Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequent malignancies worldwide, representing the fifth most common
cause of cancer in men and the seventh in women, and the third most
frequent cause of cancer-related mortality (1–3).
Globally, there are ~750,000 new cases of liver cancer reported per
year (4). Although surgical
resection provides better results in patients with HCC in early
stages, the long-term prognosis remains unsatisfactory because many
patients are diagnosed at the advanced stage, and tumors have an
inherent capacity for invasiveness and metastasis (5,6). At
present, chemotherapy is commonly used in the treatment of
metastatic HCC. However, this treatment remains ineffective due to
its toxicity to normal cells and the rapid development of
resistance. Therefore, to further explore the biology of metastasis
and develop novel therapeutics that specifically target metastasis
and metastatic progression are required (7,8).
Increasing attention has been paid to drugs among
traditional Chinese medicines, since these materials typically
share high safety profiles and can effectively prevent and control
metastasis (9,10). Rheum palmatum L. is a plant
that has been widely used in Chinese medicine as a laxative for
thousands of years. Emodin is an anthraquinone derivative from its
root and rhizome (11). It has been
reported that emodin possesses a number of biological properties
such as anti-inflammatory, antiviral and anti-proliferative effects
(12). In the context of cancer,
several studies have indicated that emodin exerts anticancer
effects on various cell lines derived from the pancreas (13), colorectum (14) and breast (15). However, so far there is little
evidence revealing the possible effect of emodin on tumor
metastasis in HCC.
Herein, in the present study, the inhibitory effect
of migration and invasion by emodin are investigated using the HCC
cell line MHCC-97H, and the molecular mechanisms underlying these
actions are explored.
Materials and methods
Cell culture and reagents
The MHCC-97H cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA), and was
cultured in high-glucose Dulbeccos modified Eagles medium with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in a humidified atmosphere with 5% CO2 at 37°C.
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinine) was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Its molecular
formula is C15H10O5 and its
molecular weight is 270.24 g/mol. It was dissolved at a
concentration of 100 mmol/l in 100% dimethylsulfoxide (DMSO;
Sigma-Aldrich; Merck Millipore) as a stock solution, stored at
−20°C, and the following concentrations were prepared: 2.5, 5,
12.5, 25, 50 and 100 mmol/l. DMSO (0.2%) was used as vehicle
control for all the experiments. MTT was purchased from
Sigma-Aldrich (Merck Millipore). The Annexin V-FITC apoptosis
detection kit (cat. no. KGA108) was purchased from KeyGen Bio-Tech
Co., Ltd., Nanjing, China. Rabbit phosphorylated (p)-Akt (cat. no.
4060S) and total (t)-Akt (cat. no. 4691S) polyclonal antibody,
rabbit p-extracellular-signal-regulated kinase (ERK)1/2 (cat. no.
4370S) and t-ERK1/2 (cat. no. 4695S) monoclonal antibody, rabbit
p-p38 (cat. no. 4511S) and t-p38 (cat. no. 9212S) monoclonal
antibody and mouse β-actin (cat. no. 3700) monoclonal antibody were
all purchased from Cell Signaling Technology, Inc. (Boston, MA,
USA). Rabbit matrix metalloproteinase (MMP)-2 (cat. no. BS1236) and
MMP-9 (cat. no. BS1241) polyclonal antibody were purchased from
Bioworld Technology, Inc. (St. Louis Park, MN, USA).
MTT assay for cell viability
Cell viability was determined by MTT assay. Briefly,
Cells were seeded into a 96-well plate at a density of
1×104 cells/well and cultured for 24 h. Emodin was then
added to the wells with final concentrations of 0, 5, 10, 25, 50,
100 and 200 µmol/l and incubated for 12, 24, 48 and 72 h at 37°C.
DMSO (0.2%) was used as a negative control. Then, 20 µl MTT
solution (5 mg/ml) was added to each well and incubated for an
additional 4 h at 37°C. The medium was carefully removed and 150 µl
DMSO was added to each well to dissolve the formazan crystals and
the absorbance was detected at 570 nm using a multiskan spectrum
microplate reader (Thermo Fisher Scientific, Inc.).
Flow cytometricanalysis of
apoptosis
Cell apoptosis was detected using the Annexin V-FITC
apoptosis detection kit according to the manufacturers
instructions. In brief, a total of 3×105 MHCC-97H cells
were seeded into each well of the 6-well plates and various
concentrations (10, 25, 50,100 µmol/l) of emodin were added to the
wells after 24 h of incubation at 37°C. The control group was
treated with the equivalent quantity of DMSO (0.2%). Cells of each
sample were harvested after an additional 12 or 24 h and suspended
in 500 µl of Annexin V binding buffer (1X). Annexin V-FiTC (5 µl)
and 5 µl of propidium iodide (PI) were added and incubated for 15
min in dark. The stained cells were analyzed by flow cytometry
using a FACS Calibur (BD Biosciences, San Jose, CA, USA).
Migration and invasion assay
Cell migration was analyzed with the aid of a
Transwell chamber (Corning Incorporated, Corning, NY, USA) with
8-µm pores and a cell invasion assay was performed using a
Corning® Matrigel® invasion chamber (Corning
Incorporated). Cells suspended in 200 µl serum-free medium were
seeded onto the upper chambers (1×105 cells/chamber for
migration and 2×105 cells/chamber for invasion) and
incubated with different concentrations of emodin (10, 25 and 50
µmol/l), and the chambers were placed into 24-well plates with
medium containing 10% serum. DMSO (0.2%) was used as a negative
control. After 24 h, the cells remaining in the upper chamber were
removed and migrated or invaded cells on the lower membrane surface
were fixed with 4% formaldehyde polymerisatum followed by staining
with 0.1% crystal violet for 20 min. The migrated or invaded cells
in six visual fields (magnification, ×200) selected randomly were
counted in each Transwell chamber under a phase-contrast
microscope.
Western blot analysis
After treatment with 50 µM emodin and DMSO (0.2%) as
a negative control, the cells were washed twice using ice-cold
phosphate-buffered saline (pH 7.4) and lysed in
radioimmunoprecipitation assay protein lysis buffer containing 1 mM
PMSF on ice. Total proteins were extracted by centrifuging the cell
lysates at 12,000 × g for 15 min at 4°C and the protein
concentration was determined using a BCA assay kit (cat. no. P0010)
Beyotime Institute of Biotechnology, Shanghai, China). A total of
30 µg protein from every sample was separated using a 10% SDS-PAGE
gel and transferred to a polyvinylidene fluoride (PVDF) membrane.
After blocking with Tris-buffered saline and Tween 20 (TBST) buffer
containing 5% skimmed milk for 1 h at room temperature, the PVDF
membrane was incubated with appropriate concentrations of primary
antibodies (dilution, 1:1,000) at 4°C overnight. After washing the
membrane with TBST three times for 15 min, the membrane was
incubated with corresponding secondary antibody labeled with
horseradish peroxidase-conjugated (goat anti-mouse, 1:5,000; goat
anti-rabbit, 1:2,000) secondary antibody for 2 h at room
temperature. Following three washes with TBST for 15 min, the
immunoreactive bands were detected using an enhanced
chemiluminescence detection kit (Sigma-Aldrich; Merck Millipore).
β-actin was used as the internal control and the relative values of
target protein were corrected in accordance with the absorbency of
the internal control.
Statistical analysis
All results were expressed as the mean ± standard
deviation of at least three independent experiments and were
analyzed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA). The statistical analysis was performed using a one-way
analysis of variance and Dunnetts test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of emodin on cell
viability
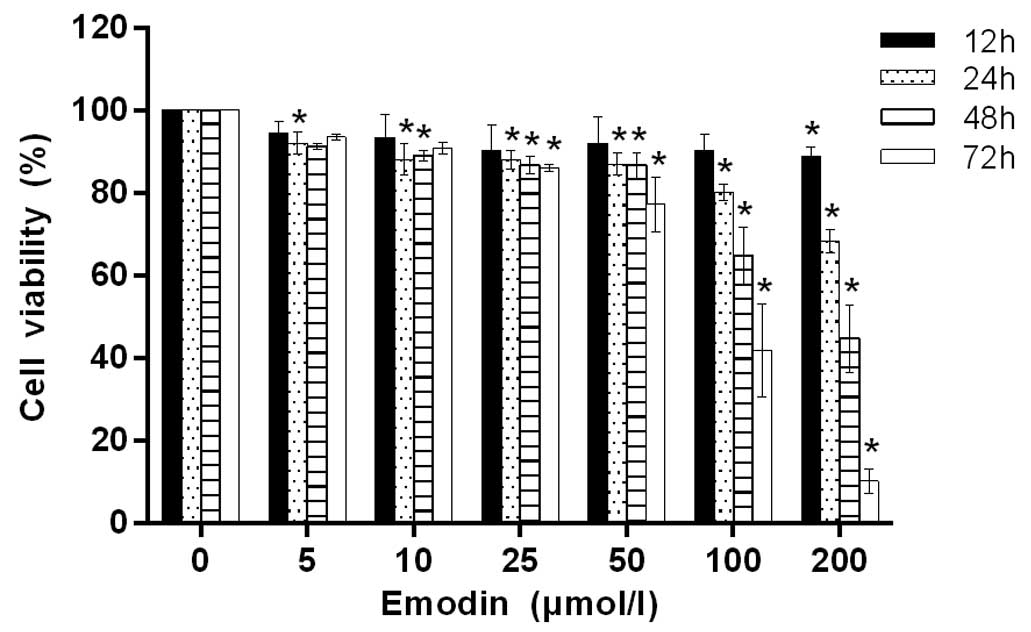
In order to investigate the antiproliferative effect
of emodin on MHCC-97H cells, MTT assay was used to quantify the
effect following treatment with emodin at different concentrations
(5, 10, 25, 50, 100 and 200 µmol/l) for 12, 24, 48 and 72 h. As
shown in Fig. 1, when the treatment
concentration was <100 µmol/l and the treatment time was <24
h, the viability of the cells changed very little. With increases
in the emodin concentration and treatment time, cell viability
decrease evidently in a concentration- and time-dependent
manner.
Effect of low dose emodin on mild
cells apoptosis
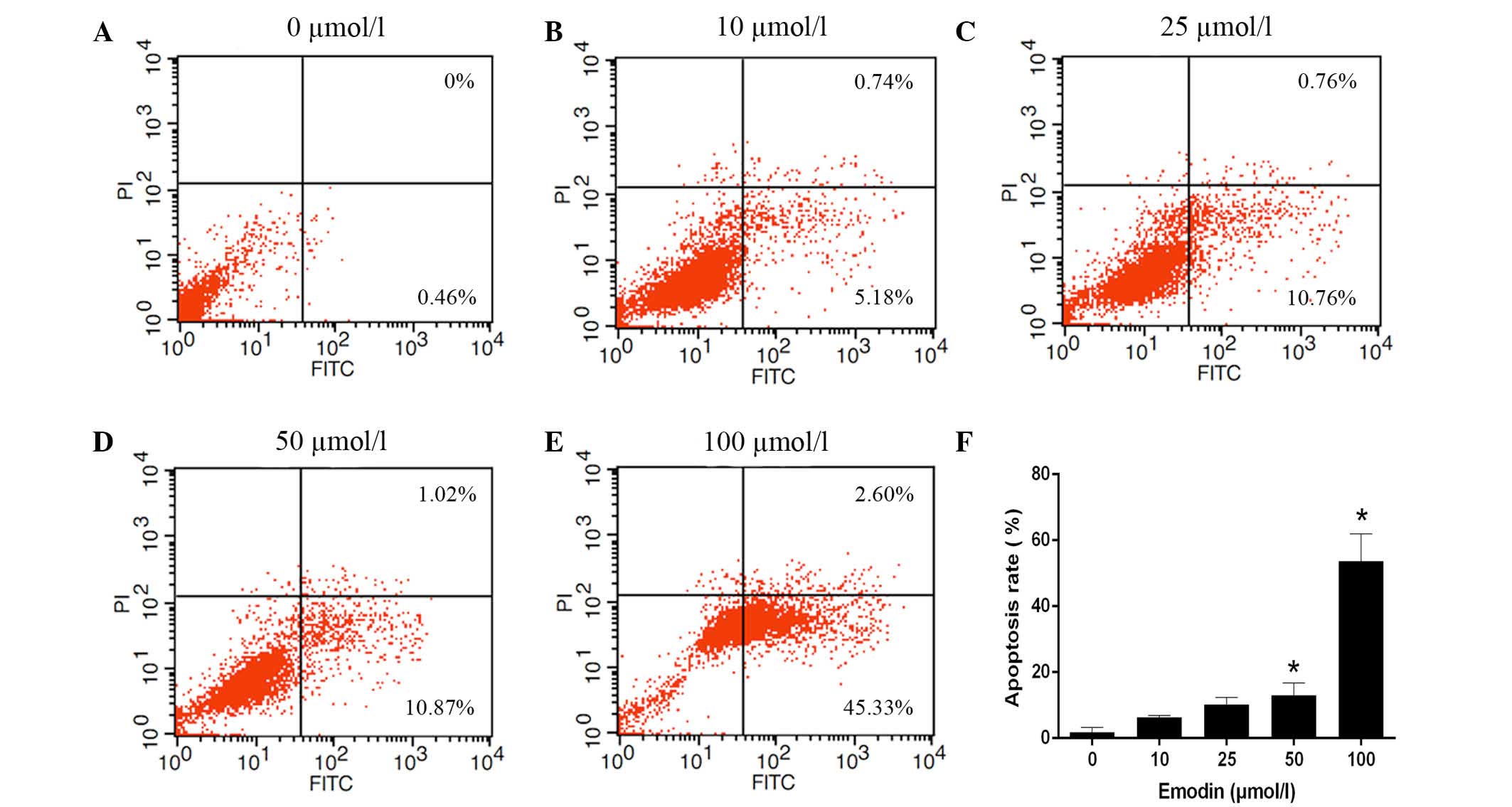
To determine the effect of emodin on apoptosis
induction in MHCC-97H cells, flow cytometry was used to assess the
cell apoptosis rate. After treatment with various concentrations of
emodin (0 µmol/l for the control, 10, 25, 50 and 100 µmol/l for the
experimental groups) for 24 h, MHCC-97H cells were stained with
FITC-Annexin V/PI. As shown in Fig.
2, the apoptosis rate of MHCC-97H cells was shown to gradually
increase as the emodin concentration increased (1.73±1.53,
6.31±0.59, 10.16±2.21, 12.99±3.77 and 53.68±8.32%, respectively).
These results suggest that emodin was able to induce apoptosis in
MHCC-97H cells, but the change was mild when the emodin
concentration was <50 µmol/l.
Effect of emodin on cell migration and
invasion
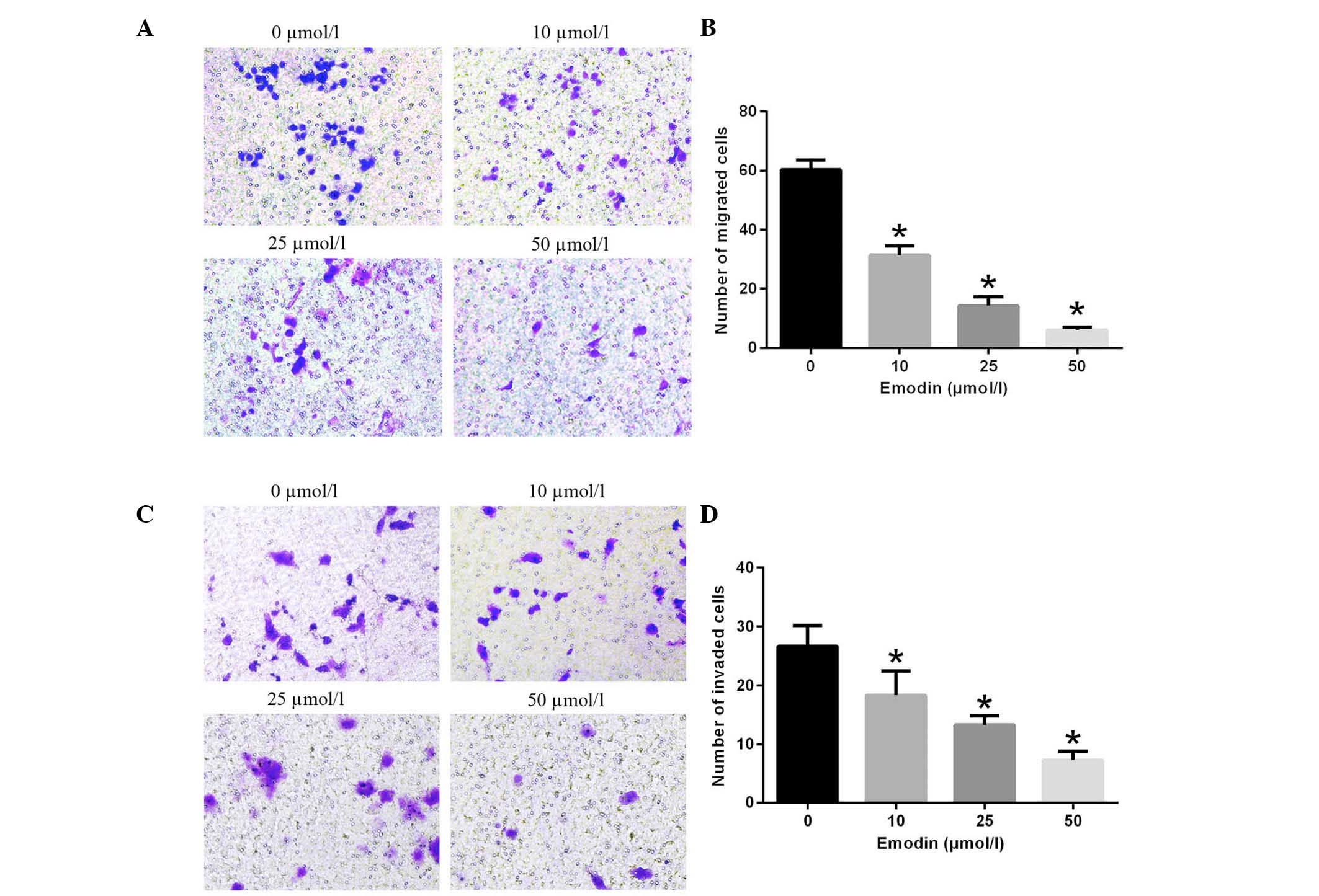
To determine the effect of emodin on cell migration
and invasion, MHCC-97H cells treated with 0, 10, 25 and 50 µmol/l
emodin were induced to migrate through the Transwell membranes and
invade in Matrigel-coated Transwells for 24 h. The number of cells
that migrated was reduced by emodin in a dose-dependent manner
(Fig. 3). In the same manner,
following treatment with emodin at concentration of 0, 10, 25 and
50 µmol/l, the numberof invasive cells was 26.67±3.51, 18.34±4.16,
13.33±1.53 and 7.33±1.53, respectively. These data clearly
demonstrate that emodin treatment significantly inhibits the
migration and invasion of MHCC-97H cells in a dose-dependent
manner.
Effect of emodin on the expression of
proteins associated with tumor invasion
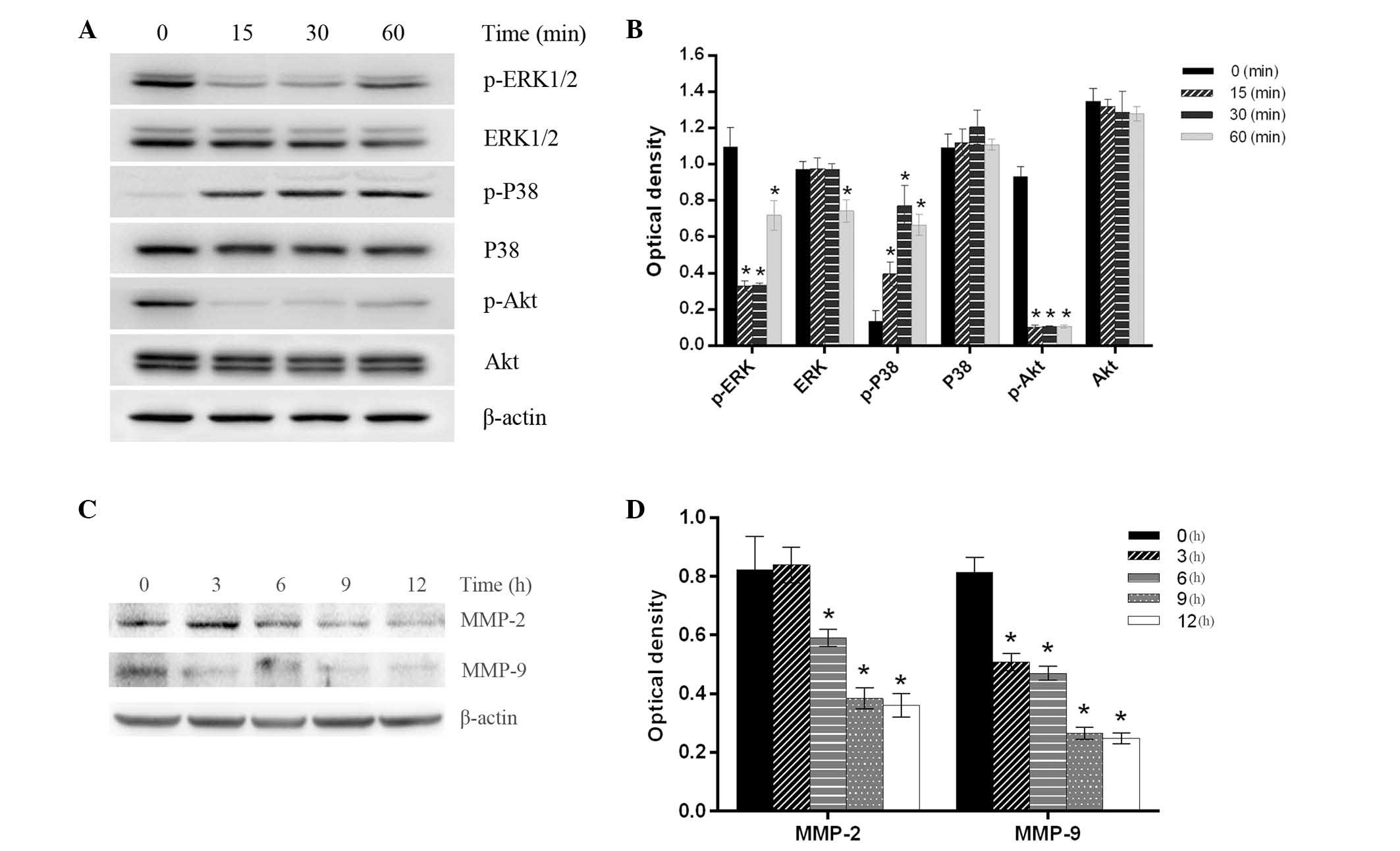
In order to investigate the probable mechanism of
the inhibition of migration and invasion induced by emodin, the
activation of metastasis-related signal pathways, such as
mitogen-activated protein kinase (MAPK) and phosphatidylinositol
3-kinase (PI3K)/Akt signaling pathways, were detected by western
blotting. The results demonstrated that following treatment with 50
µM emodin, the phosphorylated (p)-extracellular signal regulated
kinase (ERK1)/2 expression levels were significantly decreased in a
time-dependent manner until 60 min, at which point the levels began
to increase again. In addition, p-Akt expression levels were
decreased in a time-dependent manner, whereas the tendency of p-p38
expression levels were inverse. However, the ERK1/2, p38 and Akt
expression levels remained essentially unchanged, except for a mild
decrease at 60 min in ERK1/2 expression. In addition, MHCC-97H
cells were treated with 50 µmol/l emodin for 0, 3, 6, 9 and 12 h,
and it was observed that the MMP-2 and MMP-9 expression levels
decreased in a time-dependent manner (Fig. 4).
Discussion
Distant metastasis is a common occurrence in
patients with HCC and is the primary hindrance in improving overall
survival of HCC. Therefore, novel innovative therapeutic drugs are
required to improve HCC prognosis. Emodin, an anthraquinone
derivative from the root and rhizome of Rheum palmatum L.,
was found to have antitumor effects in several types of cancer by
regulating multi-molecular targets involved in tumor growth,
apoptosis and angiogenesis (16).
Recent research demonstrated that emodin may inhibit growth and
induce apoptosis in an orthotopic hepatocellular carcinoma model by
blocking activation of signal transducer and activator of
transcription 3 (17). It was also
reported that emodin may suppresses migration and invasion through
the modulation of C-X-C chemokine receptor type 4 expression in an
orthotopic model of HCC (18). In
the present study, the inhibitory effects of emodin on the
migration and invasion of HCC in vitro are investigated,
using MHCC-97H cell line as a model due to its high potential of
malignant invasion. Furthermore, the molecular mechanisms
underlying these effects are investigated.
Initially, we used a MTT assay to investigate the
antiproliferative effect of emodin in MHCC-97H cells and found that
emodin could suppress cell proliferation in a dose-and
time-dependent manner, particularly when the treatment
concentration was >100 µmol/l and the treatment time was >24
h. Then, we used flow cytometry to identify the effects of emodin
on the induction of apoptosis with <100 µmol/l emodin for 24 h
and found that its induction effect on apoptosis was mild when
emodin was <50 µmol/l. Therefore, in order to eliminate the
effect of antiproliferation and apoptosis, 0, 10, 25 and 50 µmol/l
emodin was chosen to treat MHCC-97H cells for 24 h and investigate
the cell migration and invasion ability with the aid of a Transwell
chamber. The result indicated that emodin treatment inhibited the
migration and invasion of MHCC-97H cells in a dose-dependent
manner.
The establishment and progression of metastases is a
complex, multi-step process involving the modulation of the cell
phenotype, detachment from the primary tumor, invasion through the
extracellular matrix and transportation to other normal organs
(9,19). MMPs are a class of proteolytic
enzymes which serve important roles in extracellular matrix
degradation and their abnormal expression may promote tumor
invasion and metastasis (20). MMP-2
and MMP-9 are two members of the MMP family and they are the
primary enzymes that degrade diffusely basal membrane type IV
collagen (21). Early studies have
demonstrated that MMP-2 and MMP-9 exhibited increased expression in
patients with HCC, which may be a prediction of tumor recurrence
and survival in patients with HCC following surgical resection
(22,23). In the current study, the effects of
emodin on MMP-2 and MMP-9 expression in cells were investigated and
the results suggest that emodin significantly downregulated the
expression of MMP-2 and MMP-9, indicating that MMP-2 and MMP-9 were
involved in the inhibitory impact of emodin.
MAPKs are serine-threonine protein kinases that
mediate a wide variety of cellular behaviors, including
proliferation, differentiation, apoptosis, migration and invasion
(24). In mammals, MAPKs include
ERK, p38 MAPK and c-Jun NH2-terminal kinase. Typically, ERK is
present in its non-activated forms in the cytoplasm. When exposed
to inflammatory cytokines and other factors, the ERK MAPK pathway
may be activated and mediates the cancer processes. Lin et
al (25) found that mammalian
sterile-20-like kinase 4 (MST4) promotes HCC metastasis via the
activation of the p-ERK pathway, and the combination of MST4 and
p-ERK has better power to predict the outcomes of HCC. Thus, the
effects of emodin on the expression of total and phosphorylated
ERK1/2 was investigated and the results indicated that following
exposure to 50 µmol/l emodin, the level of p-ERK1/2 was
downregulated in a time-dependent manner while t-ERK1/2 remained
unchanged. Thus, emodin may inhibit MHCC-97H cell metastasis
through the regulation of the ERK1/2 MAPK signaling pathway. p38
MAPK is an additional important signaling pathway that regulates
the proliferation of malignant tumor cells (26). The p38 MAPK signaling pathway can be
activated in response to various environmental and cellular
stresses, and a number of studies have shown that activation of
p38-MAPK signaling pathways may produce anti-apoptotic and
proliferative effects (27).
However, previous studies have suggested an opposite role of p38
MAPK in mediating cell apoptosis and growth inhibition, which
indicated that p38 MAPK serves a dual role as a regulator of cell
behavior (28,29). In the present study, it was observed
that emodin could promote the phosphorylation of p38, indicating
that the p38 MAPK signaling pathway may exert a function in
response to the type of stimulus and in a cell type specific
manner. Further investigation regarding the specific molecular
mechanism involved is required.
Akt is a serine-threonine kinase that is a primary
effector of PI3K (30). Increased
PI3K/Akt activation is suggested to change the migration and
invasion characteristics of HCC cells, and is an independent
prognostic index for patients with HCC (31,32).
MMP-2 and MMP-9 expression in HCC cells could be enhanced through
the activation of the PI3K/Akt signaling pathway, and as a result
further regulate HCC cell invasion and metastasis (33). The observations in the present study
indicated that emodin treatment inhibited the expression levels of
p-Akt in the MHCC-97H cell line in a time-dependent manner, while
no significant change existed in t-Akt expression levels. Since Akt
is a downstream target of the PI3K signaling pathway, the
inhibition of Akt phosphorylation revealed that emodin treatment
inhibited the PI3K/Akt signaling pathway. This phenomenon may
associated with the inhibition of MMP-2 and MMP-9 expression.
In conclusion, the current study is the first to use
MHCC-97H cells, which have a high malignant and invasion potential,
to demonstrate that emodin can suppress the migration and invasion
at concentrations that have little effects on proliferation
inhibition or apoptosis induction. Furthermore, emodin suppressed
the MMP-2 and MMP-9 expression, which are closely associated with
the metastasic characteristics of malignant tumors. It can be
speculated that these effects were mediated by the activation of
the p38 MAPK signaling pathway and the suppression of the ERK/MAPK
and PI3K/Akt signaling pathways. However, further studies are
required to explore and elucidate the specific molecular mechanism
by which emodin inhibits the invasion and metastasis of HCC.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 30901986), the
Foundation for the Author of National Excellent Doctoral
Dissertation of China (grant no. 201366) and the Shanghai Municipal
Natural Science Foundation (grant no. 13ZR1448900).
Glossary
Abbreviations
Abbreviations:
|
MMP
|
matrix metalloproteinase
|
|
MAPK
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular signal regulated
kinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosetti C, Turati F and La Vecchia C:
Hepatocellular carcinoma epidemiology. Best Pract Res Clin
Gastroenterol. 28:753–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: The BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baffy G: Decoding multifocal
hepatocellular carcinoma: An opportune pursuit. Hepatobiliary Surg
Nutr. 4:206–210. 2015.PubMed/NCBI
|
|
7
|
Lui GY, Kovacevic Z, Richardson V, Merlot
AM, Kalinowski DS and Richardson DR: Targeting cancer by binding
iron: Dissecting cellular signaling pathways. Oncotarget.
6:18748–18779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li F and Zhang W: Role of traditional
Chinese medicine and its chemical components in anti-tumor
metastasis. J Cancer Res Ther. 10(Suppl 1): S20–S26. 2014.
|
|
10
|
Wang X, Wang N, Cheung F, Lao L, Li C and
Feng Y: Chinese medicines for prevention and treatment of human
hepatocellular carcinoma: Current progress on pharmacological
actions and mechanisms. J Integr Med. 13:142–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun ZH and Bu P: Downregulation of
phosphatase of regenerating liver-3 is involved in the inhibition
of proliferation and apoptosis induced by emodin in the SGC-7901
human gastric carcinoma cell line. Exp Ther Med. 3:1077–1081.
2012.PubMed/NCBI
|
|
12
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Chen L, Bu HQ, Yu QJ, Jiang DD,
Pan FP, Wang Y, Liu DL and Lin SZ: Effects of emodinon the
demethylation of tumor-suppressor genes in pancreatic cancer PANC-1
cells. Oncol Rep. 33:3015–3023. 2015.PubMed/NCBI
|
|
14
|
Pooja T and Karunagaran D: Emodin
suppresses Wnt signaling in human colorectal cancer cells SW480 and
SW620. Eur J Pharmacol. 742:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Lu H, Wang S, Chen B, Liu Z, Ke X,
Liu T and Fu J: The anthraquinone derivative Emodin inhibits
angiogenesis and metastasis through downregulating Runx2 activity
in breast cancer. Int J Oncol. 46:1619–1628. 2015.PubMed/NCBI
|
|
16
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562.
2013.PubMed/NCBI
|
|
17
|
Subramaniam A, Shanmugam MK, Ong TH, Li F,
Perumal E, Chen L, Vali S, Abbasi T, Kapoor S, Ahn KS, et al:
Emodin inhibits growth and induces apoptosis in an orthotopic
hepatocellular carcinoma model by blocking activation of STAT3. Br
J Pharmacol. 170:807–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manu KA, Shanmugam MK, Ong TH, Subramaniam
A, Siveen KS, Perumal E, Samy RP, Bist P, Lim LH, Kumar AP, et al:
Emodin suppresses migration and invasion through the modulation of
CXCR4 expression in an orthotopic model of human hepatocellular
carcinoma. PloS One. 8:e570152013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polacheck WJ, Zervantonakis IK and Kamm
RD: Tumor cell migration in complex microenvironments. Cell Mol
Life Sci. 70:1335–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation-and
metastasis-sustaining neovasculature. Matrix Biol 44–46. 94–112.
2015. View Article : Google Scholar
|
|
21
|
Kessenbrock K, Wang CY and Werb Z: Matrix
metalloproteinases in stem cell regulation and cancer. Matrix Biol
44–46. 184–190. 2015. View Article : Google Scholar
|
|
22
|
Daniele A, Divella R, Quaranta M, Mattioli
V, Casamassima P, Paradiso A, Garrisi VM, Gadaleta CD,
Gadaleta-Caldarola G, Savino E, et al: Clinical and prognostic role
of circulating MMP-2 and its inhibitor TIMP-2 in HCC patients prior
to and after trans-hepatic arterial chemo-embolization. Clin
Biochem. 47:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen R, Cui J, Xu C, Xue T, Guo K, Gao D,
Liu Y, Ye S and Ren Z: The significance of MMP-9 over MMP-2 in HCC
invasiveness and recurrence of hepatocellular carcinoma after
curative resection. Ann Surg Oncol. 19(Suppl 3): S375–S384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin ZH, Wang L, Zhang JB, Liu Y, Li XQ,
Guo L, Zhang B, Zhu WW and Ye QH: MST4 promotes hepatocellular
carcinoma epithelial-mesenchymal transition and metastasis via
activation of the p-ERK pathway. Int J Oncol. 45:629–640.
2014.PubMed/NCBI
|
|
26
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olson JM and Hallahan AR: p38 MAP kinase:
A convergence point in cancer therapy. Trends Mol Med. 10:125–129.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin JJ, Su JH, Tsai CC, Chen YJ, Liao MH
and Wu YJ: 11-epi-Sinulariolide acetate reduces cell migration and
invasion of human hepatocellular carcinoma by reducing the
activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling
pathways. Mar Drug. 12:4783–4798. 2014. View Article : Google Scholar
|
|
32
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu YJ, Neoh CA, Tsao CY, Su JH and Li HH:
Sinulariolide suppresses human hepatocellular carcinoma cell
migration and invasion by inhibiting matrix metalloproteinase-2/-9
through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci.
16:16469–16482. 2015. View Article : Google Scholar : PubMed/NCBI
|