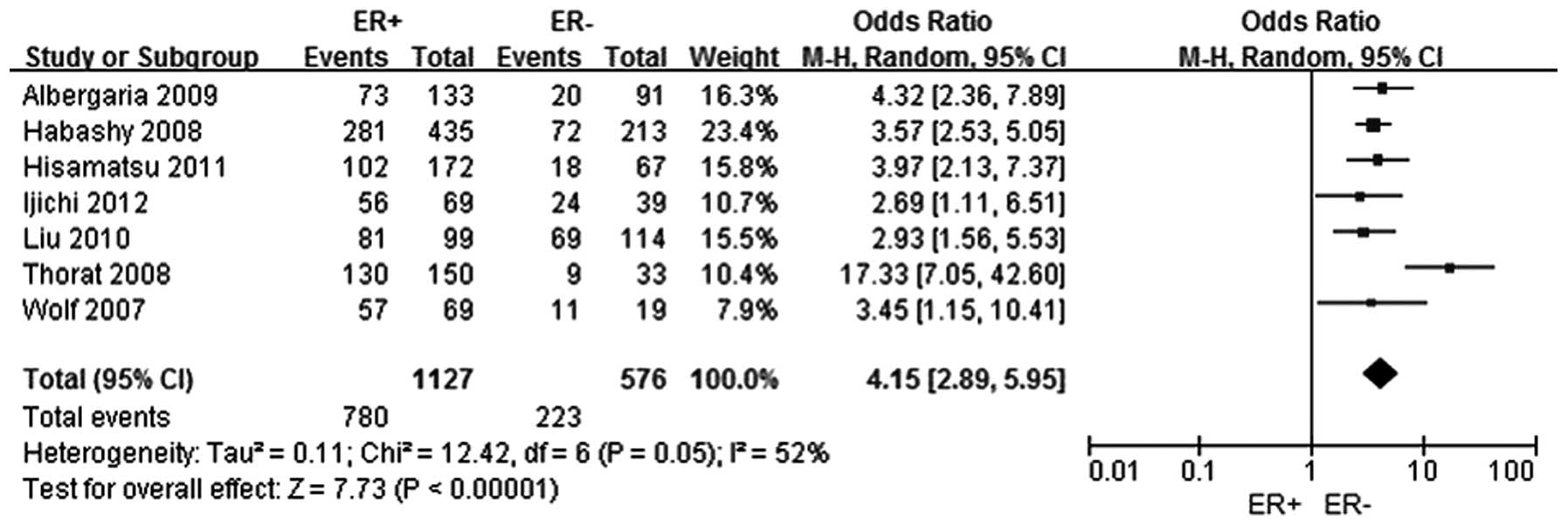
A total of seven studies were selected (5–11) to
analyze the association between FOXA1 expression and the ER status
in breast cancer. In total, 1,127 patients were included in the ER
positive group, while 576 patients comprised the ER negative group.
The FOXA1 expression level was shown to be higher in the ER
positive breast cancer group when compared with the ER negative
breast cancer group, and the difference was statistically
significant (OR, 4.15; 95% CI, 2.89–5.95; P<0.0001), as shown in
Fig. 2.
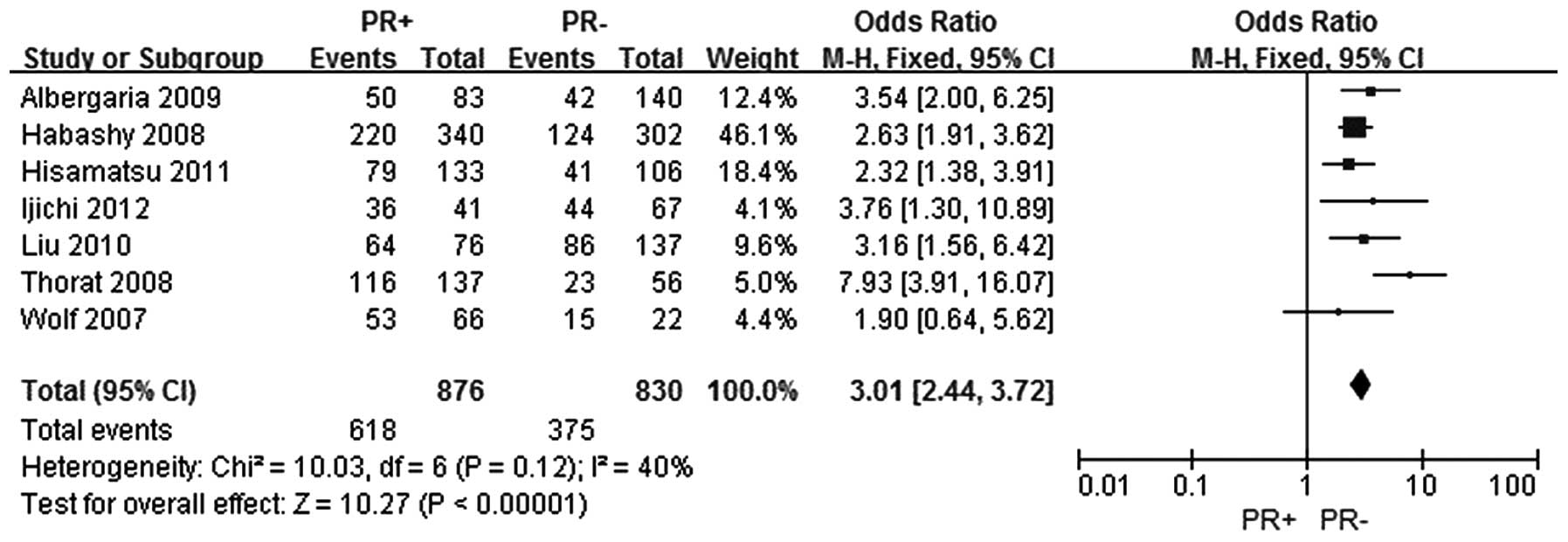
The same seven studies were included to assess the
association between FOXA1 expression and the PR status in breast
cancer (5–11). In total, 876 patients were included
in the PR positive group and 830 patients were included in the PR
negative group. The FOXA1 expression level was demonstrated to be
increased in the PR positive breast cancer group when compared with
the PR negative breast cancer group, and the difference was
statistically significant (OR, 3.01; 95% CI, 2.44–3.72;
P<0.0001), as shown in Fig.
3.
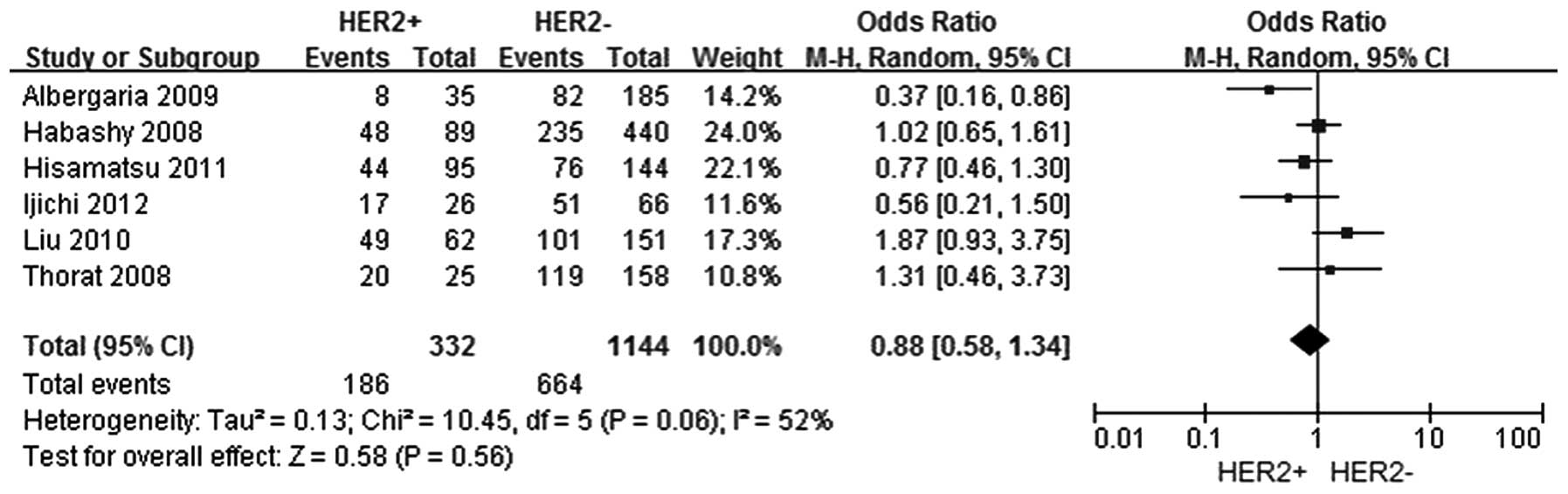
In total, six studies were selected (5–11) to
investigate the association between FOXA1 expression and the HER-2
status, among which 332 patients comprised the HER-2 positive group
and 1,144 patients were included in the HER-2 negative group.
However, no significant association was identified between FOXA1
expression and the HER-2 status in breast cancer patients (OR,
0.88; 95% CI, 0.58–1.3; P=0.56), as shown in Fig. 4.
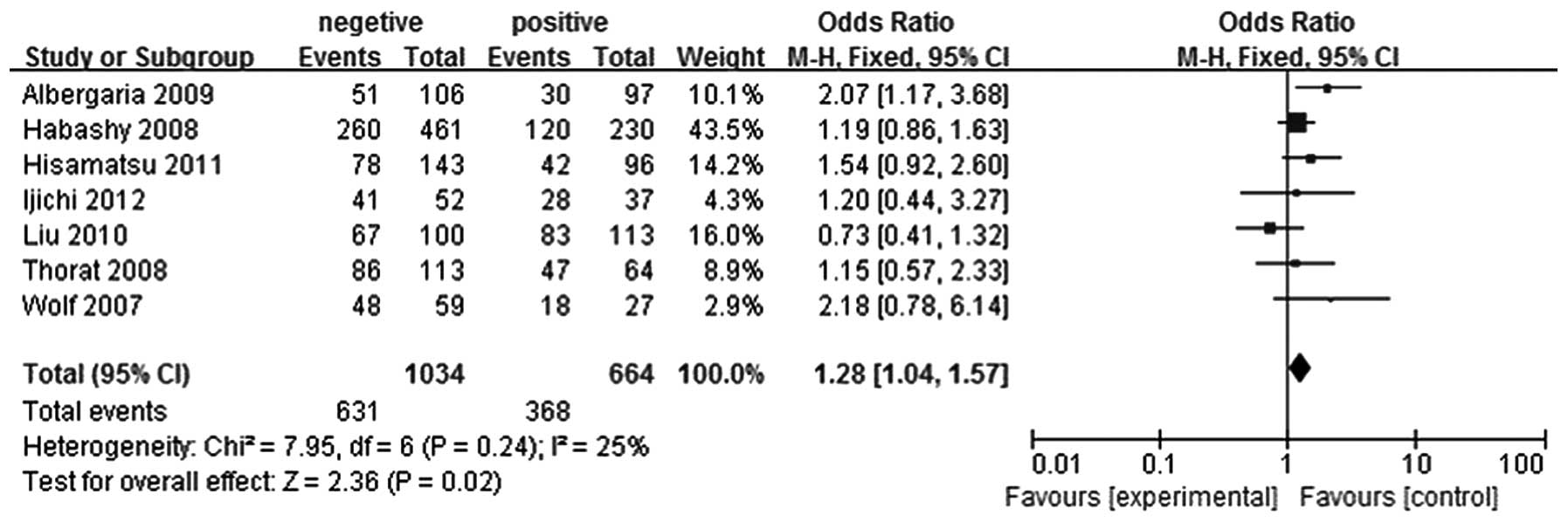
With regard to the analysis of the association
between lymph node metastasis and FOXA1 expression in breast
cancer, seven studies were selected (5–11). In
total, 664 patients were included in the lymph node metastasis
positive group and 1,034 patients were included in the lymph node
metastasis negative group. FOXA1 expression levels were shown to be
higher in the lymph node metastasis negative breast cancer group
when compared with the lymph node metastasis positive breast cancer
group (OR, 1.28; 95% CI, 1.04–1.57; P=0.02), as shown in Fig. 5.
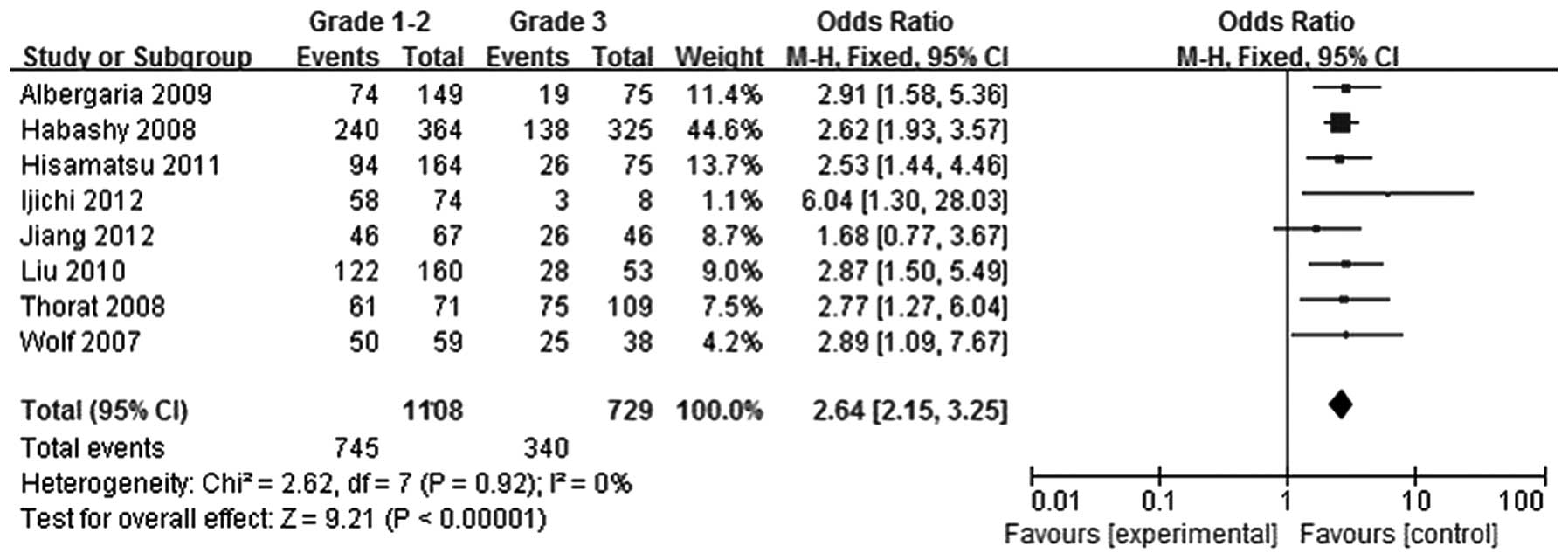
Tumors were divided according to histological grade
into a grade 1–2 group and grade 3 group. Using eight studies
(5–12), 1,108 patients were included in the
grade 1–2 group, while 729 patients were included in the grade 3
group. FOXA1 expression levels in the grade 1–2 breast cancer group
were found to be greater compared with those in the grade 3 breast
cancer group (OR, 2.64; 95% CI, 2.15–3.25; P<0.00001), as shown
in Fig. 6.
Discussion
As a member of the FOX family of transcription
factors, FOXA1 expression has been observed not only in breast
cancer, but also in colon, lung, thyroid, esophageal, prostate and
endometrial cancers (22–26). FOXA1 can bind to the promoters of
numerous genes associated with regulation of cell signaling and the
cell cycle (27). In addition, FOXA1
was identified to not only stimulate growth, but also function as a
growth inhibitor (28). When
functioning as a trigger, FOXA1 is a ‘pioneer factor’ that binds to
chromatinized DNA, and opens the chromatin to enhance the
combination of ER and target genes (29). While functioning as an inhibitor,
FOXA1 expression has been shown to block metastatic progression by
influencing the expression of the breast cancer susceptibility gene
(BRCA1)-associated cell cycle inhibitor, p27, and promoting
E-cadherin expression (30,31). These observations indicate that FOXA1
plays an important role in regulating the growth and activity of
cancer cells. In previous years, a number of studies have
demonstrated differential expression of FOXA1 in breast cancer, and
that FOXA1 plays an important role in the occurrence, development
and prognostics of breast cancer, which has attracted further
studies (7,32,33).
Certain studies have suggested that there is an association between
FOXA1 expression and prognostic factors of breast cancer, such as
ER, PR, HER-2, lymph node metastasis and histological grade
(5–9), which has subsequently lead to the
investigation of whether FOXA1 may be a significant prognostic
factor. The expression of certain receptors in breast cancer is
associated with a variety of factors, of which FOXA1 expression may
be one (5–12). The results, however, remain
controversial. The majority of studies indicate that the ER and PR
status are significantly associated with FOXA1 expression in breast
cancer; however, there is no association with the HER-2 status
(5,7,8,10–12). By
contrast, Wolf et al (6)
found that there was no association between FOXA1 expression and PR
status in breast cancer, while Albergaria et al (9) reported that FOXA1 expression was
associated with the HER-2 status. In the present meta-analysis, the
results were consistent with the majority of studies in that the
expression of FOXA1 in the ER positive or PR positive groups was
significantly higher compared with that in the ER negative or PR
negative groups. However, no statistically significant difference
was observed between the HER-2 positive and HER-2 negative groups.
With regard to the breast cancer process (lymph node metastasis and
histological grade), the result for FOXA1 expression at different
stages was also divided. Albergaria et al (9) found that FOXA1 expression was
associated with lymph node metastasis; however, this was
inconsistent with the results observed by Ijichi et al
(11) and Thorat et al
(8). Albergaria et al
(9) and Habashy et al
(7) hypothesized that FOXA1
expression was associated with histological grade, while Jiang
et al (12) demonstrated no
correlation between FOXA1 expression and histological grade. In the
current meta-analysis, the combined results indicated that FOXA1
expression was associated with lymph node metastasis and
histological grade. The expression levels of FOXA1 in the lymph
node negative or low histological grade groups were significantly
higher compared with that in the lymph node positive or high
histological grade groups, which demonstrated that FOXA1 expression
was associated with the development, cell differentiation and
prognosis of breast cancer.
Limitations of the present analysis should be
acknowledged. Firstly, the cut-off values in the present study were
not consistent. Four studies defined a score of 0–3 as FOXA1
expression negative, one study defined a score of 0–2 as FOXA1
expression negative, whereas different thresholds (median) were
used in the two additional studies. The remaining study did not
discuss cut-off values. Secondly, heterogeneity is a potential
problem that may have affected the interpretation of the results of
the meta-analysis. When investigating the correlation between FOXA1
expression and the ER status or HER-2 status, high heterogeneity
was observed in selected articles. The sources of heterogeneity may
be attributed to differences in the ethnicity and histological
type. Thirdly, the study was restricted to papers published in
English and Chinese, which is likely to have introduced bias.
Despite a number of limitations in the present study, to the best
of our knowledge, this is the first meta-analysis focusing on the
association between FOXA1 expression and the clinical
characteristics of breast cancer.
In conclusion, the results of the meta-analysis
demonstrated a significant association between FOXA1 expression and
the clinical characteristics of breast cancer, which may be
valuable to the diagnosis, treatment and prognosis of breast
cancer. However, due to the aforementioned limitations, future
studies evaluating the significance of FOXA1 expression on the
clinical characteristics of breast cancer are strongly
recommended.
Acknowledgements
This project was sponsored by the Scientific
Research Foundation for Returned Overseas Chinese Scholars by the
State Education Ministry. This study was supported by grants from
the National Natural Science Foundation of China (nos. 30873044 and
81272372).
References
|
1
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark GM: Prognostic and predictive
factors for breast cancer. Breast Cancer. 2:79–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernardo GM and Keri RA: FOXA1: A
transcription factor with parallel functions in development and
cancer. Biosci Rep. 32:113–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakshatri H and Badve S: FOXA1 in breast
cancer. Expert Rev Mol Med. 11:e82009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hisamatsu Y, Tokunaga E, Yamashita N, et
al: Impact of FOXA1 expression on the prognosis of patients with
hormone receptor-positive breast cancer. Ann Surg Oncol.
19:1145–1152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolf I, Bose S, Williamson EA, Miller CW,
Karlan BY and Koeffler HP: FOXA1: Growth inhibitor and a favorable
prognostic factor in human breast cancer. Int J Cancer.
120:1013–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habashy HO, Powe DG, Rakha EA, et al:
Forkhead-box A1 expression in breast cancer and its prognostic
significance. Eur J Cancer. 44:1541–1551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thorat MA, Marchio C, Morimiya A, Savage
K, Nakshatri H, Reis-Filho JS and Badve S: Forkhead box A1
expression in breast cancer is associated with luminal subtype and
good prognosis. J Clin Pathol. 61:327–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albergaria A, Paredes J, Sousa B, et al:
Expression of FOXA1 and GATA-3 in breast cancer: The prognostic
significance in hormone receptor-negative tumours. Breast Cancer
Res. 11:R402009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu N, Niu Y, Wang SL, Yu Q, Zhang RJ and
Liu TJ: Diagnostic and prognostic significance of FOXAl expression
in molecular subtypes of breast invasive ductal carcinomas.
Zhonghua Yi Xue Za Zhi. 90:1403–1407. 2010.(In Chinese). PubMed/NCBI
|
|
11
|
Ijichi N, Shigekawa T, Ikeda K, et al:
Association of double-positive FOXA1 and FOXP1 immunoreactivities
with favorable prognosis of tamoxifen-treated breast cancer
patients. Horm Cancer. 3:147–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang GY, Li H, He WL and Wang NX:
Prognostic significance of FOXA1 and BRCA1 expression in triple
negative breast cancer. Zhongguo Bing Li Sheng Li Za Zhi.
28:1230–1234. 2012.(In Chinese).
|
|
13
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DerSimonian R and Kacker R: Random-effects
model for meta-analysis of clinical trials: an update. Contemp Clin
Trials. 28:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ademuyiwa FO, Thorat MA, Jain RK,
Nakshatri H and Badve S: Expression of Forkhead-box protein A1, a
marker of luminal A type breast cancer, parallels low oncotype DX
21-gene recurrence scores. Mod Pathol. 23:270–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawase M, Toyama T, Takahashi S, et al:
FOXA1 expression after neoadjuvant chemotherapy is a prognostic
marker in estrogen receptor-positive breast cancer. Breast Cancer.
Jun 16–2013.(Epub ahead of print). PubMed/NCBI
|
|
17
|
Imamura Y, Sakamoto S, Endo T, et al:
FOXA1 promotes tumor progression in prostate cancer via the
insulin-like growth factor binding protein 3 pathway. PloS One.
7:e424562012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badve S, Turbin D, Thorat MA, et al: FOXA1
expression in breast cancer-correlation with luminal subtype A and
survival. Clin Cancer Res. 13:4415–4421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCune K, Mehta R, Thorat MA, Badve S and
Nakshatri H: Loss of ERα and FOXA1 expression in a progression
model of luminal type breast cancer: Insights from PyMT transgenic
mouse model. Oncol Rep. 24:1233–1239. 2010.PubMed/NCBI
|
|
20
|
Bernardo GM, Lozada KL, Miedler JD, et al:
FOXA1 is an essential determinant of ERα expression and mammary
ductal morphogenesis. Development. 137:2045–2054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hisamatsu Y, Tokunaga E, Yamashita N,
Akiyoshi S, Okada S, Nakashima Y, et al: Impact of GATA-3 and FOXA1
expression in patients with hormone
receptor-positive/HER-2-negative breast cancer. Breast Cancer. Jan
11–2014.(Epub ahead of print). PubMed/NCBI
|
|
22
|
Hosoda M, Yamamoto M, Nakano K, et al:
Differential expression of progesterone receptor, FOXA1, GATA3, and
p53 between pre- and postmenopausal women with estrogen
receptor-positive breast cancer. Breast Cancer Res Treat.
144:249–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin L, Miller CT, Contreras JI, et al: The
hepatocyte nuclear factor 3 α gene, HNF3α (FOXA1), on chromosome
band 14q13 is amplified and overexpressed in esophageal and lung
adenocarcinomas. Cancer Res. 62:5273–5279. 2002.PubMed/NCBI
|
|
24
|
Nucera C, Eeckhoute J, Finn S, et al:
FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin
Cancer Res. 15:3680–3689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbieri CE, Baca SC, Lawrence MS, et al:
Exome sequencing identifies recurrent SPOP, FOXA1 and MED12
mutations in prostate cancer. Nat Genet. 44:685–689. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe Y, Ijichi N, Ikeda K, Kayano H,
Horie-Inoue K, Takeda S and Inoue S: Forkhead box transcription
factor, forkhead box A1, shows negative association with lymph node
status in endometrial cancer, and represses cell proliferation and
migration of endometrial cancer cells. Cancer Sci. 103:806–812.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sérandour AA, Avner S, Percevault F, et
al: Epigenetic switch involved in activation of pioneer factor
FOXA1-dependent enhancers. Genome Res. 21:555–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Q, Luo Z, Xu T, et al: FOXA1: A
promising prognostic marker in breast cancer. Asian Pac J Cancer
Prev. 15:11–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beck S, Sommer P, dos Santos Silva E, Blin
N and Gött P: Hepatocyte nuclear factor 3 (winged helix domain)
activates trefoil factor gene TFF1 through a binding motif adjacent
to the TATAA box. DNA Cell Biol. 18:157–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williamson EA, Wolf I, OKelly J, Bose S,
Tanosaki S and Koeffler HP: BRCA1 and FOXA1 proteins coregulate the
expression of the cell cycle-dependent kinase inhibitor p27 (Kip1).
Oncogene. 25:1391–1399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YN, Lee WW, Wang CY, Chao TH, Chen Y
and Chen JH: Regulatory mechanisms controlling human E-cadherin
gene expression. Oncogene. 24:8277–8290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehta RJ, Jain RK, Leung S, Choo J,
Nielsen T, Huntsman D, Nakshatri H and Badve S: FOXA1 is an
independent prognostic marker for ER-positive breast cancer. Breast
Cancer Res Treat. 131:881–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakshatri H and Badve S: FOXA1 as a
therapeutic target for breast cancer. Expert Opin Ther Targets.
11:507–14. 2007. View Article : Google Scholar : PubMed/NCBI
|