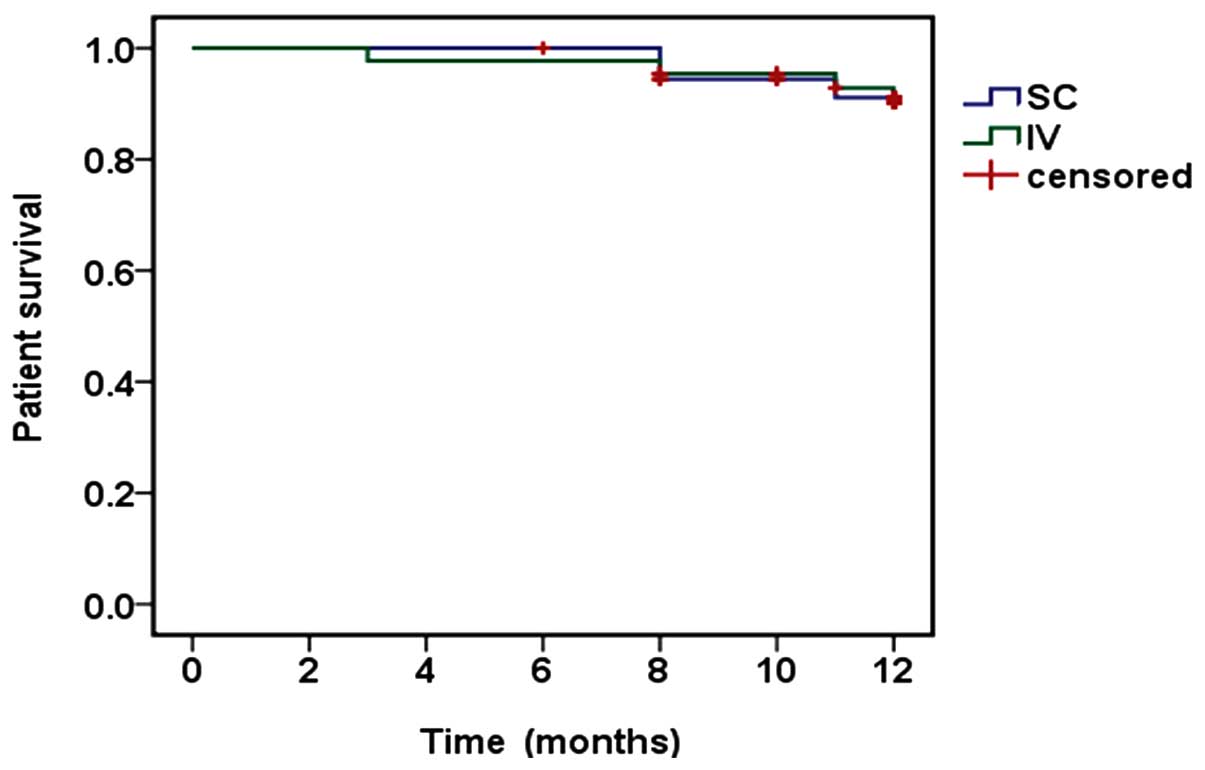
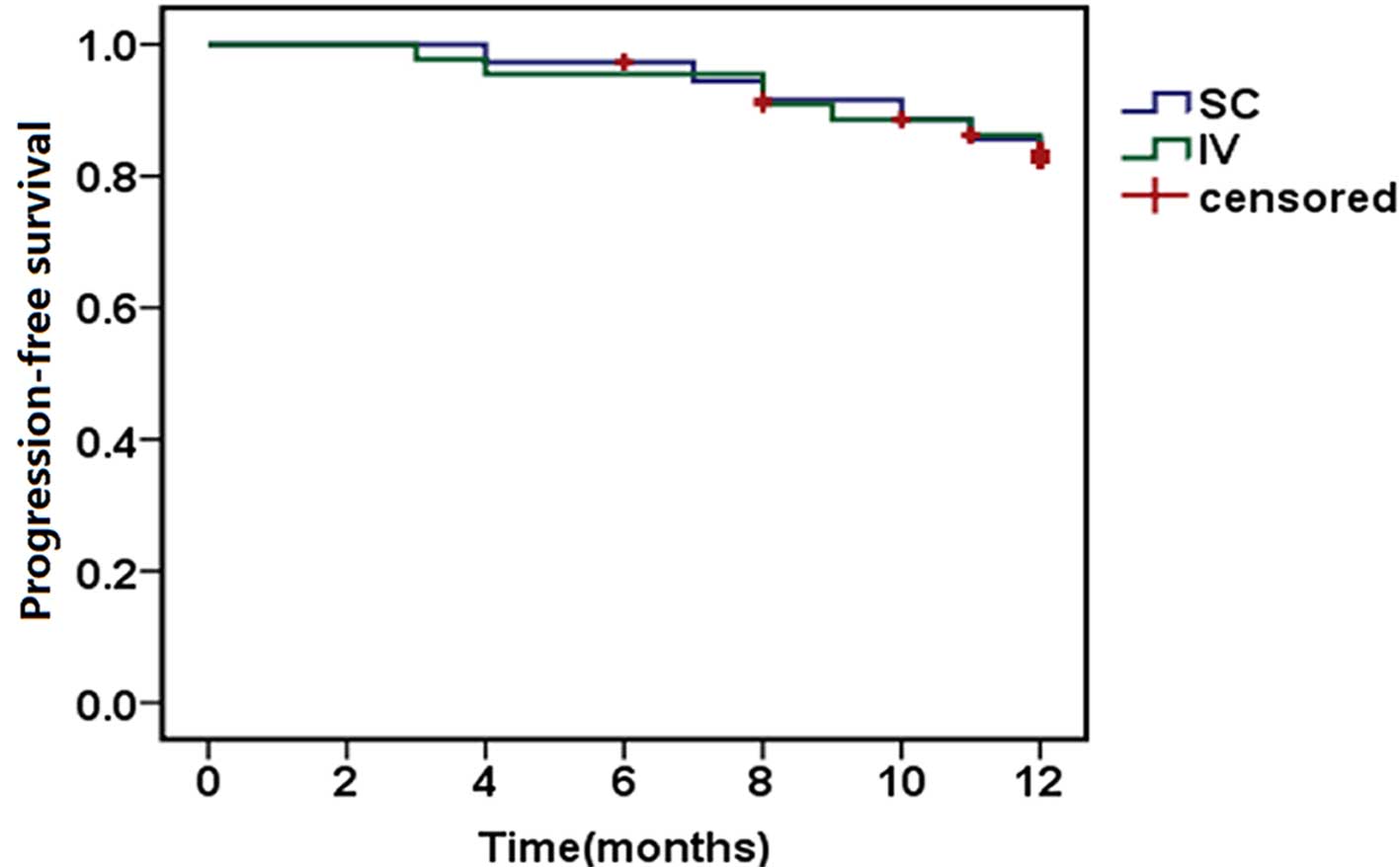
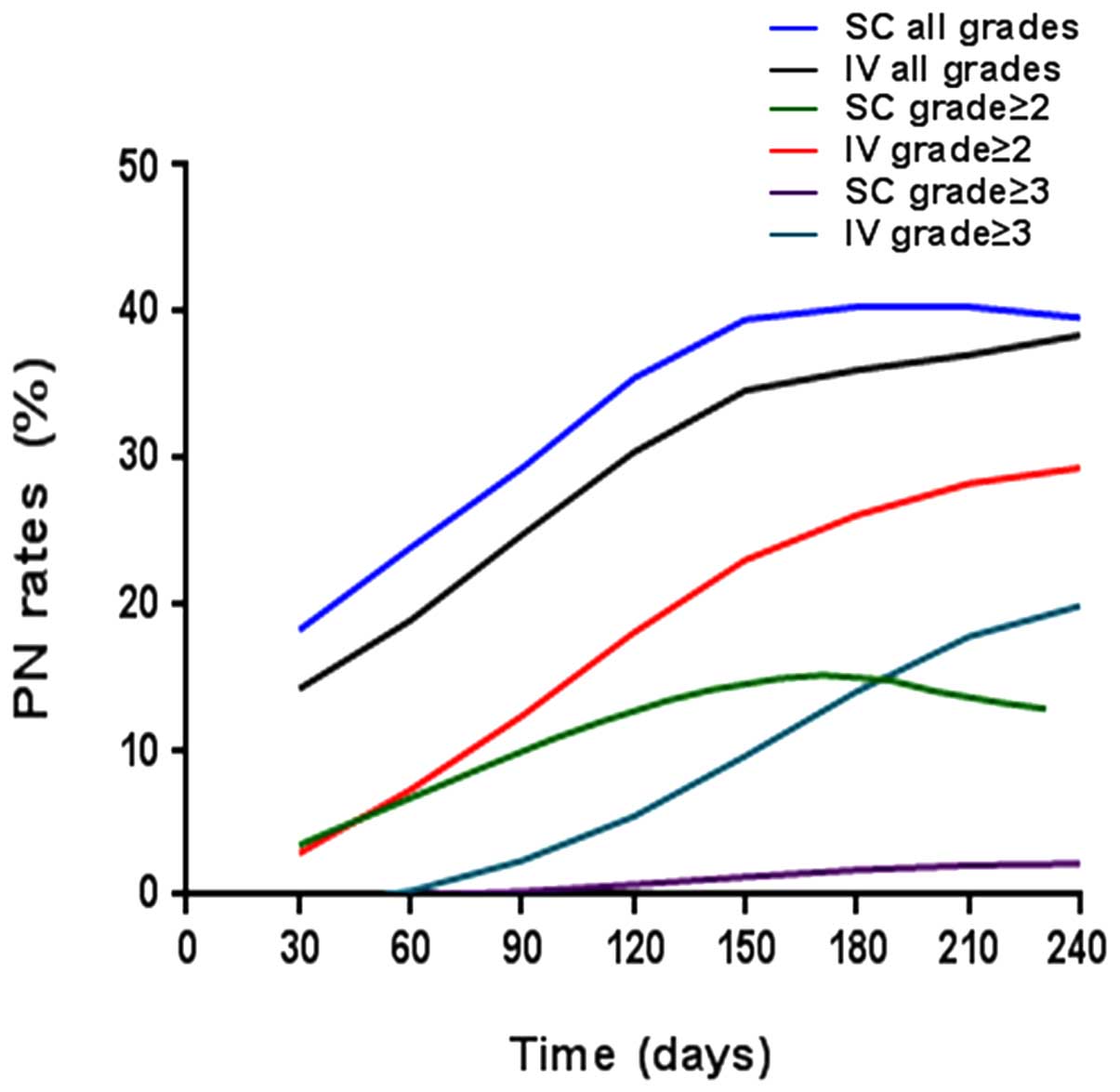
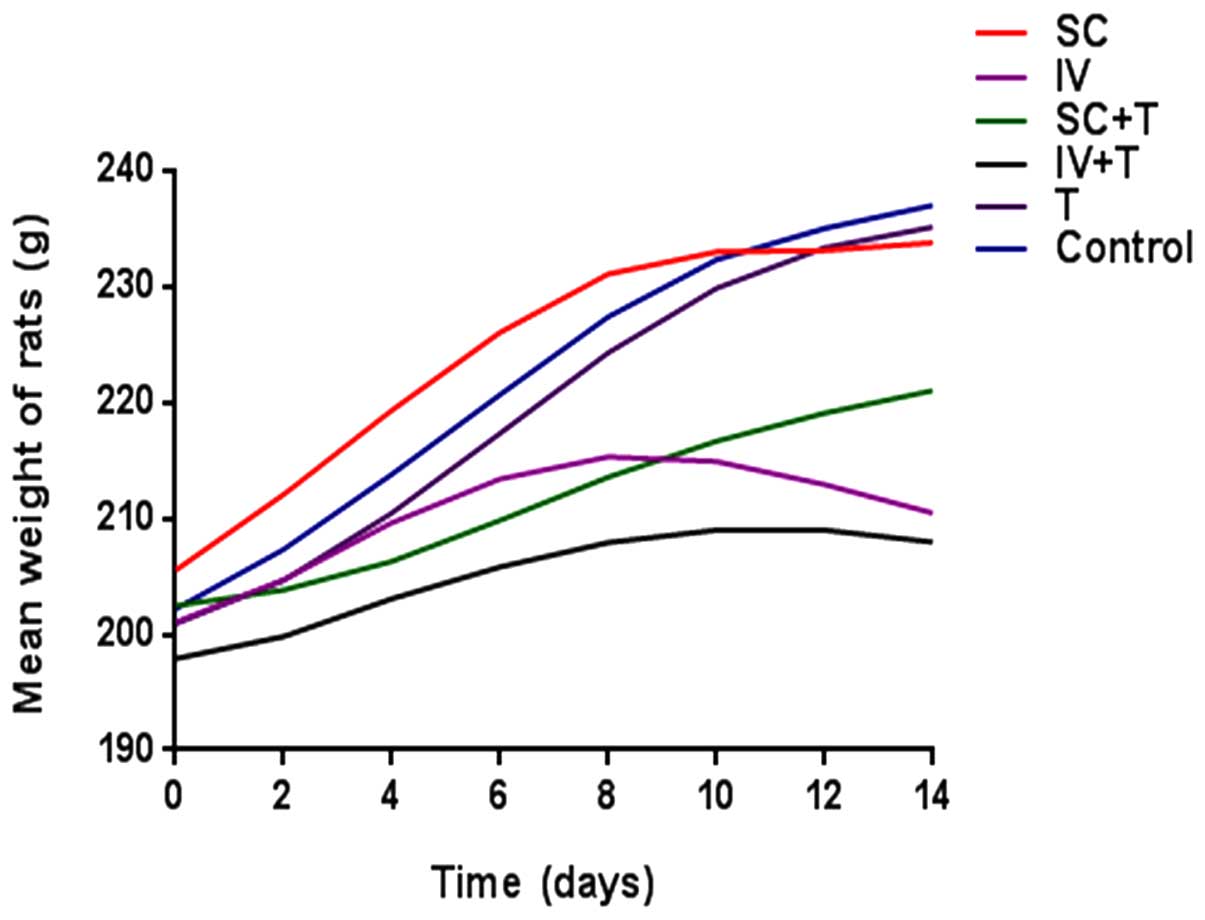
|
1
|
Phekoo KJ, Schey SA, Richards MA, Bevan
DH, Bell S, Gillett D and Møller H: Consultant Haematologists,
South Thames Haematology Specialist Committee: A population study
to define the incidence and survival of multiple myeloma in a
National Health Service Region in UK. Br J Haematol. 127:299–304.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantani L, Zamagni E, Zannetti BA, Pezzi
A, Tacchetti P, Brioli A, Mancuso K, Perrone G, Rocchi S, Tosi P
and Cavo M: Bortezomib and dexamethasone as salvage therapy in
patients with relapsed/refractory multiple myeloma: Analysis of
long-term clinical outcomes. Ann Hematol. 93:123–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moreau P, Pylypenko H, Grosicki S,
Karamanesht L, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak
T, Shubina A, et al: Subcutaneous versus intravenous administration
of bortezomib in patients with relapsed multiple myeloma: A
randomised, phase 3, non-inferiority study. Lancet Oncol.
12:431–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soriano GP, Besse L, Li N, Kraus M, Besse
A, Meeuwenoord N, Bader J, Everts B, den Dulk H, Overkleeft HS,
Florea BI and Driessen C: Proteasome inhibitor-adapted myeloma
cells are largely independent from proteasome activity and show
complex proteomic changes, in particular in redox and energy
metabolism. Leukemia. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ri M: Mechanism of action and determinants
of sensitivity to the proteasome inhibitor bortezomib in multiple
myeloma therapy. Rinsho Ketsueki. 57:537–45. 2016.PubMed/NCBI
|
|
6
|
Dimopoulos MA, Beksac M, Benboubker L,
Roddie H, Allietta N, Broer E, Couturier C, Mazier MA, Angermund R
and Facon T: Phase II study of bortezomib-dexamethasone alone or
with added cyclophosphamide or lenalidomide for sub-optimal
response as second-line treatment for patients with multiple
myeloma. Haematologica. 98:1264–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arnulf B, Pylypenko H, Grosicki S,
Karamanesht L, Leleu X, van de Velde H, Feng H, Cakana A, Deraedt W
and Moreau P: Updated survival analysis of a randomized phase III
study of subcutaneous versus intravenous bortezomib in patients
with relapsed multiple myeloma. Haematologica. 97:1925–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glasmacher A, Hahn C, Hoffmann F, Naumann
R, Goldschmidt H, von Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I
and Gorschlüter M: A systematic review of phase-II trials of
thalidomide monotherapy in patients with relapsed or refractory
multiple myeloma. Br J Haematol. 132:584–593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palumbo A, Davies F, Kropff M, Bladé J,
Delforge M, da Costa F Leal, Sanz R Garcia, Schey S, Facon T,
Morgan G and Moreau P: Consensus guidelines for the optimal
management of adverse events in newly diagnosed,
transplant-ineligible patients receiving melphalan and prednisone
in combination with thalidomide (MPT) for the treatment of multiple
myeloma. Ann Hematol. 89:803–811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, Ludwig H, et al: International uniform response
criteria for multiple myeloma. Leukemia. 20:1467–1473. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Cancer Institute, . Common
terminology criteria for adverse events, version 3.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfAccessed.
April 11–2011
|
|
12
|
Greipp PR, San MJ, Durie BG, Crowley JJ,
Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle
RA, Lahuerta JJ, et al: International staging system for multiple
myeloma. J Clin Oncol. 23:3412–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoy SM: Subcutaneous bortezomib: In
multiple myeloma. Drugs. 73:45–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grosicki S: Subcutaneous bortezomib as a
new promising way to successful maintenance therapy in multiple
myeloma. Wiad Lek. 65:167–173. 2012.(In Polish). PubMed/NCBI
|
|
15
|
Garderet L, Iacobelli S, Moreau P, Dib M,
Niederwieser D, Masszi T, Fontan T, Michallet M, Gratwohl A, Lafon
I, et al: Superiority of the triple combination of
bortezomib-thalidomide-dexamethasone over the dual combination of
thalidomide-dexamethasone in patients with multiple myeloma
progressing or relapsing after autologous transplantation: The
MMVAR/IFM 2005-04 Randomized phase III trial from the chronic
leukemia working party of the European group for blood and marrow
transplantation. J Clin Oncol. 30:2475–2482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tacchetti P, Terragnac C, Galli M, Zamagni
E, Petrucci MT, Pezzi A, Montefusco V, Martello M, Tosi P, Baldini
L, et al: Bortezomib- and thalidomide-induced peripheral neuropathy
in multiple myeloma: Clinical and molecular analyses of a phase 3
study. Am J Hematol. 89:1085–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richardson PG, Sonneveld P, Schuster MW,
Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S,
Goldschmidt H, Reece D, et al: Reversibility of symptomatic
peripheral neuropathy with bortezomib in the phase III APEX trial
in relapsed multiple myeloma: Impact of a dose-modification
guideline. Br J Haematol. 144:895–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamm W, Drach-Schauer B, Eder S and Drach
J: Bortezomib administered subcutaneously is well tolerated in
bortezomib-based combination regimens used in patients with
multiple myeloma. Oncology. 85:223–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
San Miguel JF, Schlag R, Khuageva NK,
Dimopoulos MA, Shpilberg O, Kroptt M, Spicka I, Petrucci MT,
Palumbo A, Samoilova OS, et al: Bortezomib plus melphalan and
prednisone for initial treatment of multiple myeloma. N Engl J Med.
359:906–917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wright JJ: Combination therapy of
bortezomib with novel targeted agents: An emerging treatment
strategy. Clinical Cancer Research. 16:4094–4104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
San Miguel JF, Schlag R, Khuageva NK,
Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, et al:
Continued overall survival benefit after 5 years' follow-up with
bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone
(MP) in patients with previously untreated multiple myeloma and no
increased risk of second primary malignancies: Final results of the
phase 3 VISTA trial. Blood. 118:4762011.PubMed/NCBI
|
|
22
|
Hrusovsky I, Emmerich B, von Rohr A,
Voegeli J, Taverna C, Olie RA, Pliskat H, Frohn C and Hess G:
Bortezomib retreatment in relapsed multiple myeloma - results from
a retrospective multicentre survey in Germany and Switzerland.
Oncology. 79:247–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sher T, Ailawadhi S, Miller KC, Manfredi
D, Wood M, Tan W, Wilding G, Czuczman MS, Hernandez-llizaliturri
FJ, Hong F, et al: A steroid-independent regimen of bortezomib,
liposomal doxorubicin and thalidomide demonstrate high response
rates in newly diagnosed multiple myeloma patients. Br J Haematol.
154:104–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavaletti G, Gilardini A, Canta A,
Rigamonti L, Rodriguez-Menendez V, Ceresa C, Marmirili P, Bossi M,
Oggioni N, D'Incalci M and De Coster R: Bortezomib-induced
peripheral neurotoxicity: A neurophysiological and pathological
study in the rat. Exp Neurol. 204:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Csizmadia V, Raczynski A, Csizmadia E,
Fedyk ER, Rottman J and Alden CL: Effect of an experimental
proteasome inhibitor on the cytoskeleton, cytosolic protein
turnover and induction in the neuronal cells in vitro.
Neurotoxicology. 29:232–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mangiacavalli S, Pochintesta L, Pascutto
C, Cocito F, Cazzola M, Corso A and Pompa A: Good clinical activity
and favorable toxicity profile of once weekly bortezomib,
fotemustine and dexamethasone (B-MuD) for the treatment of relapsed
multiple myeloma. Am J Hematol. 88:102–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bringhen S, Larocca A, Rossi D, Cavalli M,
Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, et
al: Efficacy and safety of once-weekly bortezomib in multiple
myeloma patients. Blood. 116:4745–4753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moore S, Atwal S, Sachchithanantham S,
Streetly M, Khan I, Percy L, Narat S, Rabin N, Johnston R, D'Sa S,
et al: Weekly intravenous bortezomib is effective and well
tolerated in relapsed/refractory myeloma. Eur J Haematol.
90:420–425. 2013. View Article : Google Scholar : PubMed/NCBI
|