Introduction
Platelet-rich plasma (PRP) is blood plasma with a
high number of platelets. As an enriched source of autologous
platelets, PRP contains several growth factors that are important
for initiating and accelerating tissue repair and regeneration.
Given these advantages, PRP therapy has recently emerged as an
innovative technique with great potential for healing chronic and
acute wounds, including diabetic wounds, bedsores, skin ulcers and
thermal burns. Fundamentally, the mechanisms underlying PRP therapy
are the molecular and cellular stimulation of normal wound healing
responses, similar to those observed during platelet activation
(1). However, it is difficult to
cure intractable diseases such as diabetic ulcers and decubitus,
since the therapeutic effect tend to differ among individuals
(2,3). Reviving a wound with impaired healing
is unmanageable because standard wound healing methods do not
always provide improved healing results. This often demands more
advanced therapies (4–6). Platelets can be activated upon two
different types of stimuli: Physical stimuli, including heat, cold
and vibration, and chemical stimuli, including collagen,
lipopolysaccharide and chitosan (7–11). The
activated platelets release biologically active substances and
growth factors, such as platelet factor 4 (PF4), von Willebrand
factor, platelet-derived growth factor (PDGF), hepatocyte growth
factor (HGF), insulin-like growth factor (IGF) and vascular
endothelial growth factor (12–14).
Chitosan is a polysaccharide derived from chitin,
which is a compound of natural origin obtained from the shell of
crabs and shellfish. Chitosan prepared from alkaline
N-deacetylation is composed of β-(1–4)-linked
D-glucosamine and N-acetyl-D-glucosamine, which are randomly
distributed. It carries a positive charge, as the free amino groups
of β-(1–4)-linked D-glucosamine are protonated at
physiological pH. Chitosan is being extensively used as a potential
biomaterial in several medical devices and health care products
owing to its biodegradability and advantageous biological
properties, including hemostatic activity (15,16),
biodegradability (15,17), antibacterial activity (18,19) and
its ability to serve as a wound dressing agent to accelerate wound
healing (16,20,21).
Given these advantages, the use of chitosan as a stimulant for
platelet activation can be highly effective in PRP therapy.
However, depending on the methods adopted for purifying chitosan
from chitin, the molecular weight (Mw) and degree of deacetylation
(DDA) vary. In other words, the function, properties and
performance of chitosan are associated with their DDA and Mw.
Given these considerations, the present study
proposed the concept of effective PRP therapy using chitosan. This
strategy relies on the fact that chitosan activates platelets and
enhances the release of growth factors into the plasma. To further
enhance the effectiveness of PRP, a basic study using 13 different
types of chitosan with varying Mw and DDA was performed.
Materials and methods
Animals and chitosan
The present study was approved by the Ethics
Committee of Animal Care and Use, National Defense Medical College
(Saitama, Japan) on July 28, 2014 (approval no., 14040) and the
protocol was in accordance with the committee's guidelines for the
care of animal subjects. Male Sprague-Dawley rats (28–48 weeks old;
weight, 500–700 g; n=4) were obtained from Japan SLC (Hamamatsu,
Japan). Following anesthesia with 3% sevoflurane (Maruishi
Pharmaceutical Co., Ltd., Osaka, Japan) inhalation, each 15-ml
blood sample collected from the tail vein was mixed with 3.13%
sodium citrate solution (10% v/v) to inhibit coagulation. The blood
sample was used for the examination as soon as it was collected.
The Mw and DDA of each chitosan sample are listed in Table I. The samples with a DDA (in %)/Mw
(in Da) of 84.2/8,600, 85.7/15,900, 50.3/28,800, 48.7/57,700,
35.5/30,000 and 33.6/57,300 (Yaizu Suisankagaku Industry Co., Ltd,
Tokyo, Japan) were purified according to a previously described
method (22). Chitosan oligomers
(dimer, trimer, tetramer, pentamer and hexamer) were purchased from
Seikagaku Co. (Tokyo, Japan), chitosan with a DDA of 87.6% and a Mw
of 247,000 Da was obtained from Primex ehf (Siglufjordur, Iceland)
and chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da was
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). As an
adjustment for the chitosan solution, 40 mg of each chitosan powder
was dissolved in 15 ml 2% acetic acid, the pH was adjusted to 4.0
with 1 M sodium bicarbonate, 2 ml of 10X concentrated Dulbecco's
phosphate-buffered saline (PBS) without calcium and magnesium was
added to adjust to the osmotic pressure of blood, and the solution
was topped up to 20 ml with distilled water. Blank sample was
adjusted as follows: 15 ml 2% acetic acid was adjusted to pH 4.0
with 1 M sodium bicarbonate, 2 ml of 10X concentrated PBS without
calcium and magnesium was added and the solution was topped up to
20 ml with distilled water.
 | Table I.Chitosan samples used in the present
study. |
Table I.
Chitosan samples used in the present
study.
| Number | Degree of
deacetylation (%) | Molecular weight
(Da) |
|---|
| 1 | 84.2 | 8,600 |
| 2 | 85.7 | 15,900 |
| 3 | 75–85 | 50,000–190,000 |
| 4 | 87.6 | 247,000 |
| 5 | 50.3 | 28,800 |
| 6 | 48.7 | 57,700 |
| 7 | 35.5 | 30,000 |
| 8 | 33.6 | 57,300 |
| II (dimer) | 100 | 413.25 |
| III (trimer) | 100 | 610.87 |
| IV (tetramer) | 100 | 808.49 |
| V (pentamer) | 100 | 1,006.11 |
| VI (hexamer) | 100 | 1,203.72 |
Determination of protein in the
plasma
In a typical process, 500 µl of blood was added to
500 µl of 0.2% chitosan solution and 25 µl of 200 mM calcium
chloride solution. The solution was gently mixed and incubated at
room temperature for 1 h. Subsequently, the mixture was centrifuged
at 10,000 × g for 15 min and the plasma was collected. The
plasma samples were used at once without freezing. The levels of
albumin, fibrinogen, PF4, PDGF-AB, PDGF-BB, IGF and HGF in the
plasma were measured using ELISA kits as follows: Rat Albumin ELISA
kit (E-25AL) and Rat Fibrinogen ELISA kit (E-25FIB), both from
Immunology Consultants Laboratory (Portland, OR, USA); ELISA kit
for Platelet Factor 4 (SEA172Ra; USCN Life Science, Wuhan, China);
Mouse/Rat PDGF-AB Quantikine ELISA kit (MHD00), Mouse/Rat PDGF-BB
Quantikine ELISA kit (MBB00) and Mouse/Rat IGF-I Quantikine ELISA
kit (MG100), all from R&D Systems, Inc. (Minneapolis, MN, USA);
and Rat HGF EIA (1Z81; Institute of Immunology, Tokyo, Japan).
Cell proliferation assay
Plasma was sterilized using a 0.2-µm filter (EMD
Millipore, Billerica, MA, USA). Human fibroblasts (NHDF-Ad) and
adipose tissue-derived stromal cells (ASCs; cat. no. PT-5006)
(Lonza Japan, Tokyo) were plated at a density of 1.0×104
cells/well in 96-well culture plates (Sumitomo Bakelite Co., Ltd.,
Tokyo, Japan) and were cultured with Dulbecco's modified Eagle's
medium including 5% plasma. Cell proliferation was examined using a
Cell Counting kit (Dojindo Co., Kumamoto, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Multiple comparisons were evaluated using analysis of
variance, as well as Tukey's and Dunnet's tests as appropriate.
Statistical analysis was conducted using JMP® 11
software (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Platelet activation
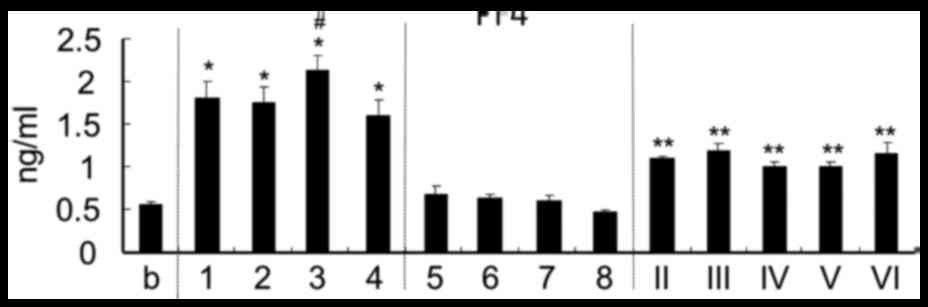
The platelet activation test was performed by
measuring PF4 secretion in plasma. Chitosan with a high DDA
(>75%) including oligomer induced a higher release of PF4 in
plasma than chitosan with a DDA of 50.3% and below (Fig. 1). In particular, chitosan with a Mw
of 50,000–190,000 Da and a DDA of 75–85% exhibited the best
activating efficiency among the 13 types of chitosan tested.
Growth factors induced by
chitosan
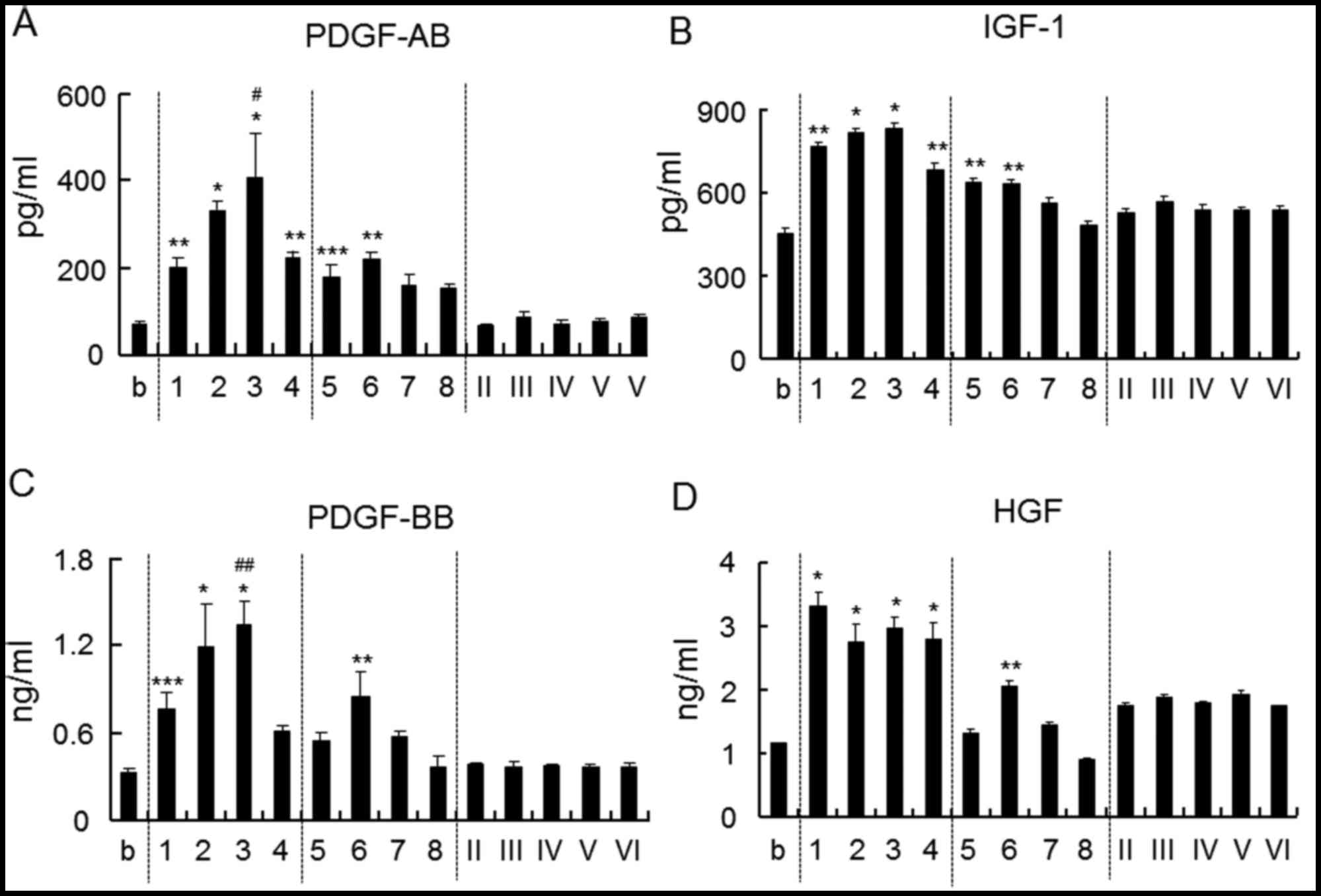
The amounts of growth factors such as PDGF-AB,
PDGF-BB, IGF-1 and HGF in plasma were measured (Fig. 2). The amount of PDGF-AB and IGF-1
increased upon the addition of chitosan with a DDA of >75% and a
Mw of >8,600 Da, as well as chitosan with a DDA of ~50% and a Mw
of 28,800 or 57,700 Da (Fig. 2A and
B). The amount of PDGF-BB and HGF was increased upon addition
of chitosan with a DDA of >75% and a Mw of >8,600 Da as well
as with a DDA of 48% and a Mw of 57,700 Da (Fig. 2C and D). Chitosan oligomer (100% DDA)
did not have any influence on the release of these growth
factors.
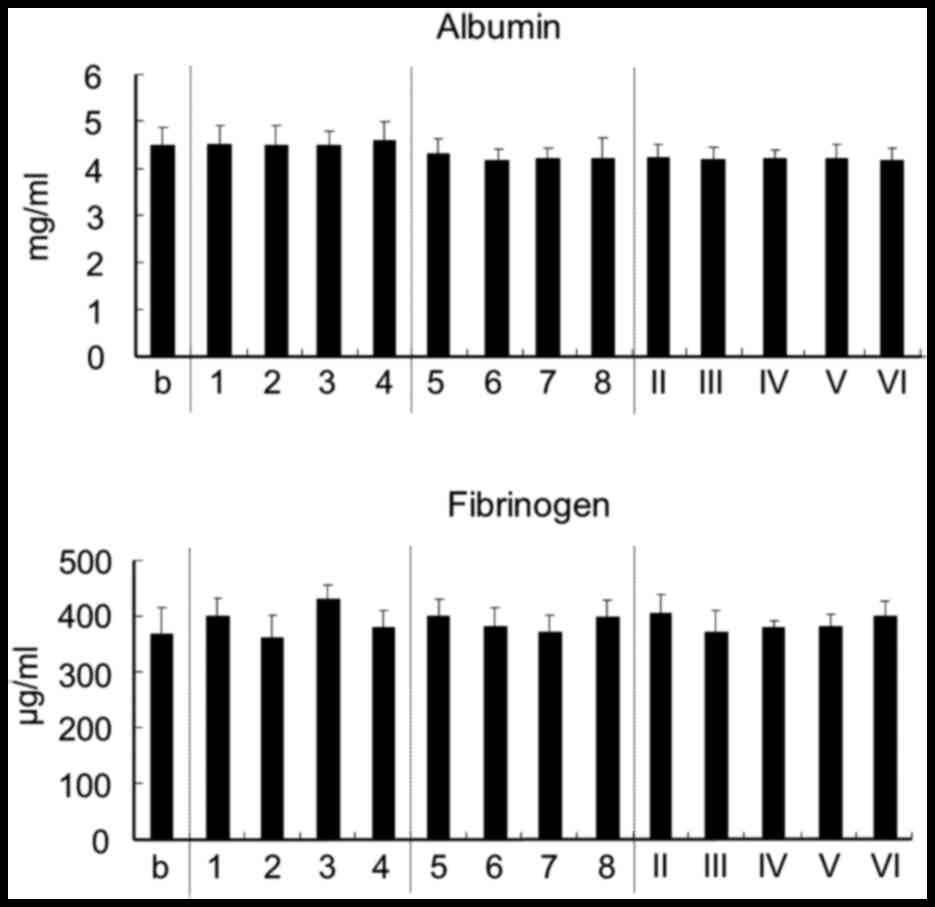
Effect on plasma protein
The effect of chitosan on plasma albumin, which
accounts for ~60% of all plasma protein, and on fibrinogen, which
has an important role in secondary hemostasis, was measured.
However, chitosan was found to have no effect on the levels of
albumin and fibrinogen in plasma (Fig.
3).
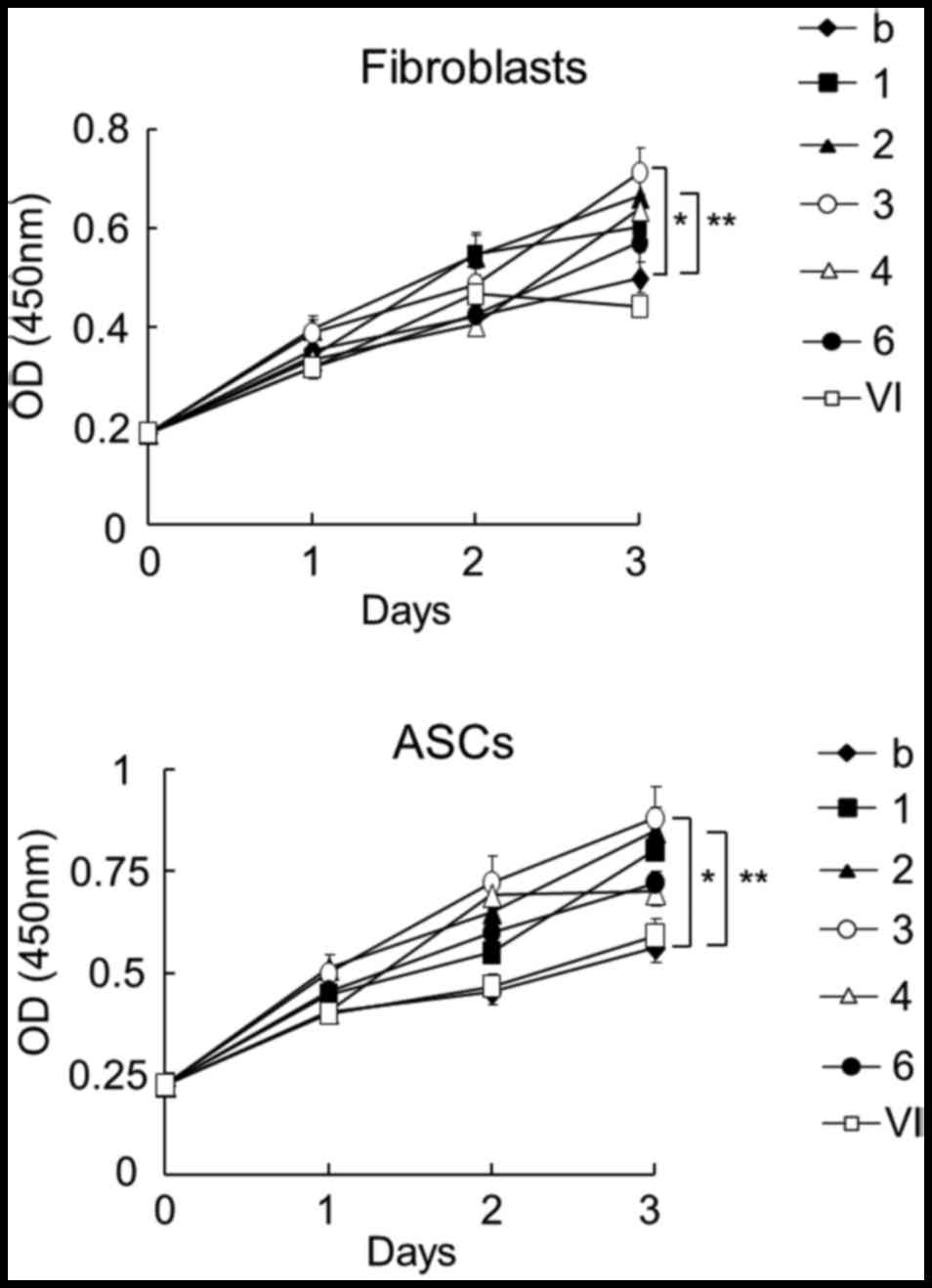
Cell proliferation
To analyze the effect of chitosan-treated plasma on
cell proliferation, human fibroblasts and ASCs were cultured using
plasma activated by chitosan (Fig.
4). Based on the results of growth factors induced by chitosan
(Fig. 2), the effects of plasma
individually treated with 7 different chitosan samples on cell
proliferation was examined. The use of plasma activated by chitosan
with a DDA of 75–85% and a Mw of 50,000–190,000 Da resulted in the
highest increase in the proliferation of fibroblasts and ASCs. The
second-highest increase was achieved using chitosan with a DDA of
85.7 % and a Mw of 15,900 Da. However, the chitosan oligomer did
not increase the cell proliferation when compared to other chitosan
samples.
Discussion
PRP therapy is a treatment process that uses a
patient's own blood to activate and release of growth factor-rich
granules at the wound site. Chitosan has been reported to improve
the efficiency of PRP therapy (23–25).
PRP-loaded chitosan scaffolds may be an appropriate carrier for PRP
applications that facilitate the sustained release of growth
factors (23). Chitosan is used as a
functional delivery aid to simultaneously support PRP, stem cells
and growth factors (24). Platelets
contain negatively charged membranes due to the presence of
negatively charged sugars, negatively charged phospholipids such as
phosphatidylethanolamine phosphatidylcholine, carbohydrates such as
sialic acid, and, to a much lesser extent, negatively charged amino
acids such as aspartate and glutamate (26–29). By
contrast, chitosan is positively charged due to the existence of
free amino groups derived from the deacetylation of
N-acetyl-D-glucosamine. Chitosan is highly positively
charged, and strongly attracts and binds to negatively charged
molecules. These interactions potentially induce platelet
activation. Normally, non-activated platelets store CD62P in the
alpha-granule membranes, but several stimulators rapidly
translocate to the platelet surface (30–33).
Platelet activated by these stimulators releases alpha-granule
constituents, such as PF4 and growth factors (12,13).
Although chitosan has previously been reported to cause platelet
activation (34–37), the effect of differences in Mw and
DDA has remained elusive. Therefore, basic studies using 13 types
of chitosan with varying Mw and DDA were performed in the present
study.
As it was assumed possible to produce more effective
treatments for intractable diseases by use of PRP in which
platelets are activated by chitosan, a preliminary experiment was
performed to assess the platelet activation and the release of
growth factors in PRP including chitosan; however, no accurate
analysis was possible. Due to the platelet activation effect of
chitosan, the PF4 levels and growth factors showed large variations
due to being affected by slight stimulations occurring during the
measurement procedure. Consequently, these factors were assayed in
plasma, which was centrifuged after chitosan was added to the whole
blood. Chitosan with a DDA of >75%, including chitosan oligomer,
significantly enhanced PF4 release; chitosan with a reasonably high
DDA therefore increased the platelet activation. This activation
can be attributed to the increase in the number of free amino
groups in chitosan with a higher DDA. A high DDA is therefore
important for high platelet activation, and additionally, a Mw of
>8,900 Da was also required for higher activation.
Platelets activated by chitosan released various
growth factors, including PDGF-AB, PDGF-BB, HGF and IGF-1. However,
the effects on the levels of growth factors were not the same among
the different types of chitosan. The release of PDGF-AB and PDGF-BB
was the highest in the presence of chitosan with a DDA of 75–85%
and a Mw of 50,000–190,000 Da. However, this was not the case for
IGF-1 and HGF, whose release was highest in the presence of
chitosan with a DDA of >75% and a Mw of 8,600–190,000 Da as well
as with a DDA of 48% and a Mw of 57,700 Da. The observed difference
in the levels of growth factors may possibly be attributed to the
charge balance or interactions of other proteins. Further studies
are required to explain for the high platelet activation observed
in chitosan with a higher DDA and lower Mw. Overall the results of
the present study demonstrated that the addition of chitosan to
blood activates platelets to release growth factors in plasma,
thereby improving the effectiveness of the PRP therapy.
Growth factors promote cell proliferation,
differentiation and angiogenesis (38,39). PRP
induces stimulation of cell growth in ASCs, periodontal ligament
and mesenchymal stem cells as well as enhancement of cellular
adhesion, proliferation and differentiation of human periodontal
ligament cells (40). In the present
study, plasma with chitosan-induced growth factor enrichment
stimulated the growth of fibroblasts and ASCs. In particular, the
proliferation was enhanced with the use of plasma containing
chitosan with a DDA of 75–85% and a Mw of 50,000–190,000 Da.
Recently, Bura et al (41)
demonstrated the feasibility and safety of autologous ASC
transplantation in patients with objectively proven critical limb
ischemia not suitable for revascularization. The use of ASCs and
PRP, which is activated by chitosan with a DDA of 75–85% and a Mw
of 50,000–190,000 Da for therapeutic application in wound healing
and complications in patients with intractable diseases such as
diabetic ulcers and decubitus, is expected to be an efficacious
approach.
Fibrinogen is an acute-phase protein that is a part
of the coagulation cascade, the end result of which is the
production of thrombin that converts fibrinogen to fibrin clots.
Surfaces of the materials coated with fibrinogen promote platelet
adhesion and activation (42).
Albumin, which accounts for ~60% of plasma protein, is negatively
charged, as are platelets (43).
Albumin combines with various internal substrates and functions to
transport them to the target tissue. In the present study, chitosan
was not found to affect fibrinogen and albumin in plasma.
Of all the 13 chitosan samples tested in the present
study, that with a DDA of 75–85% and a Mw of 50,000–190,000 Da
showed the highest platelet activation and release of growth
factors. Moreover, plasma induced by chitosan stimulated the
proliferation of human fibroblasts and ASCs. However, chitosan did
not affect the levels of fibrinogen and albumin in plasma. These
results suggested that the effectiveness of PRP can be improved by
using this type of chitosan.
Acknowledgements
The authors would like to thank Yaizu Suisankagaku
Industry Co., Ltd (Tokyo, Japan) for supplying the chitosan, as
well as Ms. Keiko Yamazaki and Ms. Reiko Yoshimoto for their
research assistance.
References
|
1
|
Ferrari M, Zia S, Valbonesi M, Henriquet
F, Venere G, Spagnolo S, Grasso MA and Panzani I: A new technique
for hemodilution, preparation of autologous platelet-rich plasma
and intraoperative blood salvage in cardiac surgery. Int J Artif
Organs. 10:47–50. 1987.PubMed/NCBI
|
|
2
|
Greer N, Foman NA, MacDonald R, Dorrian J,
Fitzgerald P, Rutks I and Wilt TJ: Advanced wound care therapies
for nonhealing diabetic, venous, and arterial ulcers: A systematic
review. Ann Intern Med. 159:532–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones KR, Fennie K and Lenihan A:
Evidence-based management of chronic wounds. Adv Skin Wound Care.
20:591–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter MJ, Fylling CP and Parnell LK: Use
of platelet rich plasma gel on wound healing: A systematic review
and meta-analysis. Eplasty. 11:e382011.PubMed/NCBI
|
|
5
|
Steed DL, Attinger C, Colaizzi T,
Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M,
Serena T, et al: Guidelines for the treatment of diabetic ulcers.
Wound Repair Regen. 14:680–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bolton LL, van Rijswijk L and Shaffer FA:
Quality wound care equals cost-effective wound care: A clinical
model. Adv Wound Care. 10:33–38. 1997.PubMed/NCBI
|
|
7
|
Zucker MB and Nachmias VT: Platelet
activation. Arteriosclerosis. 5:2–18. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winokur R and Hartwig JH: Mechanism of
shape change in chilled human platelets. Blood. 85:1796–1804.
1995.PubMed/NCBI
|
|
9
|
Kroll MH, Hellums JD, McIntire LV, Schafer
AI and Moake JL: Platelets and shear stress. Blood. 88:1525–1541.
1996.PubMed/NCBI
|
|
10
|
Brown GT, Narayanan P, Li W, Silverstein
RL and McIntyre TM: Lipopolysaccharide stimulates platelets through
an IL-1β autocrine loop. J Immunol. 191:5196–5203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Delaney MK, O'Brien KA and Du X:
Signaling during platelet adhesion and activation. Arterioscler
Thromb Vasc Biol. 30:2341–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kowalska MA, Rauova L and Poncz M: Role of
the platelet chemokine platelet factor 4 (PF4) in hemostasis and
thrombosis. Thromb Res. 125:292–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burnouf T, Goubran HA, Chen TM, Ou KL,
El-Ekiaby M and Radosevic M: Blood-derived biomaterials and
platelet growth factors in regenerative medicine. Blood Rev.
27:77–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Teramoto H and Ichihara A:
Purification and characterization of a growth factor from rat
platelets for mature parenchymal hepatocytes in primary culture.
Proc Natl Acad Sci USA. 83:6489–6493. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattori H, Amano Y, Nogami Y, Takase B and
Ishihara M: Hemostasis for severe hemorrhage with
photocrosslinkable chitosan hydrogel and calcium alginate. Ann
Biomed Eng. 38:3724–3732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ono K, Ishihara M, Ozeki Y, Deguchi H,
Sato M, Saito Y, Yura H, Sato M, Kikuchi M, Kurita A and Maehara T:
Experimental evaluation of photocrosslinkable chitosan as a
biologic adhesive with surgical applications. Surgery. 130:844–850.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wedmore I, McManus JG, Pusateri AE and
Holcomb JB: A special report on the chitosan-based hemostatic
dressing: Experience in current combat operations. J Trauma.
60:655–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarasam AR, Brown P, Khajotia SS, Dmytryk
JJ and Madihally SV: Antibacterial activity of chitosan-based
matrices on oral pathogens. J Mater Sci Mater Med. 19:1083–1090.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mellegard H, Kovács ÁT, Lindbäck T,
Christensen BE, Kuipers OP and Granum PE: Transcriptional responses
of Bacillus cereus towards challenges with the polysaccharide
chitosan. PLoS One. 6:e243042011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shigemasa Y and Minami S: Applications of
chitin and chitosan for biomaterials. Biotechnol Genet Eng Rev.
13:383–420. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park CJ, Gabrielson NP, Pack DW, Jamison
RD and Johnson AJ Wagoner: The effect of chitosan on the migration
of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials.
30:436–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hattori H and Ishihara M: Changes in blood
aggregation with differences in molecular weight and degree of
deacetylation of chitosan. Biomed Mater. 10:0150142015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kutlu B, Tiğlı Aydın RS, Akman AC,
Gümüşderelioglu M and Nohutcu RM: Platelet-rich plasma-loaded
chitosan scaffolds: Preparation and growth factor release kinetics.
J Biomed Mater Res B Appl Biomater. 101:28–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Busilacchi A, Gigante A, Mattioli-Belmonte
M, Manzotti S and Muzzarelli RA: Chitosan stabilizes platelet
growth factors and modulates stem cell differentiation toward
tissue regeneration. Carbohydr Polym. 98:665–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oktay EO, Demiralp B, Demiralp B, Senel S,
Akman A Cevdet, Eratalay K and Akincibay H: Effects of
platelet-rich plasma and chitosan combination on bone regeneration
in experimental rabbit cranial defects. J Oral Implantol.
36:175–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bosmann HB: Platelet adhesiveness and
aggregation: II. Surface sialic acid, glycoprotein:
N-acetylneuraminic acid transferase, and neuraminidase of human
blood platelets. Biochim Biophys Acta. 279:456–474. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lupu C and Calb M: Changes in the platelet
surface charge in rabbits with experimental hypercholesterolemia.
Atherosclerosis. 72:77–82. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
vd Winkel JG, Wetzels JF, van Duijnhoven
JL, Koene RA and Capel PJ: Red blood cell surface charge and alcian
blue binding. Nephrol Dial Transplant. 2:280–281. 1987.PubMed/NCBI
|
|
29
|
Briedé JJ, Heemskerk JW, Hemker HC and
Lindhout T: Heterogeneity in microparticle formation and exposure
of anionic phospholipids at the plasma membrane of single adherent
platelets. Biochim Biophys Acta. 1451:163–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mackman N, Tilly RE and Key NS: Role of
the extinsic pathway of blood coagulation in hemostasis and
thrombosis. Arterioscler Thromb Vasc Biol. 27:1687–1693. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McEver RP: Adhesive interactions of
leukocytes, platelets, and the vessel wall during hemostasis and
inflammation. Thromb Haemost. 86:746–756. 2001.PubMed/NCBI
|
|
32
|
Klinger MH: Platelets and inflammation.
Anat Embryol (Berl). 196:1–11. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hagberg IA and Lyberg T: Evaluation of
circulating platelet-leukocyte conjugates: A sensitive flow
cytometric assay well suited for clinical studies. Platelets.
11:151–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen EC, Chou TC, Gau CH, Tu HP, Chen YT
and Fu E: Releasing growth factors from activated human platelets
after chitosan stimulation: A possible bio-material for
platelet-rich plasma preparation. Clin Oral Implants Res.
17:572–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukusawa M, Abe H, Masaoka T, Orita H,
Horikawa H, Campeau JD and Washio M: The hemostatic effect of
deacetylated chitin membrane on peritoneal injury in rabbit model.
Surg Today. 22:333–338. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
International committee for
standardization in hematology, . Recommendation of measurement of
erythrocyte sedimentation rate of human blood. Am J Clin Pathol.
68:505–507. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sugamori T, Iwase H, Maeda M, Inoue Y and
Kurosawa H: Local hemostatic effects of microcrystalline partially
deacetylated chitin hydrochloride. J Biomed Mater Res. 49:225–232.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lubkowska A, Dolegowska B and Banfi G:
Growth factor content in PRP and their applicability in medicine. J
Biol Regul Homeost Agents. 26(2): Suppl 1. S3–S22. 2012.
|
|
39
|
Miyazawa K: Hepatocyte growth factor
activator (HGFA): A serine protease that links tissue injury to
activation of hepatocyte growth factor. FEBS. 277:2208–2214. 2010.
View Article : Google Scholar
|
|
40
|
Han J, Meng HX, Tang JM, Li SL, Tang Y and
Chen ZB: The effect of different platelet-rich plasma
concentrations on proliferation and differentiation of human
periodontal ligament cells in vitro. Cell Prolif. 40:241–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bura A, Planat-Benard V, Bourin P,
Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA,
Fleury S, Gadelorge M, et al: Phase I trial: The use of autologous
cultured adipose-derived stroma/stem cells to treat patients with
non-revascularizable critical limb ischemia. Cytotherapy.
16:245–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Q, Ao Q, Gong K, Zhang L, Hu M, Gong Y
and Zhang X: Preparation and characterization of chitosan-heparin
composite matrices for blood contacting tissue engineering. Biomed
Mater. 5:0550012010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Busher JT: Serum albumin and
globulinClinical Methods: The History, Physical and Laboratory
Examinations. Walker KH, Hall DW and Hurst WJ: 3rd. Butterworth
Publishers; Boston: pp. 497–499. 1990
|