Introduction
The endoplasmic reticulum (ER) is the main site for
the synthesis, processing and transport of the majority of
intracellular, cell surface and extracellular proteins. ER
functions may be disturbed by different factors, such as the
accumulation of unfolded proteins and changes in Ca2+
homeostasis (1,2), which result in ER stress (ERS). The
accumulation of misfolded proteins within the ERS triggers a
pro-survival adaptation, the unfolded protein response (UPR)
(3). Protein aggregates and
Ca2+ homeostasis have been reported to accumulate
following transient cerebral ischemia (4–6).
Furthermore, aggregate formation coincided with the time course of
cell death (4). Cumulative evidence
suggests that signaling markers of the UPR are activated following
cerebral ischemia (7–10). ERS is the key link of cerebral
ischemia-reperfusion injury (CIRI) (8).
Curcumin is a low molecular weight polyphenol
isolated from Curcuma longa, and has attracted increasing
interest due to its roles against oxidative stress, inhibiting the
release of inflammatory cytokines, anti-apoptosis and anti-tumor
agents (11,12). Curcumin has neuroprotective effects
against neurological diseases, such as cerebral ischemia,
Parkinson's disease and Alzheimer's disease (13–17).
Therefore, the present study investigated the dynamic changes of
ERS apoptosis-related proteins, growth arrest and DNA
damage-inducible 153 (GADD153) gene and caspase-12 in the CIRI
process and the impact of curcumin pretreatment on the expression
levels of GADD153 and caspase-12. The aim of the present study was
to explore the effects of curcumin on ERS and to determine its
neuroprotective effects.
Materials and methods
Animals and grouping
A total of 76healthy adult male Wistar rats
(weighing 240–270 g, 7–8 weeks old) were provided by the Animal
Experimental Center, School of Medicine, Shandong University
(Jinan, China; certificate no. Ludongzhi 20001003). The rats were
housed in a temperature (20±2°C) and humidity-controlled (50–60%)
facility with a 12 h light/dark cycle and access to food and water
ad libitum. First, 6 rats were randomly selected and divided
into the sham operation group (group S) and the normal group (group
N; n=3 per group). The remaining rats were used to create a CIRI
model using the Longa method (18),
and grouped into the dimethyl sulfoxide (DMSO) control group (group
D) and the curcumin treatment group (group C; n=27 per group). For
the rats in groups D and C, 12 (T1), 24 (T2) and 72 h (T3) of
reperfusion were performed after 2-h ischemia (n=9 in each
subgroup). The present study was carried out in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (19). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Shandong Provincial Hospital (Jinan, China).
Establishment of a CIRI model
The rats in groups C and D were used to create a
focal middle CIRI model using the modified Longa method (18). Each rat was anesthetized by
intraperitoneally injecting 10% chloral hydrate (300 mg/kg;
Shanghai Chemical Reagent Co., Ltd., Shanghai, China). After
ligation of the distal end of the external carotid artery, one
small incision was cut on the external carotid artery and one
fishing line (0.25 mm in diameter, with the tip coated in silica
gel) was inserted into the internal carotid artery for ~20 mm, and
ligated. A total of 2 h later, the line bolt was gently pulled out
by 15 mm as so to re-supply the blood flow to prepare the
reperfusion model. In group S, the line was inserted~10 mm into the
external carotid artery, and the rest of the procedure was the same
as for groups C and D. Group N received no treatment.
The Longa nerve function score was used to evaluate
the success of model preparation (18). Rats with a Longa score within 0 and 4
points were discarded, and the rats in which model preparation was
successful (mean success rate=71%) were randomly selected for the
supplement. Rats in group C were intraperitoneally injected with
curcumin solution (150 mg/kg; concentration, 7.5 mg/ml, dissolved
in DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 2 h prior
to modeling. Rats in group D were intraperitoneally injected with
an equal volume of 10% DMSO solution (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) 2 h prior to modeling as the solvent
control.
Reperfusion and sampling
The 54 rats modeled successfully were
intraperitoneally injected with 10% chloral hydrate (400 mg/kg) and
left atrial appendage perfusion was performed with 0.9% NaCl at T1,
T2 and T3. At 2 h post-surgery, group S were anesthetized and
perfused. Meanwhile, group N was anesthetized and perfused
immediately. The brain tissue from the left cerebral hemisphere to
the pituitary stalk was sampled, quickly frozen in liquid nitrogen,
and stored at −70°C for the immunoblotting test. For the
immunohistochemical staining and immunofluorescence double staining
test, rats were perfused with 0.9% NaCl and paraformaldehyde (4%),
respectively, and the whole brain tissue was fixed with 4%
paraformaldehyde at 4°C for 24 h. The brain tissue from the left
cerebral hemisphere to the pituitary stalk was subsequently
sampled.
Immunohistochemistry
Conventional paraffin embedding was performed on the
brain tissue and slices of 2–3 µm in thickness were created.
Immunohistochemical staining was performed using a SABC kit (cat.
no. SA1022; Wuhan Boster Biological Technology, Ltd., Wuhan,
China), according to the manufacturer's instructions. The positive
cells were characterized using an inverted microscope to observe
brown stained cells in the cytoplasm and/or nucleus. A total of 10
random visual fields (magnification, ×40) of each of five slices of
the cortex and corpus striatum in each rat were observed to
calculate the rate of positive cells: Positive cells=(positive
cells/total counted cells) ×100%. The mean number of positive cells
in each group was then calculated: Mean number of positive cells in
each group=total number of positive cells in each group/the number
of the rats in each group. The standard deviation was then
calculated.
Immunofluorescence double
staining
The brain tissue was precipitated in 30% sucrose
solution, sliced (~40-µm thick), stored at −20°C and the
immunofluorescence double staining of GADD153 and caspase-12 was
prepared by the section-bleaching method (20). Following blocking using 10% donkey
serum (Guangzhou Ruite Biotechnology Co., Ltd., Guangzhou, China)
at room temperature for 30 min, the slices were rinsed 3 times for
3 min with PBS and incubated with rabbit polyclonal GADD153
antibody (cat. no. sc-575) and goat polyclonal caspase-12
antibody (cat. no. sc-12395; both 1:150; both Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Subsequently, the slices
were rinsed 5 times for 5 min with PBS and incubated for 1 h in
darkness with donkey anti-rabbit immunoglobulin (Ig)G-Dylight 549
(cat. no. 711-505-152;Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA; 1:200) and donkey anti-goat IgG-Dylight 488
(cat. no. 705-485-003;, Jackson ImmunoResearch Laboratories, Inc.;
1:200) at 37°C.Observation and photography was performed using an
immunofluorescence microscope. The GADD153 positive cells exhibited
red fluorescence, and the caspase-12 positive cells exhibited green
fluorescence, so the double-labeled positive cells exhibited yellow
fluorescence. A total of 10 random visual fields (magnification,
×40) of each of five slices of the cortex and corpus striatum in
each rat were observed to calculate the rate of positive cells and
the average was calculated using the same method.
Western blotting
The left frontal and parietal lobes and the striatum
were frozen in liquid nitrogen and stored at −70°C for western
blotting. The tissue samples were homogenized in a lysis buffer
[0.1 mol/l NaCl, 0.01 M Tris-HCl (pH 7.5), 1 mM EDTA and 1 µg/ml
aprotinin], and then the homogenates were centrifuged at 12,000 × g
for 5 min at 4°C. A Bradford assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to determine the protein concentration,
and western blotting was performed with a 5% acrylamide stacking
gel and a 14% acrylamide resolving gel, with 80 µg protein per
lane. Proteins were electrotransferred onto nitrocellulose
membranes. Nonspecific protein binding to the nitrocellulose
membrane was reduced by blocking the membranes with blocking buffer
(5% nonfat dry milk, 2.7 mM KCl, 137 mM NaCl, 8 mM NaHPO4, 1.4 mM
KPO4 and 0.1 % Tween 20) for 1 h at 37°C. Membranes were
subsequently incubated with anti-β-actin (cat. no. RDP-105-025,
1:500; Chemicon; Merck KGaA), antiGADD153 (cat. no. SC-575; 1:500;
Santa Cruz Biotechnology, Inc.) and anti-caspase-12 (cat. no.
SC-12395; 1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary anti-mouse IgG, (cat. no. ZDR-5307),
HRP-anti-rabbit IgG (cat. no. ZDR-5306) and HRP-anti-goat IgG (cat.
no. ZDR-5308; all 1:1,000; ZSGB-BIO, Beijing, China) for 1 h at
37°C. Bands were visualized using DAB reagent at room temperature
for 2 min). The membrane was scanned with an imaging densitometer
(SigmaScan program 4; Systat Software, Inc., San Jose, CA, USA),
and the optical density was quantified.
Statistical analysis
SPSS v. 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. All results are expressed as the mean
± standard deviation. The individual groups were tested for
differences using one-way analysis of variance with repeated
measures, followed by Fisher's least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Success rate of rat modeling
Among the 70 rats subjected to the modeling, 54 rats
were successfully modeled, with a success rate of 71% (data not
shown). In total, 5 rats lost consciousness (3 due to large area
cerebral infarction, 1 due to subarachnoid hemorrhage, 1 due to
anesthesia) and 6 rats succumbed in modeling (1 due to anesthesia
death, 2 due to subarachnoid hemorrhage, 2 due to large area
cerebral infarction, 1 due to pulmonary edema and hemorrhage, 1 due
to unknown causes), and these 11 rats inhaled overdoses of
isoflurane via chamber inhalation and mortality was confirmed. Of
these, 5 rats exhibited no symptoms of neurological deficits
following surgery. Prior to immunohistochemistry, double staining
and western blotting, the 54 rats were sacrificed by chamber
inhalation of overdoses of isoflurane and mortality was
confirmed.
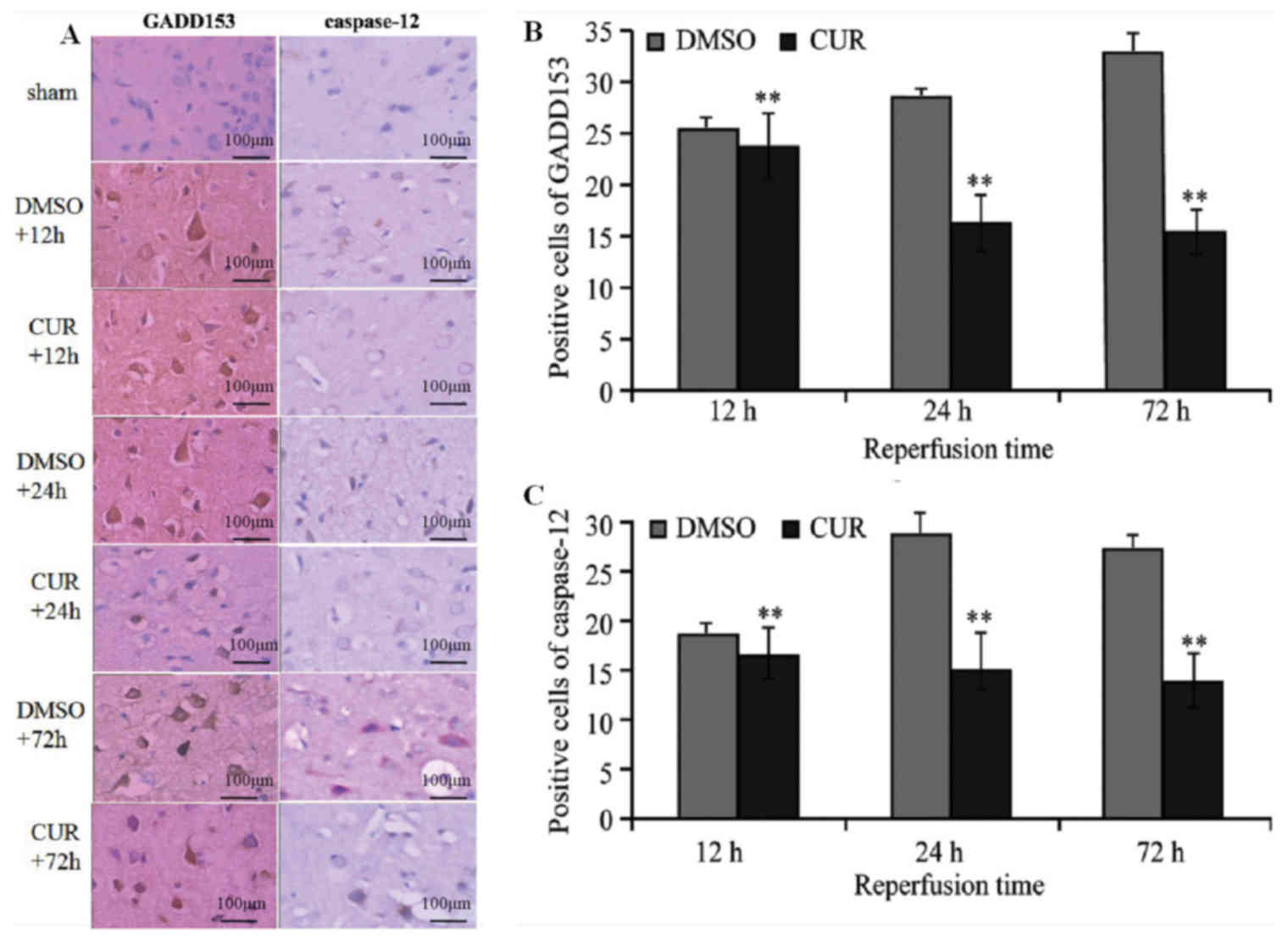
Immunohistochemical staining
GADD153 and caspase-12 were not expressed in groups
N and S (data not shown), while tan granular deposits were observed
in the neurons, as well as the nuclei and cytoplasm of glial cells
in group C and D at T1, T2 and T3 (Fig.
1A). Group D exhibited significantly more GADD153- and
caspase-12-positive cells at T1 compared with group C (P<0.01).
The number of GADD153-positive cells in group D increased at T2 and
reached a peak at T3, whereas the number of caspase-12-positive
cells in group D peaked at T2. Compared with group D, the
expression of GADD153 and caspase-12 in group C at T2 and T3 were
significantly decreased (P<0.01; Fig.
1B and C).
Immunofluorescence double
staining
GADD153 and caspase-12 were not expressed in groups
N and S (data not shown), while group C and D exhibited GADD153-
and caspase-12-positive staining (single or double staining) at the
three time points (Fig. 2A).
Group D exhibited single-stained GADD153- and
caspase-12-positive cells at T1, as well as small amounts of
dual-stained GADD153- and caspase-12-positive cells, which peaked
at T2. The number of single-stained caspase-12-positive cells was
reduced while that of single-stained GADD153-positive cells was
high, and the total number of dual-stained cells was reduced in
Group D at T3 (72 h). Compared with group D, the numbers of
dual-stained GADD153 and caspase-12 positive cells in group C at T2
and T3 were significantly decreased (P<0.01; Fig. 2B).
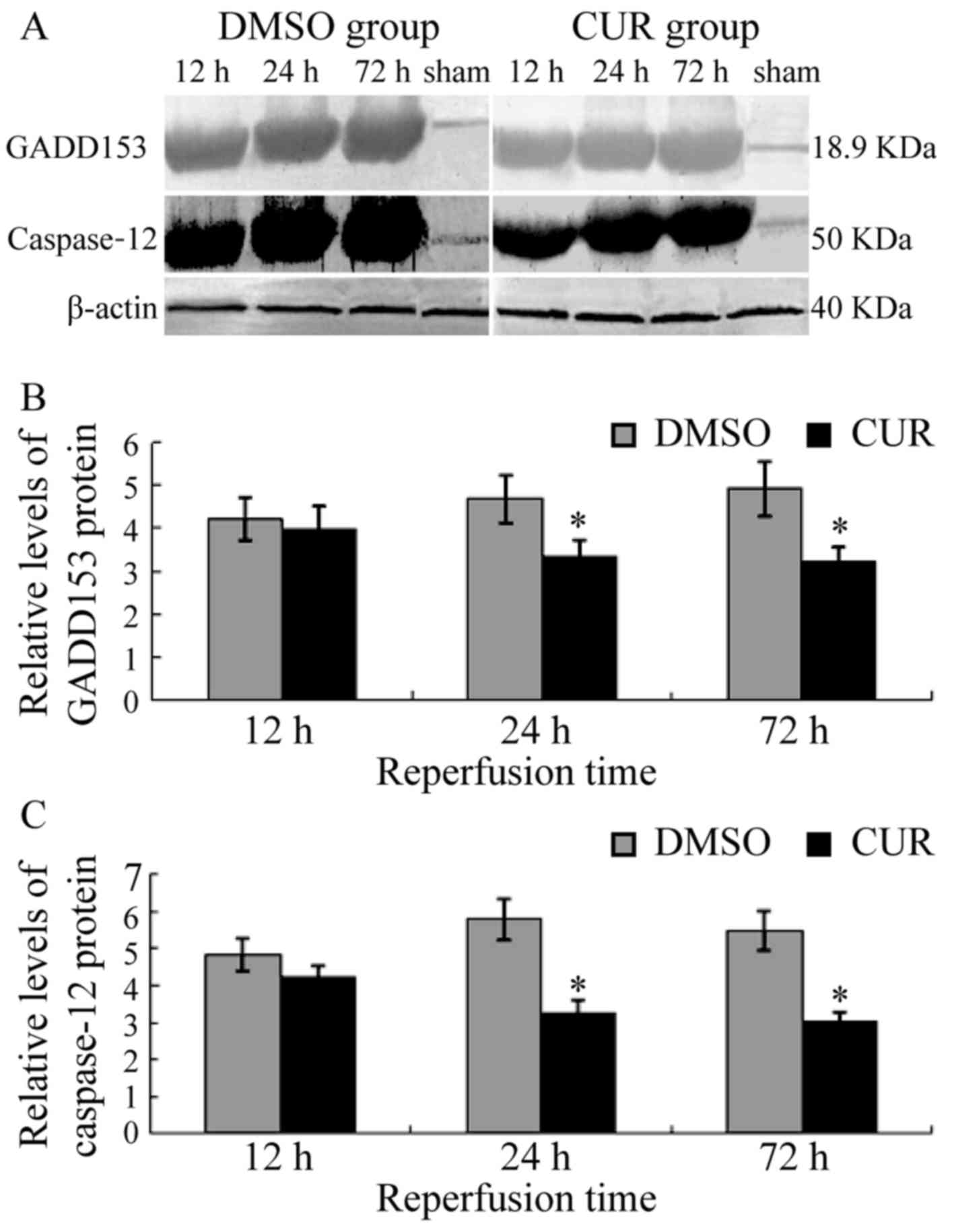
Western blot
GADD153 and caspase-12 were not expressed in the
brain tissue of groups N and S (data not shown). Group D exhibited
the expression of GADD153 at T1, which was upregulated gradually
and exhibited a high level until T3. Caspase-12 was expressed in
group D at T1, reached a peak at T2 and slightly decreased at T3.
Compared with group D, the expression levels of GADD153 and
caspase-12 in group C were significantly decreased at T2 and T3
(P<0.05; Fig. 3).
Discussion
Studies have suggested that multiple causes of ERS
occur in neurons following CIRI, including depletion of ER
Ca2+, aggregation of proteins, decreased protein
degradation and accumulation of lipid peroxidation products in ER
and Golgi structures (21). The
evidence summarized highlights early ER signaling events triggered
by ischemia contribute to the restoration of homeostasis (22,23).
However, prolonged ERS may trigger pro-apoptotic processes and lead
to apoptosis.
ERS-mediated apoptosis occurs via a variety of ways,
among which GADD153 and caspase-12 are two important pathways
(24). GADD153 is an ERS-inducing
protein that participates in ERS-related apoptosis (25). GADD153 is an important intermediate
signaling molecule associated with ERS and apoptosis, and it may
promote apoptosis by various pathways, such as affecting
intracellular Ca2+ metabolism and downregulating B-cell
lymphoma 2 (26–28). Caspase-12 belongs to the conserved
caspase family, which is another major factor in regulating
ERS-mediated apoptosis. Caspase-12 is located in the cytoplasm of
the ER and may be activated by excessive ERS, including the damage
of ER Ca2+ balance or excessive protein deposition in
the ER; however, non-ERS-mediated apoptosis does not involve
activation of caspase-12 (29).
Therefore, caspase-12 is inherent in ERS-mediated apoptosis. When
ERS occurs, caspase-12 is released from the ER, thus further
activating the caspase cascade and leading to apoptosis (29).
Curcumin is a polyphenolic compound and the active
ingredient of C. longa (30).
Its chemical structure is C21H20O6
(molecular weight, 368.37 g/mol), including the enol type and keto
type. Numerous studies have demonstrated that curcumin has
anti-apoptotic effects. A study by Jutooru et al (31) indicated that curcumin may have an
anti-apoptotic role through reducing nuclear factor (NF)-κB
activity and the expression of its regulatory gene. A study by
Chhunchha et al (32)
demonstrated that curcumin may increase the expression of
peroxiredoxin 6 (prdx6), thereby reducing hypoxia-induced
hippocampal ERS and the oxidative stress response, and reducing the
apoptosis of hippocampal cells, eliciting neuroprotective
effects.
The results of the present study demonstrated that
GADD153 and caspase-12 were expressed in the neurons and glial
cells of rats with CIRI, which also indicated dynamic changes with
time. At 12 h of reperfusion, the expression levels of GADD153 and
caspase-12 were increased, caspase-12 peaked at 24 h, while GADD153
peaked at the 72 h. The results of immunofluorescence staining
demonstrated that GADD153 was increased markedly compared with
caspase-12 at T1, while both were markedly increased at T2 (more
positive dual-stained cells); however, at T3, GADD153 was decreased
while caspase-12 increased (positive dual-stained cells were
decreased), suggesting that the activation of GADD153 occupies the
main status in early perfusion stages, which activates various
apoptosis factors. As CIRI worsens, caspase-12 is largely
activated. GADD153 and caspase-12 then act together to promote the
progression of ERS and aggravate apoptosis. The present study
further demonstrated that ERS is involved in the pathological
process of CIRI.
The expression levels of GADD153 and caspase-12 in
group C at T1 were not significantly different to those in group D;
however, they were significantly decreased at T2 and T3, suggesting
that curcumin may reduce the expression levels of ERS
apoptosis-related proteins GADD153 and caspase-12, thus reducing
ERS and having neuroprotective effects. Previous studies have
demonstrated that curcumin may reduce blood-brain barrier damage in
rats with focal cerebral ischemia and have neuroprotective effects
(13). A study by Jiang et al
(14) reported, based on the SH-SY5Y
cell line of Parkinson's disease, that curcumin may induce
macro-autophagy, thus exhibiting neuroprotective effects. Curcumin
may also protect amyloid β-induced mitochondrial and synaptic
toxicities in Alzheimer's disease (15). However, the inhibitory mechanism of
curcumin on ERS is not clear, and this may be realized through
inhibiting the ERS cascade by decreasing the activity of NF-κB,
increasing the expression of prdx6 or activating the Sirtuin type 1
pathway (26–34). Therefore, further research is
required for clarification.
The results of the present study suggest that ERS is
associated with the pathological progression of CIRI. Curcumin may
decrease the expression levels of the above two factors, thus
exhibiting protective effects against CIRI in rats.
References
|
1
|
Verkhratsky A: Physiology and
pathophysiology of the calcium store in the endoplasmic reticulum
of neurons. Physiol Rev. 85:201–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI
|
|
4
|
Hu BR, Martone ME, Jones YZ and Liu CL:
Protein aggregation after transient cerebral ischemia. J Neurosci.
20:3191–3199. 2000.PubMed/NCBI
|
|
5
|
Ge P, Luo Y, Liu CL and Hu B: Protein
aggregation and proteasome dysfunction after brain ischemia.
Stroke. 38:3230–3236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohno K, Higuchi T, Ohta S, Kohno K, Kumon
Y and Sakaki S: Neuroprotective nitric oxide synthase inhibitor
reduces intracellular calcium accumulation following transient
global ischemia in the gerbil. Neurosci Lett. 224:17–20. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts GG, Di Loreto MJ, Marshall M, Wang
J and DeGracia DJ: Hippocampal cellular stress responses after
global brain ischemia and reperfusion. Antioxid Redox Signal.
9:2265–2275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibata M, Hattori H, Sasaki T, Gotoh J,
Hamada J and Fukuuchi Y: Activation of caspase-12 by endoplasmic
reticulum stress induced by transient middle cerebral artery
occlusion in mice. Neuroscience. 118:491–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tajiri S, Oyadomari S, Yano S, Morioka M,
Gotoh T, Hamada JI, Ushio Y and Mori M: Ischemic-induced neuronal
cell death is mediated by the endoplasmic reticulum stress pathway
involving CHOP. Cell Death Differ. 11:403–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou H, Beevers CS and Huang S: The
targets of curcumin. Curr Drug Targets. 12:332–347. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onder A, Kapan M, Gümüş M, Yüksel H, Böyük
A, Alp H, Başarili MK and Firat U: The protective effects of
curcumin on intestine and remote organs against mesenteric
ischemia/reperfusion injury. Turk J Gastroenterol. 23:141–147.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang J, Wang W, Sun YJ, Hu M, Li F and
Zhu DY: Neuroprotective effect of curcumin on focal cerebral
ischemic rats by preventing blood-brain barrier damage. Eur J
Pharmacol. 561:54–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian
LP, Liu J, Ding JQ and Chen SD: Curcumin ameliorates the
neurodegenerative pathology in A53T α-synuclein cell model of
Parkinson's disease through the downregulation of mTOR/p70S6K
signaling and the recovery of macroautophagy. J Neuroimmune
Pharmacol. 8:356–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reddy PH, Manczak M, Yin X, Grady MC,
Mitchell A, Kandimalla R and Kuruva CS: Protective effects of a
natural product, curcumin, against amyloid β induced mitochondrial
and synaptic toxicities in Alzheimer's disease. J Investig Med.
64:1220–1234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng HL, Dang HZ, Fan H, Chen XP, Rao YX,
Ren Y, Yang JD, Shi J, Wang PW and Tian JZ: Curcumin ameliorates
insulin signaling pathway in brain of Alzheimer's disease
transgenic mice. Int J Immunopathol Pharmacol. 29:734–741. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pluta R, Bogucka-Kocka A, Ułamek-Kozioł M,
Furmaga-Jabłońska W, Januszewski S, Brzozowska J, Jabłoński M and
Kocki J: Neurogenesis and neuroprotection in postischemic brain
neurodegeneration with Alzheimer phenotype: Is there a role for
curcumin? Folia Neuropathol. 53:89–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the care and use of laboratory animalsGuide for
the Care & Use of Laboratory Animals. 8th. Washington (DC):
National Academies Press (US); 2011, PubMed/NCBI
|
|
20
|
Yao H, Gao J, Feng YB, Pang ZY and Chi ZF:
2R, 4R-APDC decreases cell proliferation in the dentate gyrus of
adult rats: The effect of 2R, 4R-APDC on cell proliferation.
Neuroreport. 18:1459–1462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeGracia DJ and Montie HL: Cerebral
ischemia and the unfolded protein response. J Neurochem. 91:1–8.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyce M, Bryant KF, Jousse C, Long K,
Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D and Yuan
J: A selective inhibitor of eIF2alpha dephosphorylation protects
cells from ER stress. Science. 307:935–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee DY, Lee KS, Lee HJ, Kim DH, Noh YH, Yu
K, Jung HY, Lee SH, Lee JY, Youn YC, et al: Activation of PERK
signaling attenuates Abeta-mediated ER stress. PLoS One.
5:e104892010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamkanfi M, Kalai M and Vandenabeele P:
Caspase-12: An overview. Cell Death Differ. 11:365–368. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Mongillo M, Chin KT, Harding H, Ron
D, Marks AR and Tabas I: Role of ERO1-mediated stimulation of
inositol 1,4,5-triphosphate receptor activity in endoplasmic
reticulum stress-induced apoptosis. J Cell Biol. 186:783–792. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Timmins JM, Ozcan L, Seimon TA, Li G,
Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson
ME and Tabas I: Calcium/calmodulin-dependent protein kinase II
links endoplasmic reticulum stress with Fas and mitochondrial
apoptosis pathways. J Clin Invest. 119:2925–2941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl-2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jutooru I, Chadalapaka G, Lei P and Safe
S: Inhibition of NFkappaB and pancreatic cancer cell and tumor
growth by curcumin is dependent on specificity protein
downregulation. J Biol Chem. 285:25332–25344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chhunchha B, Fatma N, Kubo E, Rai P, Singh
SP and Singh DP: Curcumin abates hypoxia-induced oxidative stress
based-ER stress-mediated cell death in mouse hippocampal cells
(HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell
Physiol. 304:C636–C655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Q, Jia N, Wang W, Jin H, Xu J and Hu
H: Activation of SIRT1 by curcumin blocks the neurotoxicity of
amyloid-β25–35 in rat cortical neurons. Biochem Biophys Res Commun.
448:89–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Zhang B, Huang F, Liu B and Xie Y:
Curcumin inhibits lipolysis via suppression of ER stress in adipose
tissue and prevents hepatic insulin resistance. J Lipid Res.
57:1243–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|