Introduction
Allergic inflammation is caused by allergens and
results in diseases, including rhinitis, asthma and conjunctivitis.
It impacts the quality of life and costs large medical expenditures
to attenuate the hypersensitive symptoms. In total, 10–20% of the
population worldwide suffers from allergies, and this number
annually increases (1). Similarly to
effector cells, mast cells are important in allergic inflammation
through the production and secretion of allergic mediators,
including histamine, chemokines, cytokines and growth factors
(2). When exposed to allergens the
stimulation leads to the production of immunoglobulin E (IgE)
within min, which binds to the IgE receptor on the surface of mast
cells with high affinity (3) and
induces IgE-dependent acute hypersensitivity reactions.
Furthermore, crosslinking of antigen-IgE triggers the IgE
receptor-mediated activation of mast cells and induces the
degranulation of mast cells through granule membrane fusion
(4). This is followed by the release
of allergic inflammatory mediators, including histamine,
chemokines, cytokines and eicosanoids for the change of membrane
permeability (5). As a major
allergic mediator, histamine is the most important molecule in
acute allergy and acts by binding to the histamine H1
receptor, which manifests edema, warmth and erythema by causing
vasodilation, increasing vascular permeability and leukocyte
recruitment (6). Additionally,
inflammatory cytokines such as tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-4, affect the chronic inflammatory phase
by enhancing B cell survival or T cell activation (7). Furthermore, nuclear factor (NF)-κB is
an important transcriptional factor in inflammation and activated
NF-κB is able to regulate gene expression of inflammatory cytokine
genes, including TNF-α, IL-1β and IL-4 (8). Finally, rat basophilic leukemia
(RBL)-2H3 cells are suitable for in vitro studies of mast
cell-mediated allergic inflammation, which involves the
degranulation and expression of inflammatory cytokines (9,10).
Angelica dahurica (A. dahurica; Fisch.
ex Hoffm.) Benth. et Hook. f. ex Franch. et Sav (Umbelliferae) is a
plant that belongs to the Angelica genus and is distributed
in Northern and Northeastern China. In Traditional Chinese
Medicine, the roots of A. dahurica have been used to treat
headache, rhinitis, cold and toothache amongst others. Furthermore,
pharmacological studies have shown that it has antimicrobial
(11), hepatoprotective (12), antioxidative (13) and cholinesterase inhibitory
activities (14), and activates
dendritic cells (15) and the
actions of lipolytic hormones (16).
Furthermore, it inhibits the production of prostaglandin
E2 (17) and nitric oxide
(NO) (18,19), and reduces the release of histamine
(20). In addition, the ethanolic
extract of A. dahurica displayed anti-inflammatory effects
by the upregulation of heme oxygenase-1, particularly in mice with
asthma (21,22). Phytochemical investigations have
revealed that there were coumarins (23,24) in
A. dahurica as well as lignans (25), polyacetylene (26) and polysaccharides (15). The aim of the present study was to
search bioactive phytochemicals for the treatment of allergic
inflammatory diseases. Various chemical constituents in A.
dahurica were isolated and relevant effects on allergic
inflammation were evaluated.
Materials and methods
Plant materials
The roots of A. dahurica were purchased from
Bozhou Materia Medica Market (Bozhou, China) in 2012 and identified
at that institution. The voucher specimen (M20120907) was deposited
in The Firs People's Hospital of Jingmen City (Jingmen, China).
Chemicals and reagents
Water was prepared from distilled water by a Milli-Q
system (EMD Millipore, Billerica, MA, USA). Anti-dinitrophenyl
(DNP)-IgE (D8406) and DNP-human serum albumin (D-5059-10) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
media and reagents for the cell culture were supplied by Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). ELISA kits for
TNF-α (H052), IL-1β (H002) and IL-4 (H005) were purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The
luciferase assay system including NF-κB luciferase reporter plasmid
pGL4.32 and the Renilla luciferase reporter vector plasmid
pRL-TK was supplied by Promega Corporation (Madison, WI, USA). The
Lipofectamine® 2000 transfection reagent was purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). The other
solvents employed in the present study were of analytical purity
grade. Sephadex LH-20 was purchased from GE Healthcare Life
Sciences (Chalfont, UK), Toyopearl HW-40C from Tosoh Corp. (Tokyo,
Japan), silica gel from Qingdao Ocean Chemical Co., Ltd. (Qingdao,
China) and octadecylsilane (ODS) was supplied by Daiso Chemical
Co., Ltd. (Osaka, Japan). Porous resin D101 was obtained
from Tianjin Blosorb Biotechnology Co., Ltd. (Tianjin, China).
Extraction and isolation
The dried roots of A. dahurica (10.0 kg) were
ground and extracted with 95% ethanol (20 l each time) under reflux
for 5 h three times. Following evaporation of the solvent under
reduced pressure, the residue was obtained and suspended in water
(5.0 l) followed by successive partition with petroleum ether (PE;
5.0 l each time), ethyl acetate (EA; 5.0 l each time) and
n-butanol (n-BuOH; 5.0 l each time) for three times.
Subsequently, the solvents were removed and three parts were
obtained.
The PE part (110.0 g) was subjected to column
chromatography (CC) on a silica gel eluted with gradient PE/EA
(from 100:0 to 20:80, v/v) and 6 fractions were obtained according
to the thin-layer chromatography (TLC) assay results. Briefly, the
samples in EA were loaded on the TLC plates (Qingdao Jiyida Silica
Reagent Co., Ltd., Qingdao, China) and eluted with PE/EA (70:30,
v/v). The spots were visualized under ultraviolet light. Fraction 3
was further separated by silica gel CC to produce compound 14 (25.0
mg) as colorless needle-like crystals. Fraction 5 was also isolated
on a silica gel CC to obtain compound 13 (10.5 mg), and fraction 6
was subjected to HW-40C and eluted with isocratic dichloromethane
methanol (DCM/MeOH; 3:1, v/v) to produce compound 1 (33.0 mg).
The EA extract (260.0 g) was subjected to silica gel
CC and eluted by gradient DCM/MeOH (from 100:0 to 0:100, v/v).
Furthermore, the eluate was combined into 12 fractions based on the
TLC assay. Fraction 2 was subjected to the HW-40C CC eluted with
isocratic DCM/MeOH (2:1, v/v) and then recrystallized to produce
compounds 4 (33.7 mg) and 6 (15.5 mg). Fractions 3 and 4 were
combined and separated on the silica gel CC with gradient DCM/MeOH
(from 90:0 to 50:50, v/v) to produce four subfractions.
Subfractions 2 and 4 were further purified with Sephadex LH-20
eluted with DCM/MeOH (50:50, v/v) and compounds 1 (81.0 mg) and 2
(63.0 mg) were obtained. Fraction 6 was repeatedly subjected to
silica gel CC with DCM/MeOH as the eluent, and the eluates were
subsequently crystalized to produce compounds 7 (21.0 mg), 5 (18.0
mg) and 11 (11.0 mg). Fraction 8 was disposed by the silica gel CC
with gradient DCM/MeOH and subsequently Sephadex LH-20 CC eluted
with isocratic DCM/MeOH (1:1, v/v) to produce compounds 9 (9.5 mg)
and 10 (13.0 mg). Fraction 9 was purified by the Sephadex LH-20 CC
directly and then preparative TLC to obtain compound 12 (8.5 mg).
Finally, fraction 11 was separated on an ODS CC eluted with
MeOH/H2O (50:50 to 95:5, v/v) and purified by Sephadex
LH-20 CC eluted with MeOH to gain compounds 3 (12.5 mg) and 8 (13.0
mg).
The n-BuOH extract (130.0 g) was subjected to
porous resin D101 CC with gradient
ethanol/H2O (10:90, 30:60, 90:10 and 100:0, v/v) to
produce 4 fractions. Fraction 1 was separated on ODS CC eluted with
MeOH/H2O (from 30:70 to 95:5, v/v) to produce compounds
1 (16.5 mg), 3 (10.5 mg) and 4 (13.0 mg). Furthermore, fraction 2
was also subjected to ODS CC to produce compound 15 (26.0 mg).
The compounds isolated were dissolved in DMSO and
the nuclear magnetic resonance (NMR) spectra were recorded on a
Bruker DRX400 NMR spectrometer (Bruker, Billerica, MA, USA) to
elucidate their chemical structures. Mass spectrometry was
performed on Agilent LC-MS with 1260 LC system and 6400 Series
Triple Quadrupole mass spectrometer equipped with electrospray
ionization (Agilent Technologies, Santa Clara, CA, USA). The
structures were determined through comparing the data obtained from
NMR and MS with those in previously published literature (see
Results).
Cell culture
RBL-2H3 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified 5% CO2
atmosphere.
Determination of released
histamine
As the levels of histamine represented the degree of
mast cell degranulation, the histamine contents in culture media
were measured. The o-phthaldialdehyde spectrofluorometric
procedure was employed as previously described (26). For the IgE-mediated histamine
release, RBL-2H3 cells (1×105 cells/well in a 96-well
plate) were sensitized with anti-DNP IgE in PBS (10 µg/ml) and
incubated at 37°C overnight. Next, the cells were pretreated with
compounds obtained from the plant (20 µM in PBS containing 0.1%
DMSO) or PBS containing 0.1% DMSO at 37°C for 1 h prior to the
challenge with DNP-human serum albumin (HSA; 500 ng/ml). The cells
were then separated from the media by centrifugation at 10,000 ×
g for 5 min at 4°C. Furthermore, the fluorescent intensity
was recorded by a fluorescent plate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA) at an excitation wavelength of 360 nm and an
emission wavelength of 440 nm.
Molecular modeling for screening the
histamine H1 receptor antagonists
Molecular docking is able to provide the visual
detail of the interaction between a ligand and a biomacromolecule.
In the present study the Surflex-Dock Sybyl v2.0 program (Tripos
Inc., St. Louis, MO, USA) was used. 3D structures of the compounds
obtained were prepared by Sybyl sketch and optimized by Tripos
force field. The crystal structure of the histamine H1
receptor was retrieved from the RSCB Protein Data Bank (www.rcsb.org/pdb/home/home.do) (PDB
code: 3RZE), and hydrogen atoms and charges were added. After
removing the water, the docking process was performed (27).
Measurements for levels of TNF-α,
IL-1β and IL-4
The levels of inflammatory cytokines in the media
from RBL-2H3 cells challenged with DNP-HSA were determined by
ELISA. ELISA was performed on a 96-well plate using ELISA kits
according to the manufacturer's instructions. After terminating the
reaction of the substrate through the addition of stop buffer (2N
H2SO4), the absorbance was recorded on a
spectrophotometer at a wavelength of 450 nm.
Dual luciferase reporter assay for
NF-κB activity
RBL-2H3 cells (1×106 cells/well in a
24-well plate) were transfected with both the NF-κB luciferase
reporter plasmid pGL4.32 and the Renilla luciferase reporter
vector plasmid pRL-TK at 100 and 9.6 ng per well, respectively.
Following transfection using lipofectamine® 2000 at 37°C
for 24 h, the medium was replaced with fresh serum-free medium.
Cells were treated with compounds obtained from the plant (20 µM in
PBS containing 0.1% DMSO) or PBS containing 0.1% DMSO before
stimulation. Cells were subsequently washed with PBS twice and
lysed with PLB for 20 min according to the manufacturer's
instructions for the luciferase reporter assay system. The Promega
protocol was run on the GloMax-Multi JR detection system (Promega
Corporation). Briefly, 50 µl luciferase assay reagent was mixed
with 10 µl lysate. The fluorescence intensity of firefly luciferase
was determined. Then 50 µl Stop & Glo® reagent was
added into the mixture and the fluorescence intensity of
Renilla luciferase was measured. Furthermore, the relative
luciferase activity was measured by normalizing the firefly
luciferase activity against the internal control (Renilla
luciferase).
Physicochemical properties of the
compounds
Lipophilicity/hydrophilicity has a strong influence
on the absorption, distribution, metabolism and excretion
properties of pharmacological agents in vivo. Hydrophobic
agents tend to bind to hydrophobic sites whereas hydrophilic ones
favor hydrophilic positions (28).
Therefore, the lipophilicity/hydrophilicity expressed as logp was
theoretically calculated through the physicochemical module of the
Sybyl program while inputting the chemical structures to elucidate
the physicochemical properties of these compounds. Generally, the
values of logp for the drugs are between 2 and 5, which suggests
that the drugs may be easily delivered to binding sites.
The polar surface area (PSA) derived from the Sybyl
program as above is defined as the surface sum over all polar
atoms, primarily oxygen and nitrogen atoms, also including their
attached hydrogen atoms. It is typically used to optimize a drug's
ability to permeate cells. Furthermore, molecules with PSA values
of <140 Å2 tend to be poor at permeating cell
membranes.
Statistical analysis
All values are expressed as the mean ± standard
deviation, and GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used for statistical analysis. Significant
differences of experimental data from different groups were
compared by one way analysis of variance followed by Dunnet's test
for multiple comparisons and Student's t-test for single
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Phytochemical investigation
Phytochemical investigation on the roots of A.
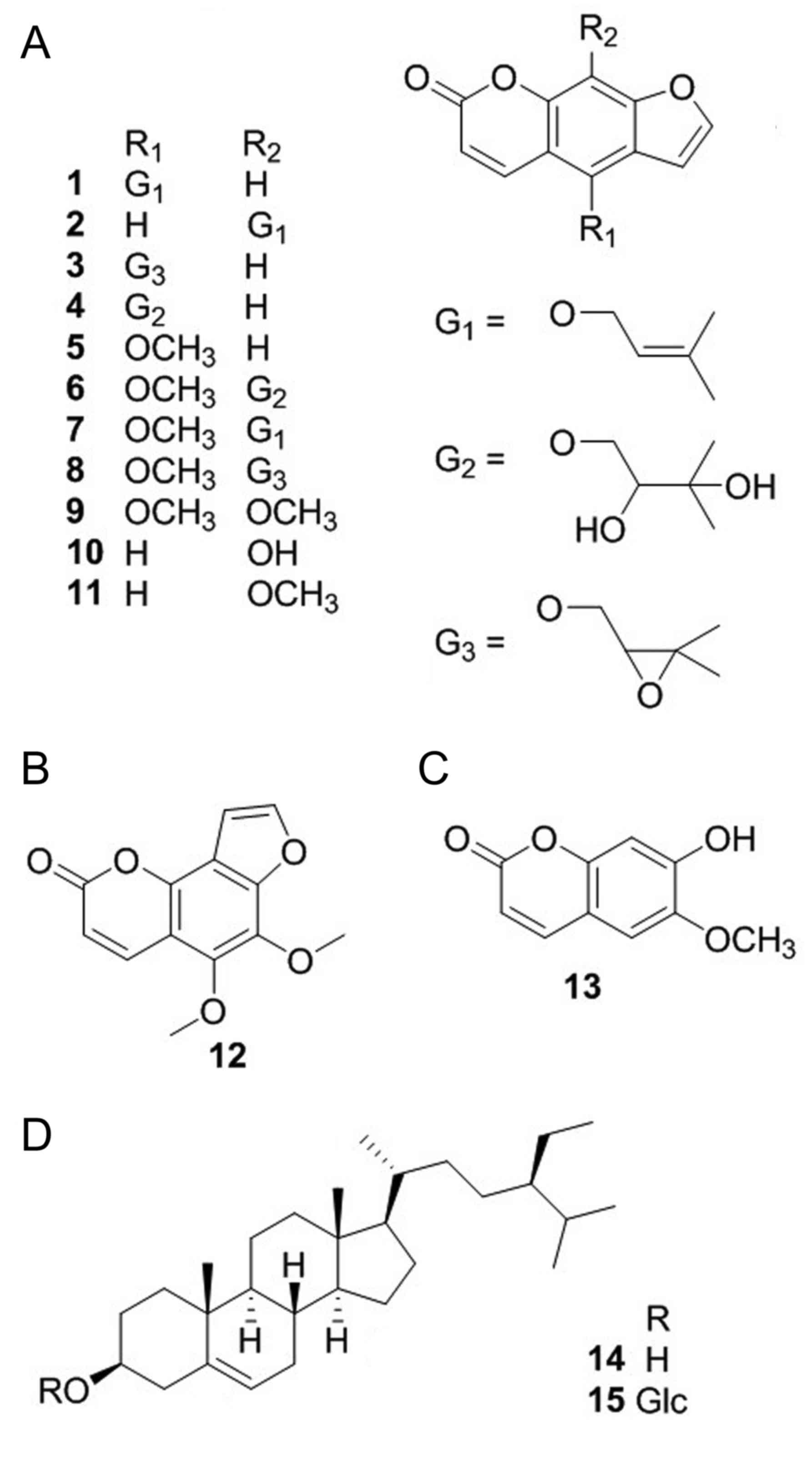
dahurica has led to the isolation of 15 compounds. As shown in
Fig. 1, their structures were
identified as isoimperatorin (1)
(29), imperatorin (2) (29),
oxypeucedanin (3) (30), oxypeucedanin hydrate (4) (29),
bergapten (5) (29), byakangelicin (6) (29),
phellopterin (7) (29), byakangelicol (8) (31),
isopimpinellin (9) (32), xanthotoxol (10) (29),
xanthotoxin (11) (29), pimpinellin (12) (31),
scopoletin (13) (31), β-sitosterol (14) (29)
and daucosterol (15) (29) on the basis of spectra analysis
including 1H-nuclear magnetic resonance (NMR),
13C-NMR and mass spectrometry in combination with the
data comparison from previously published studies (29–32).
Contents of released histamine
The contents of released histamine in the media for
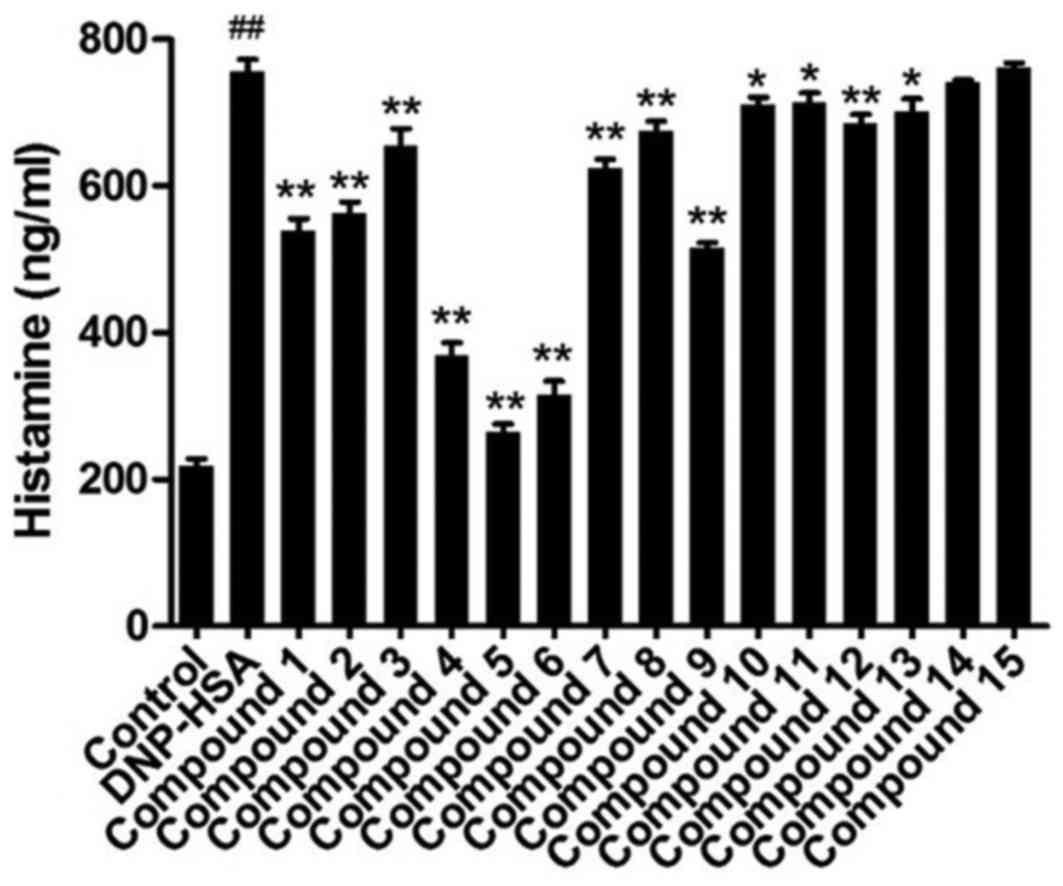
RBL-2H3 cells indicated mast cell degranulation. As shown in
Fig. 2, anti-DNP IgE-sensitized
RBL-2H3 cells released a significantly increased level of histamine
when DNP-HSA was added, compared with control cells. Compounds 1–13
significantly reduced the content of histamine in the media,
compared with DNP-HSA cells, compound 5 induced the greatest
decrease of the three.
Docking studies for the coumarins with
histamine H1 receptor
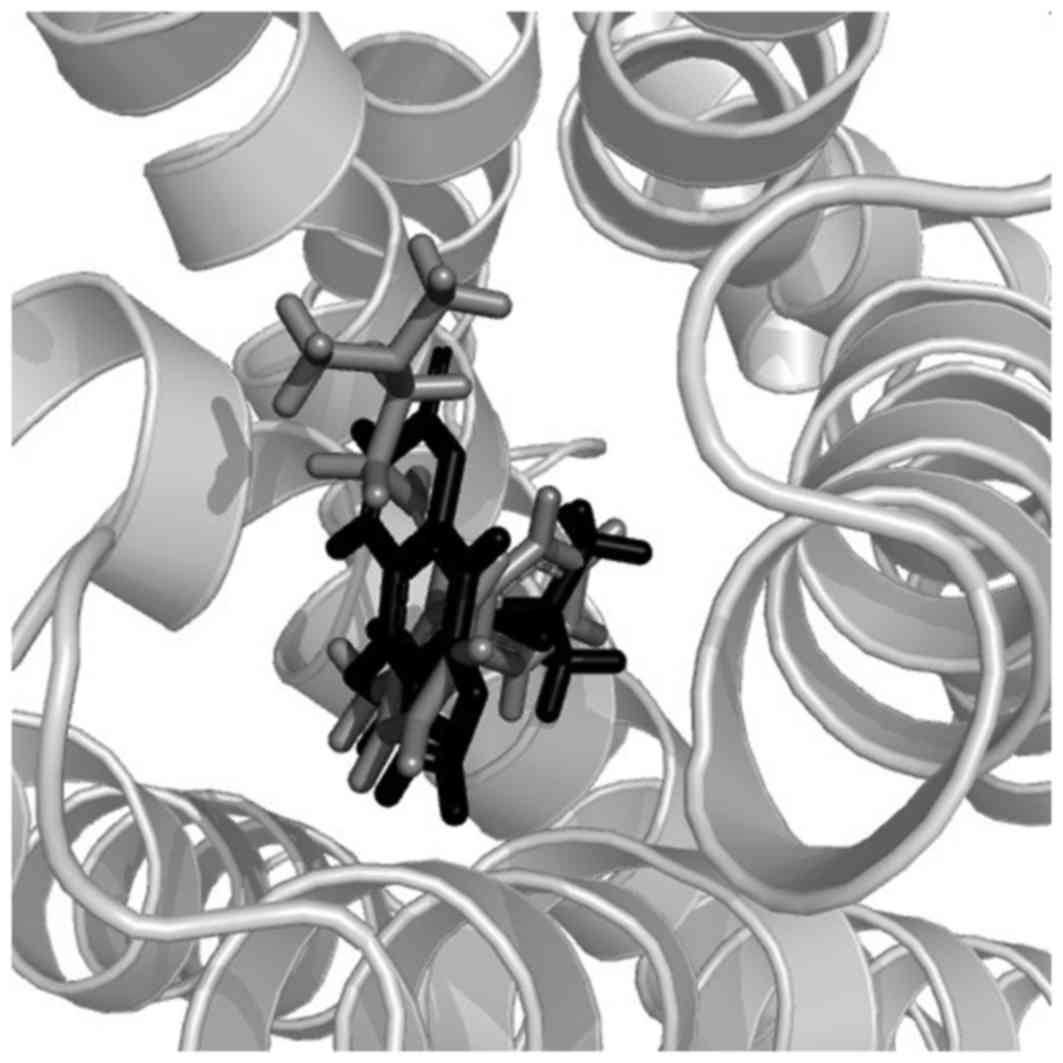
Molecular docking revealed the interaction between
the histamine H1 receptor and isolated coumarins. Out of
all the coumarins, compound 3 exhibited a more potent affinity to
the histamine H1 receptor than the others. Its total
score (a measure of its affinity recorded by the Sybyl program) was
8.46, which was higher than for doxepin (a ligand in the crystal
structure of the histamine H1 receptor) which had a
total score of 7.57. In addition, compounds 1, 2, 4 and 6 exhibited
scores of 6.11, 6.36, 6.60 and 6.60, respectively, whereas the
scores for compounds 7 and 8 were 5.81 and 5.77, respectively. As
shown in Fig. 3, compound 3 is able
to fully enter the binding pocket of histamine H1
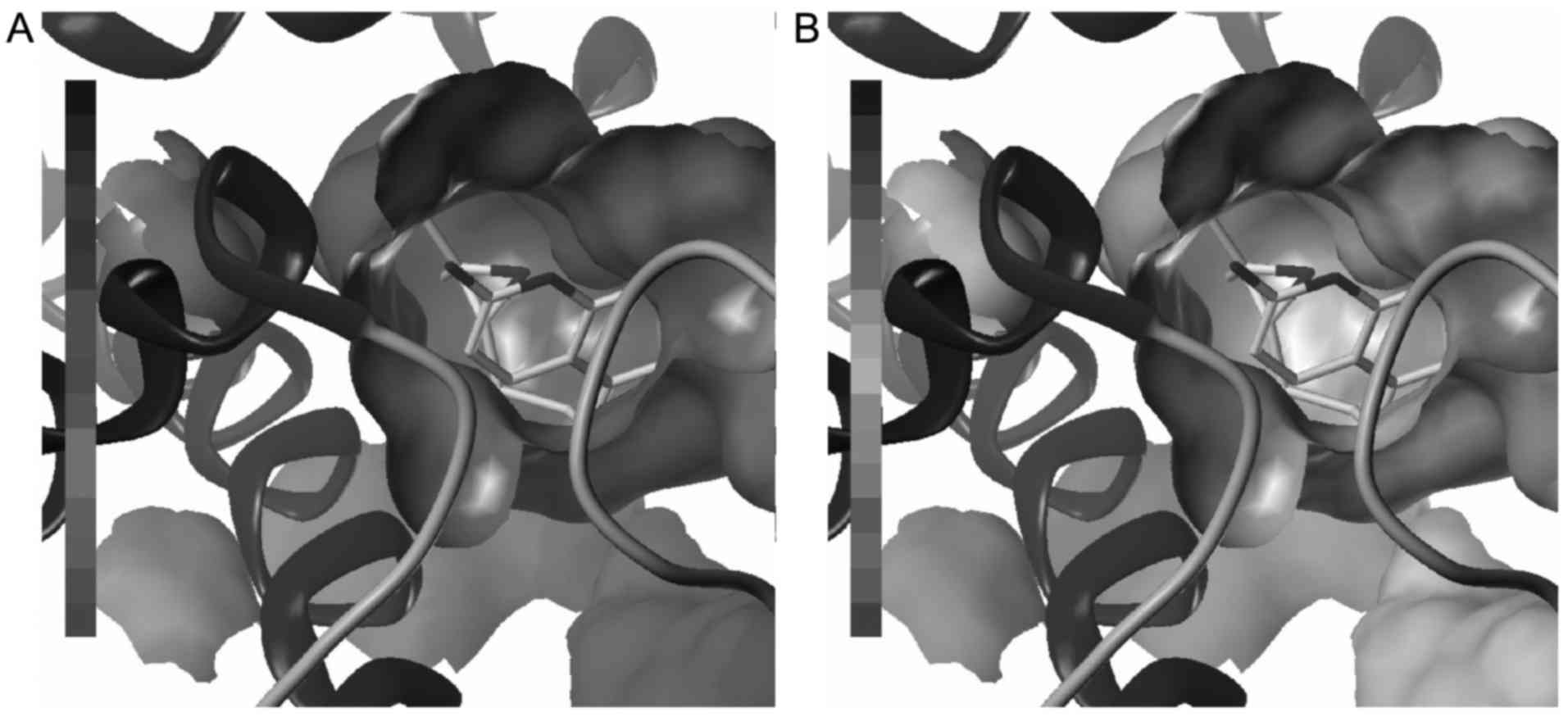
receptor and present a similar pose with doxepin. Hydrophobic and
electrostatic interactions are important for the formation of the
ligand-receptor complex (Fig. 4),
although there are no evident hydrogen bonds between small
molecules and biomacromolecules.
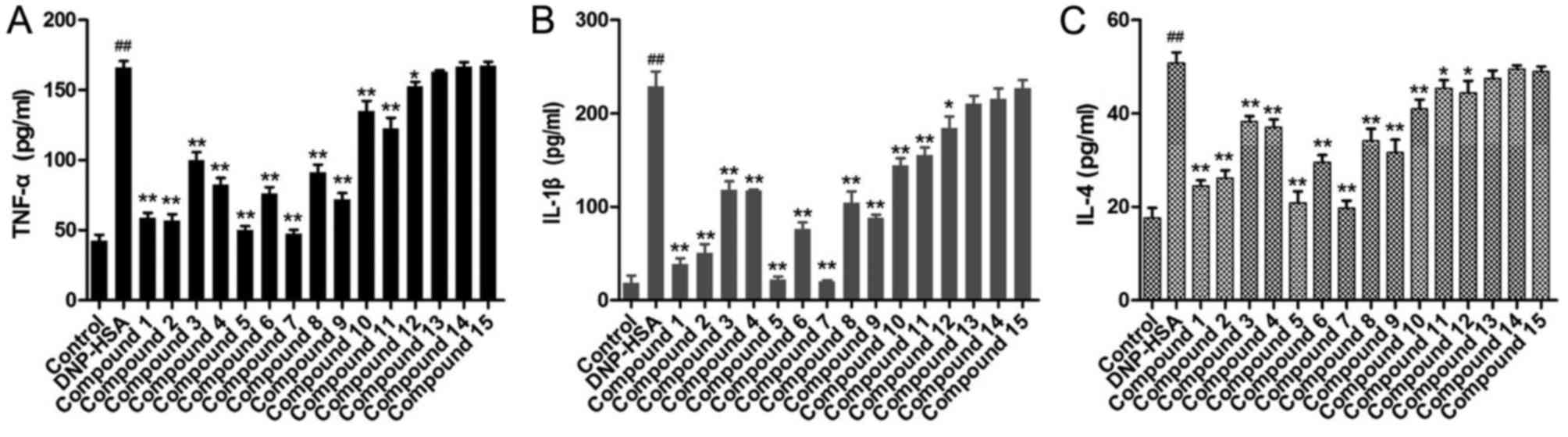
Levels of inflammatory cytokines
To further evaluate the compounds from A.
dahurica on chronic allergic inflammation, the levels of TNF-α,
IL-1β and IL-4 in the media for RBL-2H3 cells were determined
(Fig. 5). Following challenge with
DNP-HSA, the levels of TNF-α, IL-1β and IL-4 in the media
significantly increased compared with control cells. When
pretreated with the obtained compounds, the levels of TNF-α, IL-1β
and IL-4 for compounds 1–12 were significantly decreased compared
with DNP-HSA cells, to different extents. Compounds 1, 2, 5 and 7
induced the greatest decrease in levels of these inflammatory
cytokines. However, compounds 13–15 exhibited no significant
difference on TNF-α, IL-1β and IL-4.
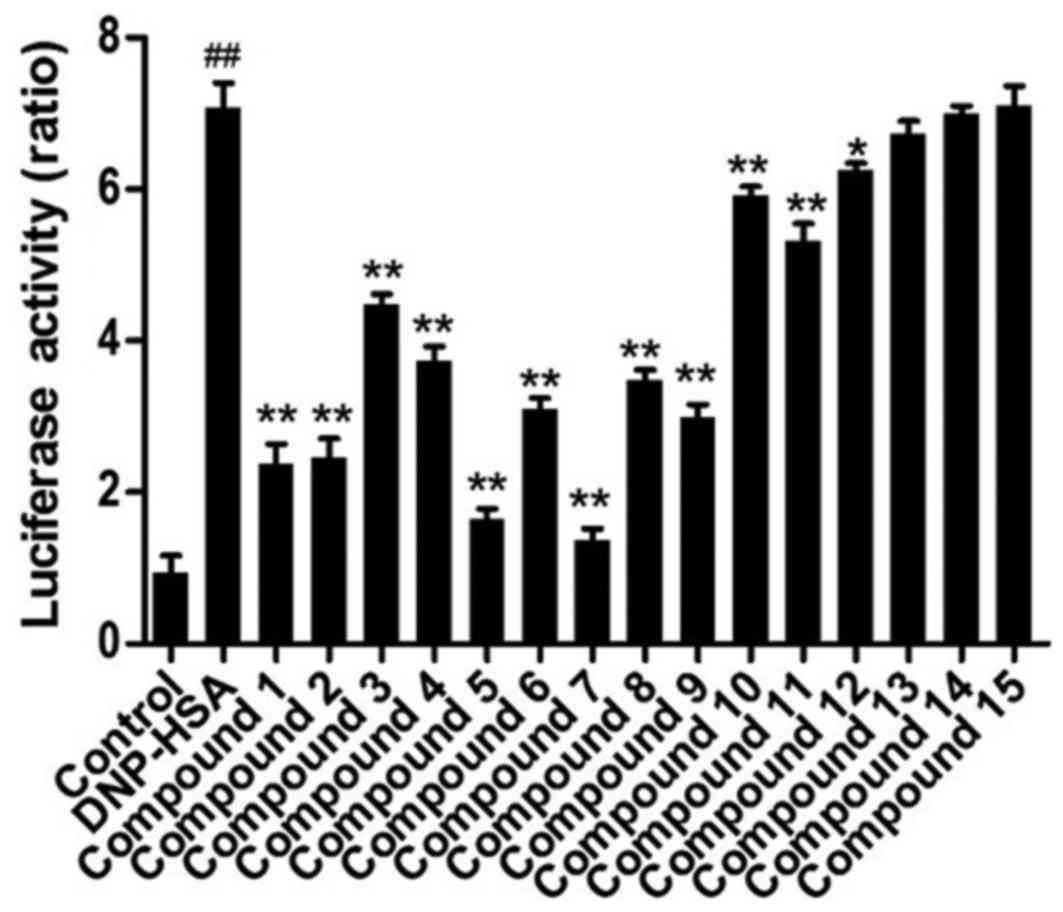
Activation of NF-κB
In order to further confirm the effects of these
compounds on the expression of inflammatory cytokine genes, the
activation of NF-κB was investigated following treatment with these
compounds. As a result, the activation of NF-κB was significantly
increased in RBL-2H3 cells stimulated by DNP-HSA and significantly
ameliorated when compounds 1–12 were administered (Fig. 6). Of all the compounds, compounds 5
and 7 exhibited the greatest potency, followed by compounds 1 and
2.
Physicochemical properties of the
compounds
As shown in Table I,
the values of logp for the identified compounds 1–3, 5 and 7–11
were between 2 and 5. Conversely, the values of compounds 4, 6 and
12–15 were not within that range. In addition, the PSA values of
these compounds were <140 besides compounds 6 and 15. However,
the PSA of compound 15 was markedly higher than that of compound
6.
 | Table I.Physicochemical properties of
compounds isolated from Angelica dahurica. |
Table I.
Physicochemical properties of
compounds isolated from Angelica dahurica.
| Compound | MW (Da) | logp | PSA
(Å2) |
|---|
| 1 | 270.28 | 4.01 | 94.86 |
| 2 | 270.28 | 4.01 | 75.46 |
| 3 | 300.31 | 3.25 | 121.64 |
| 4 | 318.32 | 1.71 | 139.58 |
| 5 | 216.19 | 2.31 | 96.88 |
| 6 | 348.35 | 1.74 | 142.93 |
| 7 | 300.31 | 4.04 | 78.03 |
| 8 | 330.33 | 3.28 | 97.99 |
| 9 | 246.22 | 2.33 | 86.29 |
| 10 | 202.16 | 2.18 | 139.43 |
| 11 | 216.19 | 2.31 | 81.86 |
| 12 | 246.22 | 1.98 | 85.57 |
| 13 | 192.19 | 1.31 | 115.89 |
| 14 | 365.32 | −6.08 | 65.77 |
| 15 | 520.40 | −3.23 | 227.64 |
Discussion
A phytochemical investigation on the roots of A.
dahurica resulted in the identification of 15 compounds. Of all
the compounds, there were 13 coumarins identified. In the
structures of compounds 1–11 the furan ring was fused at the C-6
and C-7 positions of the coumarin scaffold, and at the C-7 and C-8
positions in compound 12 to form furanocoumarins.
Pharmacological evaluation of these compounds has
shown that compounds 1–13 are able to affect the release of
histamine and these results indicate that the constituents in A.
dahurica are able to inhibit the degranulation of mast cells.
Furthermore, compounds 1–12 are able to reduce the secretion of
TNF-α, IL-1β and IL-4. It may be demonstrated that the
6,7-furanocoumarin scaffold is necessary and the methoxyl group at
the C-5 position contributes largely to the decrease of histamine
release, which is in accordance with the in vivo results for
several coumarins of A. dahurica (20). Although isopentane-derived groups are
likely to reduce the inhibition of histamine release, the
2,3-dihydroxyisopentane group has little effects on this.
Similarly, the 6,7-furanocoumarin scaffold is necessary and the
methoxyl group at the C-5 position largely contributes to reduce
the secretion of TNF-α, IL-1β and IL-4, which is in accordance with
the previous results on anti-inflammatory coumarins in A.
dahurica (18,33,34). The
isopentenyl group also shows a positive effect on the secretion of
cytokines, which makes compounds 1 and 2 display potent activity.
In addition, the present study has assessed the inhibitory effects
of these compounds on NF-κB activation. The results are also
consistent with the inhibitory effects on the secretion of TNF-α,
IL-1β and IL-4. Finally, the results imply that these compounds
inhibit expression of inflammatory cytokine genes by decreasing the
activation of NF-κB.
Virtual screening by docking indicates that compound
3 is a potent histamine H1 receptor antagonist together
with some weak ligands to that protein compared with the intrinsic
ligand. This may contribute to the molecular shape of compound 3
and its polarization. In addition, calculated logp and PSA
support the majority of furanocoumarins in A. dahurica that
are able to permeate the cell membrane and be delivered to the
binding sites. Substituted groups also contribute to the
logp values according to their polarities, and groups
affecting the logp values are epoxy isopentenyl,
3,4-dihydroxy isopentenyl, methoxyl and isopentenyl. Similarly, the
substituted groups affect the PSA value of the whole molecule. Of
all these groups, 3,4-dihydroxy isopentanyl contributes mostly to
PSA due to its abundance of oxygen atoms, resulting in compound 4
having a PSA ~140 and that of compound 6 >140. Furthermore, The
PSA of compound 10 is close to 140, which may be attributed to the
intramolecular hydrogen bond between the hydroxyl and neighbor
oxygen.
In conclusion, the present study suggests that
coumarins in A. dahurica are able to alleviate acute allergy
following chronic inflammatory reaction and indicates that
anti-allergic inflammation of this medicinal plant results from the
multiple effects of these compounds.
Acknowledgements
The authors would like to thank Dr Y Wang at Xiamen
University (Xiamen, China) for his assistance in molecular modeling
and calculation.
References
|
1
|
Erb KJ: Can helminths or helminth-derived
products be used in humans to prevent or treat allergic diseases?
Trends Immunol. 30:75–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kraft S and Kinet JP: New developments in
FcepsilonRI regulation, function and inhibition. Nat Rev Immunol.
7:365–378. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castle JD, Guo Z and Liu L: Function of
the t-SNARE SNAP-23 and secretory carrier membrane proteins
(SCAMPs) in exocytosis in mast cells. Mol Immunol. 38:1337–1340.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galli SJ, Kalesnikoff J, Grimbaldeston MA,
Piliponsky AM, Williams CM and Tsai M: Mast cells as ‘tunable’
effector and immunoregulatory cells: Recent advances. Annu Rev
Immunol. 23:749–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kay AB: Allergy and allergic diseases.
First of two parts. New Eng J Med. 344:30–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: Involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharm.
254:56–64. 2011. View Article : Google Scholar
|
|
10
|
Je IG, Kim DS, Kim SW, Lee S, Lee HS, Park
EK, Khang D and Kim SH: Tyrosol suppresses allergic inflammation by
inhibiting the activation of phosphoinositide 3-kinase in mast
cells. PLoS One. 10:e01298292015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwon YS, Kobayashi A, Kajiyama SI, Kawazu
K, Kanzaki H and Kim CM: Antimicrobial constituents of Angelica
dahurica roots. Phytochemistry. 44:887–889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh H, Lee HS, Kim T, Chai KY, Chung HT,
Kwon TO, Jun JY, Jeong OS, Kim YC and Yun YG: Furocoumarins from
Angelica dahurica with heptaprotective activity on tacrine-induced
cytotoxicity in Hep G2 cells. Planta Med. 68:463–464. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Y, Zhang Y, Wang N and He L:
Antioxidant effect of imperatorin from Angelica dahurica in
hypertension via inhibiting NADPH oxidase activation and MAPK
pathway. J Am Soc Hypertens. 8:527–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seo WD, Kim JY, Ryu HW, Kim JH, Han SI, Ra
JE, Seo KH, Jang KC and Lee JH: Identification and characterisation
of coumarins from the roots of Angelica dahurica and their
inhibitory effects against cholinesterase. J Funct Foods.
5:1421–1431. 2013. View Article : Google Scholar
|
|
15
|
Kim HS, Shin BR, Lee HK, Park YS, Liu Q,
Kim SY, Lee MK, Hong JT, Kim Y and Han SB: Dendritic cell
activation by polysaccharide isolated from Angelica dahurica. Food
Chem Toxicol. 55:241–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura Y, Ohminami H, Arichi H, Okuda H,
Baba K, Kozawa M and Arichi S: Effects of various coumarins from
roots of Angelica dahurica on actions of adrenaline, ACTH and
insulin in fat cells. Planta Med. 45:183–187. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ban HS, Lim SS, Suzuki K, Jung SH, Lee S,
Lee YS, Shin KH and Ohuchi K: Inhibitory effects of furanocoumarins
isolated from the roots of Angelica dahurica on prostaglandin E2
production. Planta Med. 69:408–412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng GG, Wei W, Yang XW, Zhang YB, Xu W,
Gong NB, Lü Y and Wang FF: New coumarins from the roots of Angelica
dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO
production in LPS-activated RAW264.7 cells. Fitoterapia.
101:194–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang WQ, Song YL, Zhu ZX, Su C, Zhang X,
Wang J, Shi SP and Tu PF: Anti-inflammatory dimeric furanocoumarins
from the roots of Angelica dahurica. Fitoterapia. 105:187–193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kimura Y and Okuda H: Histamine-release
effectors from Angelica dahurica var. Dahurica root. J Nat Prod.
60:249–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son
JK and Shin HK: Anti-inflammatory activity of Angelica dahurica
ethanolic extract on RAW264.7 cells via upregulation of heme
oxygenase-1. Food Chem Toxicol. 49:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MY, Seo CS, Lee JA, Lee NH, Kim JH, Ha
H, Zheng MS, Son JK and Shin HK: Anti-asthmatic effects of Angelica
dahurica against ovalbumin-induced airway inflammation via
upregulation of heme oxygenase-1. Food Chem Toxicol. 49:829–837.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiao SY, Yao XS and Wang ZY: Coumarins of
the roots of Angelica dahurica. Planta Med. 62:5841996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang J, Zhou L, Sun J, Han J and Guo DA:
Chromatographic fingerprint analysis and characterization of
furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn
technique. J Pharm Biomed Anal. 47:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao XZ, Feng X, Jia XD, Dong YF and Wang
M: Neolignan glycoside from Angelica dahurica. Chin Chem Lett.
18:168–170. 2007. View Article : Google Scholar
|
|
26
|
Lechner D, Stavri M, Oluwatuyi M,
Pereda-Miranda R and Gibbons S: The anti-staphylococcal activity of
Angelica dahurica (Bai Zhi). Phytochemistry. 65:331–335. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao XR, Huo XK, Dong PP, Wang C, Huang
SS, Zhang BJ, Zhang HL, Deng S, Liu KX and Ma XC: Inhibitory
effects of highly oxygenated lanostane derivatives from the fungus
Ganoderma lucidum on P-glycoprotein and α-glucosidase. J Nat Prod.
78:1868–1876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Damu GL, Cui SF, Peng XM, Wen QM, Cai GX
and Zhou CH: Synthesis and bioactive evaluation of a novel series
of coumarinazoles. Bioorg Med Chem Lett. 24:3605–3608. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang B, Xu L, Zou Z and Yang S: Chemical
constituents isolated from Angelica dahurica var. formosana. Chin
Trad Herb Drugs. 36:1132–1137. 2005.
|
|
30
|
Hata K, Kozawa M, Yen K and Kimura Y:
Pharmacognostical studies on umbelliferous plants. XX. Studies on
Chinese drug ‘bvaku-shi’. 5. On the coumarins of the roots of
Angelica formosana Boiss. and A. anomala Lall. Yakugaku Zasshi.
83:611–614. 1963.(In Japanese).
|
|
31
|
Wang MY, Jia MR, Ma YY, Tang SW, Jiang GH
and Li XB: Studies on analgestic components of RadixAngelicae
dahuricae. Chin Pharm J. 40:583–587. 2005.(In Chinese).
|
|
32
|
Sun X, Zhang C, Li J, Feng J, Zhou H, Fu S
and Chen W: Chemical constituents from Peucedanum decursivum. Chin
Trad Herb Drugs. 44:2044–2047. 2013.(In Chinese).
|
|
33
|
Jin MH, Moon TC, Hong TG, Park KM, Son JK
and Chang HW:
5-Methoxy-8-(2-hydroxy-3-buthoxy-3-methylbutyloxy)-psoralen
isolated from Angelica dahurica inhibits cyclooxygenase-2 and
5-lipoxygenase in mouse bone marrow-derived mast cells. Arch Pharm
Res. 31:617–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moon TC, Jin MH, Son JK and Chang HW: The
effects of isoimperatorin isolated from Angelicae dahuricae on
cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived
mast cells. Arch Pharm Res. 31:210–215. 2008. View Article : Google Scholar : PubMed/NCBI
|