Introduction
Knee osteoarthritis (KOA), also known as
degenerative osteoarthropathy, is a group of heterogeneous diseases
caused by integrity failure of articular cartilage and lesions of
the subchondral bone plate of articular cartilage (1,2).
According to the joint distribution, KOA can be divided into local
KOA and general KOA. KOA is characterized by joint pain, joint
stiffness, limitation of motion and is caused by friction noise of
joint motion (3). This disease is
mostly found in middle-aged people and its incidence increases with
age (4), posing a threat to the
health and quality of life of middle-aged and elderly people. The
pathogenesis of KOA has not been fully elucidated, but it is
generally believed that KOA is primarily caused by biomechanical
and genetic factors, as well as exogenous factors, including
traumatism, bacterial infection or gene mutation, which cause
changes in the metabolism of the synovial membrane, cartilage and
bone and generate inflammation and structural damage (5,6).
Risk factors of KOA include being female, increased
age, obesity, a genetic history of KOA and trauma. However, the
specific pathogenesis remains unclear. It has previously been
reported that adipocytokines secreted by adipocytes and
inflammatory factors serve an important role in the development of
KOA (7). Previous studies have found
that KOA can increase the production of pro-inflammatory cytokines
and adipocytokines, including interleukin (IL)-6, IL-17, tumor
necrosis factor (TNF)-α and other inflammatory cytokines (8,9). It has
also been reported that the occurrence of KOA is not a simple
degenerative change of articular cartilage involving
non-inflammatory factors: Obesity-induced metabolic inflammation
and inflammatory cytokines serve a key role in the pathogenesis of
KOA (10).
Nuclear factor (NF)-κB is a protein complex that can
bind specifically to the immunoglobulin K-chain gene enhancer
sequence (11). As the central
transcription factor of inflammation and immune reactions, NF-κB
can be activated by IL-1, TNF-α and other cytokines and quickly
induces the expression of multiple genes through a series of
reactions that mainly involve cytokines, inflammatory enzymes and
matrix metalloproteinases (12). It
has been indicated that the signal transduction pathway of NF-κB in
chondrocytes is activated in a rat model of osteoarthritis
(13).
Sivelestat sodium hydrate is a specific inhibitor of
neutrophil elastase that was approved for use in Japan in 2002 and
is clinically used for patients with acute lung injury accompanied
by systematic systemic inflammatory response syndrome (14). However, previous studies have
indicated that sivelestat sodium hydrate can also protect the
heart, lung, liver, kidney, nerve tissue, spinal cord tissue and
other organs from functional injury (15,16). The
goal of the current study was to investigate whether sivelestat
sodium hydrate improves post-traumatic KOA through NF-κB in a rat
model.
Materials and methods
Animals and study design
Ten-week-old male Sprague-Dawley rats (300–325 g;
10–12 weeks old; n=30) were caged in pairs in a ventilated animal
room at a controlled temperature (20–25°C) and humidity (40–60%),
with a 12 h light/dark cycle. They were provided with food and
water ad libitum. Rats were randomized into three groups
(n=8 per group): A sham group, KOA model group and sivelestat
sodium hydrate (ONO-5046) group. Rats from the sham group received
normal saline (500 µl) via intraperitoneal (ip) injection. KOA
model rats from the model group received normal saline (ip). KOA
model rats from the ONO-5046 group received 10 mg/kg/once weekly
ONO-5046 (ip; Sigma-Aldrich; Merck kGaA, Darmstadt, Germany) for 4
weeks. Ethical approval was received from the Medical Ethics
Committee of Weihai Central Hospital (Weihai, China).
Study design
In order to establish a KOA model, rat was
anesthetized using 35 mg/kg pentobarbital (Sigma-Aldrich) the right
medial meniscotibial ligaments were cut and attention was paid not
to injure the articular cartilage during the procedure, as
previously described (2).
Post-operation, animals were allowed unrestricted activity, ad
libitum access to food and water, and housed were under
standard conditions (20–25°C and 40–60% humidity). The right hind
knee joints from the sham group were sham-operated using the same
approach without inducing medial meniscotibial ligament injury.
Analysis of structural joint
changes
After rats were anesthetized using 35 mg/kg
pentobarbital (Sigma-Aldrich; Merck KGgA), rats were sacrificed via
decollation, the limbs (0.5 cm) were harvested from all three
groups, washed with PBS, and embedded in paraffin following
fixation for 72 h using 4% paraformaldehyde at room temperature and
decalcification using 10% formaldehyde. Right knee joints were cut
into serial frontal sections (5-µM thick) and stained using 0.04%
toluidine blue from six different depths spanning 0.4 µm of the
joint. Structural joint changes were assessed using the
Osteoarthritis Research Society International for cartilage
degeneration score (17).
General exploratory motor
behavior
Each rat underwent an open field test (AccuScan
Instruments, Omnitech Electronics, Inc., Columbus, OH, USA) in a
transparent Plexiglas cage (height, 33 cm; width, 42 cm; length, 42
cm) for 30 min. This assessment was done after sivelestat sodium
hydrate or control treatment. A total of 18 different variables of
exploratory motor behavior were assessed, including frequency and
duration of horizontal, sedentary, stereotypic, revolution movement
and vertical activities. Vertical episode count was one of 18
variables used to assess dynamic pain-related behavior.
Static weight bearing
In a conventional restrainer and separate
transducers, rats were habituated to a relatively static position
and the average weight on each hindlimb over 5 sec was recorded for
five trials. Between the left (contralateral control) and right
(ipsilateral) hindlimbs, changes in the hind paw weight bearing
distribution were utilized as an index of joint pain-like symptoms
in the knees that had undergone surgery. KOA pain and percentage of
ipsilateral weight bearing was subsequently calculated as weight on
the ipsilateral hind limb divided by weight on both hind limbs
multiplied.
Determination of TNF-α and IL-6
production, high mobility group box 1 (HMGB1) secretion and
nitrite/nitrate DNA binding activity of NF-κB p50/p65
Blood was collected at 4 weeks after treatment with
sivelestat sodium hydrate while rats were under anesthesia (35
mg/kg pentobarbital) and centrifuged at 12,000 × g for 10 min at
4°C. The supernatant was collected and used to determine the levels
of TNF-α (EM010-96) and IL-6 (EM004-96) production and using
commercial enzyme-linked immunosorbent assay (ELISA; ExCell Bio,
Taichang, China) kits and an ELISA reader (Bio-Rad Laboratories,
Inc.) at 405 nm. Nitrite concentrations were measured using a
commercial kit (A038; Nanjing Jiancheng Biology Engineering
Institute, Nanjing, China) and an ELISA reader (Bio-Rad
Laboratories, Inc.) at 540 nm. HMGB1 secretion were measured using
a commercial kit (E-EL-R0505c; Elabscience Biotechnology Co., Ltd.,
Wuhan, China) at 450 nM.
Western blot analysis
Arthrosis tissue samples were collected at 4 weeks
after treatment with sivelestat sodium hydrate and homogenized
using a radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Nanjing China). The supernatant was
collected following centrifugation at 12,000 × g for 10 min at 4°C
and protein concentration was determined using BCA assay (Beyotime
Institute of Biotechnology, Nanjing China). Proteins (50 µg) were
separated on a 10% SDS polyacrylamide gel and transferred to a
polyvinylidene difluoride membrane. The membranes were incubated
overnight at 4°C with anti-inducible nitric oxide synthase (iNOS;
sc-8309; 1:500), anti-NF-κB (sc-109; 1:500), anti-phosphorylated
inhibitor of κB (p-IkB; sc-7977; 1:500) and anti-β-actin (sc-7210;
1:2,000) (all from Santa Cruz Biotechnology) following blocking
with 5% skim milk powder at 37°C for 1 h. Membranes were then
washed twice with TBS with 0.1% Tween-20 and incubated for 1 h with
peroxidase-conjugated secondary antibodies (7074; 1:5,000; Cell
Signaling Technology, Inc.) at 37°C. Protein was measured using
Bio-Rad Laboratories 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean using SPSS.17.0 (SPSS, Inc., Chicago, IL, USA). Data
were analyzed using the Mann-Whitney U test for comparison between
two independent groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sivelestat sodium hydrate suppresses
structural changes of the joint in KOA rats
The chemical structure of sivelestat sodium hydrate
is presented in Fig. 1. Structural
changes of the joint were assessed in the sham, model and ONO-5046
groups (Fig. 2). Cartilage
degeneration in the model group was significantly greater than that
of the sham group (P<0.01). However, following 4 weeks
treatment, cartilage degeneration was significantly reduced in the
ONO-5046 group compared with the model group (both P<0.01;
Fig. 2).
Sivelestat sodium hydrate increases
vertical episode count in KOA rats
At 4 weeks after treatment, vertical episode count
of the joint was significantly lower in the model group compared
with the sham group (P<0.01). However, treatment with sivelestat
sodium hydrate significantly increased the vertical episode count
in KOA rats compared with the model group (P<0.01; Fig. 3).
Sivelestat sodium hydrate increases
ipsilateral static weight bearing of the joint in KOA rats
At 4 weeks after treatment, ipsilateral static
weight bearing of the joint was significantly reduced in the KOA
model group rats compared with the sham group (P<0.01).
Treatment with sivelestat sodium hydrate significantly increased
the ipsilateral static weight bearing of the joint compared with
the model group (P<0.01; Fig.
4).
Sivelestat sodium hydrate suppresses
TNF-α and IL-6 production in the joint of KOA rats
To investigate whether sivelestat sodium hydrate
affects inflammation in KOA rats, TNF-α and IL-6 production was
measured by ELISA. There was a significant increase in both TNF-α
and IL-6 production in the model group compared with the sham
control group (P<0.01). However, administration of sivelestat
sodium hydrate significantly inhibited TNF-α and IL-6 production
compared with KOA model rats (P<0.01; Fig. 5).
Sivelestat sodium hydrate suppresses
serum nitrite levels and iNOS protein expression in the joints of
KOA rats
The effect of sivelestat sodium hydrate on serum
nitrite levels and iNOS protein expression in the joints of KOA
rats was analyzed using ELISA and western blotting, respectively.
Serum nitrite levels and iNOS protein expression in KOA model rats
were significantly higher compared with the sham group (both
P<0.01). However, sivelestat sodium hydrate administration
significantly reduced the serum nitrite level and iNOS protein
expression, compared with the model group (both P<0.01; Fig. 6).
Sivelestat sodium hydrate suppresses
HMGB1 secretion in the joints of KOA rats
To investigate the effect of sivelestat sodium
hydrate on HMGB1 secretion in the joints of KOA rats, HMGB1
secretion was detected by ELISA. There was a significant increase
in HMGB1 secretion in the model group as compared with the sham
group (P<0.01). Following treatment with sivelestat sodium
hydrate, HMGB1 secretion was significantly suppressed compared with
the model group (P<0.01; Fig.
7).
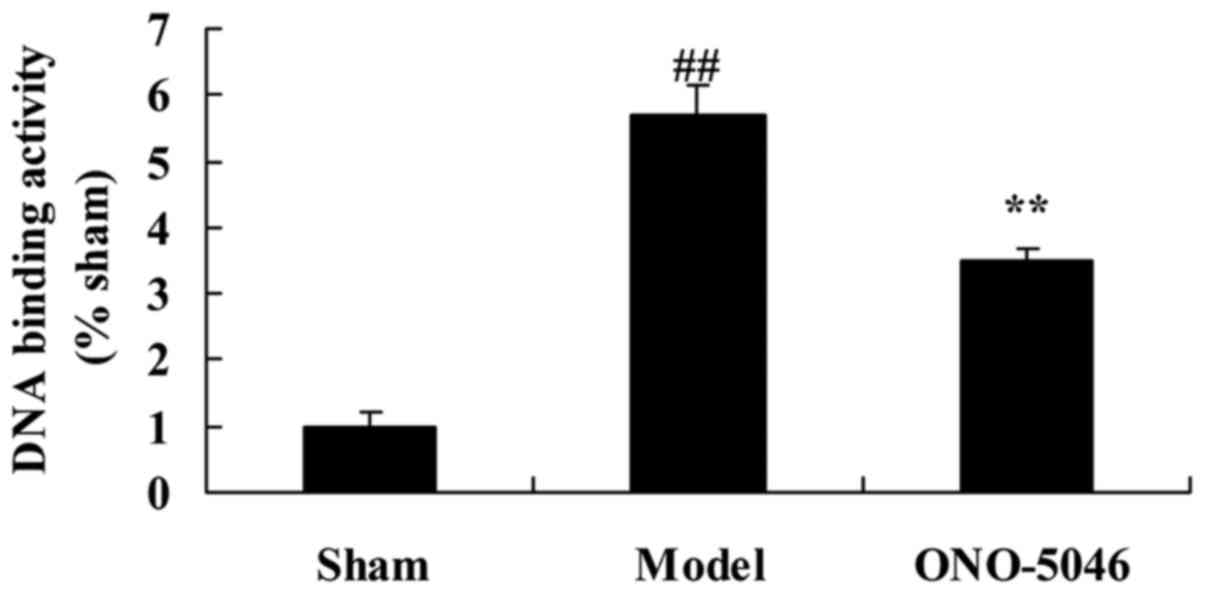
Sivelestat sodium hydrate suppresses
p50/p65 DNA binding activity in the joints of KOA rats
To investigate the effect of sivelestat sodium
hydrate on p50/p65 DNA binding activity in the joints of KOA rats,
p50/p65 DNA binding activity was evaluated by ELISA. The level of
p50/p65 DNA binding activity was significantly increased in the
model group compared with the sham group (P<0.01). By contrast,
p50/p65 DNA binding activity in KOA rats treated with sivelestat
sodium hydrate was significantly inhibited, compared with the model
group (P<0.01; Fig. 8).
Sivelestat sodium hydrate suppresses
NF-κB protein expression in the joints of KOA rats
To examine the anti-inflammation effect of
sivelestat sodium hydrate on KOA, NF-κB protein expression was
analyzed using western blotting. NF-κB protein expression was
significantly increased in the model group compared with the sham
group (P<0.01). However, treatment with sivelestat sodium
hydrate significantly reduced KOA-induced NF-κB protein expression
in KOA rats compared with the model group (P<0.01; Fig. 9).
Sivelestat sodium hydrate suppresses
p-IκB protein expression in the joints of KOA rats
To further analyze the anti-inflammation effect of
sivelestat sodium hydrate on NF-κB, p-IκB protein expression was
detected using western blotting. The results indicated that p-IκB
protein expression was significantly higher in the model group
compared with the sham group (P<0.01). Following sivelestat
sodium hydrate treatment, p-IκB protein expression in KOA rats was
significantly suppressed compared with the model group (P<0.01;
Fig. 10).
Discussion
KOA is a degenerative disease characterized by the
progressive loss of cartilage accompanied by subchondral bone
destruction, marginal osteophyte formation and joint space
narrowing (18). According estimates
from the World Health Organization, the prevalence of symptomatic
KOA among people >60 years old worldwide is 9.6% for males and
18% for females, and the disability rate may be as high as 53%
(19). KOA tends to develop in
joints that undergo heavy weight bearing and continuous activity
(20). The results of the current
study indicated that treatment with sivelestat sodium hydrate
significantly inhibits the induction of structural changes,
increases vertical episode count and increases ipsilateral static
weight bearing of the joint in KOA rats.
KOA-related cytokines include the inflammatory
cytokines, which mediate matrix damage and inhibit the
proliferation of chondrocytes, such as IL-1β, TNF-α and nitric
oxide (NO) (21). IL-1 serves an
important role in the pathogenesis of KOA; it is one of the most
influential cytokines leading to the functional decline of
cartilage (22). IL-1β and IL-6 also
has a wide range of biological effects that may act on a localized
area or affect the whole body, and it is involved in inflammatory
reactions (23). In acute and
chronic inflammation, IL-1 may cause systemic symptoms, such as
fever, muscle consumption and the synthesis of proteins in the
acute phase, and local tissue inflammation and destructive lesions
(24). In the current study, it was
demonstrated that administration of sivelestat sodium hydrate in
rats with KOA significantly inhibited TNF-α and IL-6 production
compared with model rats. Yoshikawa et al (25) reported that sivelestat sodium hydrate
reduced radiation-induced lung injury in mice by inhibiting
neutrophil elastase activity and excessive inflammatory reactions.
Therefore, sivelestat sodium hydrate improves post-traumatic KOA
and may also have anti-inflammatory properties.
At present, the pathogenesis of KOA remains unclear,
but it has been confirmed that NO serves an important role in the
development of KOA (26). NO is an
important regulatory factor involved in the differentiation and
apoptosis of articular cartilage cells (27). It can inhibit the proliferation of
chondrocytes, induce the apoptosis of chondrocytes, inhibit the
synthesis of proteoglycan and type II collagen by chondrocytes and
promote the decomposition of cartilage matrix (27). In KOA, iNOS is the key enzyme that
induces the production of NO (26).
The current data suggests that sivelestat sodium hydrate
significantly reduces serum nitrite levels and iNOS protein
expression in KOA rats. Hagiwara et al (14) demonstrated that sivelestat sodium
hydrate reduces levels of inflammatory mediators by inhibiting
NF-κB.
NF-κB is a central transcription factor involved in
inflammatory and immune reactions. It usually exists in the
cytoplasm in the form of a p50-p65 heterodimer, which is
predominantly in an inactivated state, bound to the inhibitory
protein IκB (28). Under appropriate
stimuli, NF-κB in the cytoplasm dissociates from IκB and enters the
cell nucleus, a process known as nuclear translocation (29,30).
NF-κB can be activated by many factors, including TNFs and ILs.
Once in the cell nucleus, activated NF-κB binds with the specific
sequences of the promoter/enhancer regions of target genes and
regulates gene expression (31,32). The
activated NF-κB signal transduction pathway may lead to the
transcription of a series of downstream target genes, which trigger
biological effects including the production of inflammatory factors
and the induction of chondrocyte apoptosis (13). The results of the present study also
indicated that sivelestat sodium hydrate suppresses p50/p65 DNA
binding activity, NF-κB and p-IkB protein expression in the joints
of rats with post-traumatic KOA. This is in accordance with a study
by Hagiwara et al (33),
which demonstrated that sivelestat sodium hydrate reduces the
levels of inflammatory mediators by inhibiting NF-κB.
HMGB1 is a type of alarmin that can activate the
innate immunity and acquired immune response and is involved in the
development of many diseases (34).
Binding between the DNA-binding domain of HMGB1 in the cell nucleus
and a minor groove in the double helix can cause DNA structural
changes that affect DNA transcription, repair, replication and
nucleosome stability (35).
Extracellular HMGB1 secretion may promote an inflammatory reaction
by binding to the HMGB1 receptor (36). It has previously been demonstrated
that extracellular HMGB1 is a potential induction factor of
inflammation, and HMGB1 monoclonal antibodies are effective at
treating hepatonecrosis caused by septicopyemia, stroke and other
conditions (37,38). Furthermore, it has been reported that
arthritis can be induced by injecting HMGB1 into rat joints, and
that HMGB1-targeted treatment can effectively prevent the
development of arthritis in experimental animal models (35). The current findings indicate that
treatment with sivelestat sodium hydrate significantly suppresses
HMGB1 secretion in KOA rats compared with the model group. In
addition, Hagiwara et al (14) reported that sivelestat sodium hydrate
reduces lung injury following endotoxin-induced shock through
suppression of HMGB1 in rats.
In conclusion, the results of the current study
indicate that sivelestat sodium hydrate inhibits the induction of
structural changes, increases vertical episode count and
ipsilateral static weight bearing of the joint in KOA rats, and may
exert an anti-inflammatory effect due to inhibition of HMGB1 and
NF-κB, as well as NO secretion. Further studies will be required to
elucidate whether sivelestat sodium hydrate induces an
anti-inflammatory effect on KOA or other diseases.
References
|
1
|
Provenza JR, Shinjo SK, Silva JM, Peron CR
and Rocha FA: Combined glucosamine and chondroitin sulfate, once or
three times daily, provides clinically relevant analgesia in knee
osteoarthritis. Clin Rheumatol. 34:1455–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glasson SS, Blanchet TJ and Morris EA: The
surgical destabilization of the medial meniscus (DMM) model of
osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage.
15:1061–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tok F, Aydemir K, Peker F, Safaz I,
Taskaynatan MA and Ozgül A: The effects of electrical stimulation
combined with continuous passive motion versus isometric exercise
on symptoms, functional capacity, quality of life and balance in
knee osteoarthritis: Randomized clinical trial. Rheumatol Int.
31:177–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thorp LE, Wimmer MA, Block JA, Moisio KC,
Shott S, Goker B and Sumner DR: Bone mineral density in the
proximal tibia varies as a function of static alignment and knee
adduction angular momentum in individuals with medial knee
osteoarthritis. Bone. 39:1116–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trombini-Souza F, Kimura A, Ribeiro AP,
Butugan M, Akashi P, Pássaro AC, Arnone AC and Sacco IC:
Inexpensive footwear decreases joint loading in elderly women with
knee osteoarthritis. Gait Posture. 34:126–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi T, Yoshihara Y, Yamada H and
Fujikawa K: Procollagen IIC-peptide as a marker for assessing
mechanical risk factors of knee osteoarthritis: Effect of obesity
and varus alignment. Ann Rheum Dis. 59:982–984. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sundman EA, Cole BJ, Karas V, Valle C
Della, Tetreault MW, Mohammed HO and Fortier LA: The
anti-inflammatory and matrix restorative mechanisms of
platelet-rich plasma in osteoarthritis. Am J Sports Med. 42:35–41.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clockaerts S, Bastiaansen-Jenniskens YM,
Feijt C, Verhaar JA, Somville J, De Clerck LS and Van Osch GJ:
Peroxisome proliferator activated receptor alpha activation
decreases inflammatory and destructive responses in osteoarthritic
cartilage. Osteoarthritis Cartilage. 19:895–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Liu J, Wu DI, Pang X, Zhao J and
Zhang X: Pro-inflammatory effect of fibrinogen on vascular smooth
muscle cells by regulating the expression of PPARα, PPARγ and
MMP-9. Biomed Rep. 3:513–518. 2015.PubMed/NCBI
|
|
10
|
Bevers K, Zweers MC, Vriezekolk JE,
Bijlsma JW and den Broeder AA: Are ultrasonographic signs of
inflammation predictors for response to intra-articular
glucocorticoids in knee osteoarthritis? Clin Exp Rheumatol.
32:930–934. 2014.PubMed/NCBI
|
|
11
|
Shi C, Zhu X, Wang J and Long D:
Intromitochondrial IκB/NF-κB signaling pathway is involved in
amyloid β peptide-induced mitochondrial dysfunction. J Bioenerg
Biomembr. 46:371–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hagiwara S, Iwasaka H, Togo K and Noguchi
T: A neutrophil elastase inhibitor, sivelestat, reduces lung injury
following endotoxin-induced shock in rats by inhibiting HMGB1.
Inflammation. 31:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishiyama J, Matsuda M, Ando S, Hirasawa
M, Suzuki T and Makuuchi H: The effects of the early administration
of sivelestat sodium, a selective neutrophil elastase inhibitor, on
the postoperative course after radical surgery for esophageal
cancer. Surg Today. 42:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masaoka N, Watanabe M and Nakajima Y: The
effects of sivelestat sodium hydrate on uterine contraction and the
concentration of maternal and fetal blood cytokines in a sheep
model of intra-amniotic infection induced by lipopolysaccharide. J
Matern Fetal Neonatal Med. 24:1013–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamilton CB, Pest MA, Pitelka V,
Ratneswaran A, Beier F and Chesworth BM: Weight-bearing asymmetry
and vertical activity differences in a rat model of post-traumatic
knee osteoarthritis. Osteoarthritis Cartilage. 23:1178–1185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helminen EE, Sinikallio SH, Valjakka AL,
Väisänen-Rouvali RH and Arokoski JP: Effectiveness of a
cognitive-behavioural group intervention for knee osteoarthritis
pain: A randomized controlled trial. Clin Rehabil. 29:868–881.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bodick N, Lufkin J, Willwerth C, Kumar A,
Bolognese J, Schoonmaker C, Ballal R, Hunter D and Clayman M: An
intra-articular, extended-release formulation of triamcinolone
acetonide prolongs and amplifies analgesic effect in patients with
osteoarthritis of the knee: A randomized clinical trial. J Bone
Joint Surg Am. 97:877–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bezalel T, Carmeli E and Katz-Leurer M:
The effect of a group education programme on pain and function
through knowledge acquisition and home-based exercise among
patients with knee osteoarthritis: A parallel randomised
single-blind clinical trial. Physiotherapy. 96:137–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bingham CO III, Sebba AI, Rubin BR, Ruoff
GE, Kremer J, Bird S, Smugar SS, Fitzgerald BJ, O'Brien K and
Tershakovec AM: Efficacy and safety of etoricoxib 30 mg and
celecoxib 200 mg in the treatment of osteoarthritis in two
identically designed, randomized, placebo-controlled,
non-inferiority studies. Rheumatology (Oxford). 46:496–507. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyer KA, Angst MS, Asay J, Giori NJ and
Andriacchi TP: Sensitivity of gait parameters to the effects of
anti-inflammatory and opioid treatments in knee osteoarthritis
patients. J Orthop Res. 30:1118–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian CF, Lv FH, Wang M and Gu XS: Serum
β-human chorionic gonadotropin and interleukin-1 as diagnostic
biomarkers for the premature rupture of membranes and
chorioamnionitis. Biomed Rep. 2:905–909. 2014.PubMed/NCBI
|
|
24
|
Simao AP, Avelar NC, Tossige-Gomes R,
Neves CD, Mendonça VA, Miranda AS, Teixeira MM, Teixeira AL,
Andrade AP, Coimbra CC and Lacerda AC: Functional performance and
inflammatory cytokines after squat exercises and whole-body
vibration in elderly individuals with knee osteoarthritis. Arch
Phys Med Rehabil. 93:1692–1700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshikawa N, Inomata T, Okada Y, Shimbo T,
Takahashi M, Akita K, Uesugi Y and Narumi Y: Sivelestat sodium
hydrate reduces radiation-induced lung injury in mice by inhibiting
neutrophil elastase. Mol Med Rep. 7:1091–1095. 2013.PubMed/NCBI
|
|
26
|
More AS, Kumari RR, Gupta G, Lingaraju MC,
Balaganur V, Pathak NN, Kumar D, Kumar D, Sharma AK and Tandan SK:
Effect of iNOS inhibitor S-methylisothiourea in monosodium
iodoacetate-induced osteoathritic pain: Implication for
osteoarthritis therapy. Pharmacol Biochem Behav. 103:764–772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jarvinen K, Vuolteenaho K, Nieminen R,
Moilanen T, Knowles RG and Moilanen E: Selective iNOS inhibitor
1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10
in OA cartilage. Survey of the effects of 1400W on inflammatory
mediators produced by OA cartilage as detected by protein antibody
array. Clin Exp Rheumatol. 26:275–282. 2008.PubMed/NCBI
|
|
28
|
Hu H, Yang B, Li Y, Zhang S and Li Z:
Blocking of the P2X7 receptor inhibits the activation of the MMP-13
and NF-κB pathways in the cartilage tissue of rats with
osteoarthritis. Int J Mol Med. 38:1922–1932. 2016.PubMed/NCBI
|
|
29
|
Neurath MF, Becker C and Barbulescu K:
Role of NF-kappaB in immune and inflammatory responses in the gut.
Gut. 43:856–860. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshikawa H, Kurokawa M, Ozaki N, Nara K,
Atou K, Takada E, Kamochi H and Suzuki N: Nicotine inhibits the
production of proinflammatory mediators in human monocytes by
suppression of I-kappaB phosphorylation and nuclear factor-kappaB
transcriptional activity through nicotinic acetylcholine receptor
alpha7. Clin Exp Immunol. 146:116–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Choi J and Kim S: Effective
suppression of pro-inflammatory molecules by DHCA via IKK-NF-κB
pathway, in vitro and in vivo. Br J Pharmacol. 172:3353–3369. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng CC, Chen YH, Chang WL, Yang SP,
Chang DM, Lai JH and Ho LJ: Phytoestrogen bavachin mediates
anti-inflammation targeting Ikappa B kinase-I kappaB
alpha-NF-kappaB signaling pathway in chondrocytes in vitro. Eur J
Pharmacol. 636:181–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hagiwara S, Iwasaka H, Hidaka S, Hasegawa
A and Noguchi T: Neutrophil elastase inhibitor (sivelestat) reduces
the levels of inflammatory mediators by inhibiting NF-kB. Inflamm
Res. 58:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng M, Liu H, Zhang D, Liu Y, Wang C,
Liu F and Chen J: HMGB1 enhances the AGE-induced expression of CTGF
and TGF-β via RAGE-dependent signaling in renal tubular epithelial
cells. Am J Nephrol. 41:257–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Štros M, Polanská E, Kučírek M and
Pospíšilová S: Histone H1 differentially inhibits DNA bending by
reduced and oxidized HMGB1 protein. PLoS One. 10:e01387742015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Palone F, Vitali R, Cucchiara S,
Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A and
Stronati L: Role of HMGB1 as a suitable biomarker of subclinical
intestinal inflammation and mucosal healing in patients with
inflammatory bowel disease. Inflamm Bowel Dis. 20:1448–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Tochio N, Takeuchi A, Uewaki J,
Kobayashi N and Tate S: Redox-sensitive structural change in the
A-domain of HMGB1 and its implication for the binding to cisplatin
modified DNA. Biochem Biophys Res Commun. 441:701–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee W, Ku SK, Na DH and Bae JS:
Anti-Inflammatory effects of lysozyme against HMGB1 in human
endothelial cells and in mice. Inflammation. 38:1911–1924. 2015.
View Article : Google Scholar : PubMed/NCBI
|