Introduction
The immune system includes macrophages, natural
killer cells, and T lymphocytes, and is essential for regulating
pregnancy in mammals (1). In
particular, macrophages are crucial for regulating the physiology
of pregnancy, including ovarian function, maternal recognition of
the fetus, implantation, placentation, and parturition (2). For example, macrophage depletion
following conception causes embryo implantation arrest, which is
associated with decreased plasma progesterone due to the disruption
of the luteal microvascular network (3). Macrophages secrete a variety of
cytokines during pregnancy, including pro-inflammatory cytokines
[interleukin (IL)-6, tumor necrosis factor (TNF)-α and
interferon-γ] and anti-inflammatory cytokines (IL-4 and IL-10)
(1). However, infection and/or
endogenous danger signals induce excessive inflammatory responses
in macrophages, which may potentially result in pregnancy
complications including preeclampsia, preterm delivery, and poor
fetal growth (4–7).
It has been reported that bacterial infection during
pregnancy is the prevailing cause of preterm labor and affects
5–10% of all pregnancies (8,9). The cell walls of gram-negative bacteria
contain lipopolysaccharide (LPS), which is known to induce
inflammation and has been associated with adverse developmental
outcomes (7,10). Maternal exposure to LPS has been
demonstrated to induce embryonic resorptions, fetal death, and
fetal absorption in mice (7,10). Additionally, it has previously been
reported that endogenous danger signals may contribute to the
pathogenesis of pregnancy complications, such as preeclampsia and
preterm labor (11–14). Advanced glycation end products (AGE)
are heterogeneous, reactive, and irreversibly crosslinking
molecules formed by the non-enzymatic glycation of proteins,
lipids, and nucleic acids (11), and
are one of the endogenous danger signals in pregnancy. Similar to
the adverse effects of LPS, AGE have been implicated in the
pathogenesis of infertility as they reduce the formation of
blastocysts (15,16). In general, AGE and/or LPS interact
with the AGE receptor (RAGE) and/or Toll-like receptor 4 (TLR4) to
induce inflammatory responses (cytokine production) (17,18).
The phytoalexin resveratrol
(3,5,4′-trihydroxystilbene) is a non-flavonoid polyphenolic
compound that provides the cardiovascular benefits of red wine
(19,20). It has been demonstrated that
resveratrol has multipotent benefits, including anti-cancer and
anti-oxidant effects, inflammation reduction, and metabolic and
vascular function improvement (19–23).
Resveratrol may therefore be a powerful tool for protecting against
the deleterious effects of excessive caloric intake, modulating
energy balance, and promoting good health and longevity (22,24).
Furthermore, the beneficial effects of resveratrol are putatively
assumed to be due to sirtuin 1 (SIRT1), cAMP, or 5′ adenosine
monophosphate-activated protein kinase (AMPK) pathway activation
(22,25).
Understanding how AGE and LPS induce inflammation,
and how resveratrol exerts its effects, is important, not only for
potential insights into the biological causes of
inflammation-related diseases but also to allow for the development
of pharmacological agents that have similar effects with
resveratrol. In the present study, the effects of AGE and LPS on
inflammatory cytokine production in a mouse J774 cell line were
investigated. The potential effect of resveratrol on AGE- and
LPS-induced inflammation in macrophages was also examined.
Materials and methods
Cell culture and experimental
conditions
Murine J774 macrophages were obtained from RIKEN
BioResource Center (Tsukuba, Japan) and cultured at 37°C until
confluence was reached in Dulbecco's modified Eagle's medium/F-12
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented
with antibiotics including amphotericin B and gentamicin
(Sigma-Aldrich, St Louis, MA, USA), and 5% fetal bovine serum (ICN
Pharmaceuticals, Inc., Costa Mesa, CA, USA). The J774 cells were
seeded at a concentration of 2.5×105 cells/well in a
24-well culture plate (Thermo Fisher Scientific Inc.).
To examine cytokine secretion, mRNA expression and
protein expression, cells were washed twice with PBS and treated
for 24 h with the following 7 groups: Different concentratins of
bovine serum albumin (BSA; 100, 200, or 400 µg/ml; BioVision, Inc.,
Milpitas, CA, USA), different concentrations of AGE-BSA (100, 200,
or 400 µg/ml; BioVision, Inc.), and LPS (0.1 µg/ml; Sigma-Aldrich).
BSA at the same concentration as each AGE-BSA treatment was used as
vehicle control. Supernatants were collected for ELISA (described
in the western blotting section), RNA was collected for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
using ISOGEN (Nippon Gene Co., Ltd., Tokyo, Japan) according to the
manufacturer's protocol, and cell lysates were collected for
western blot analysis using radioimmunoprecipitation assay buffer
(described later). Samples were stored at −20 or −80°C before
analysis.
In the experiments studying receptor inhibition in
AGE- or LPS-induced IL-6 secretion, J774 macrophages were
stimulated with either AGE or LPS in the absence or presence of
either RAGE antagonist (10 µM; FPS-XM1; Merck Millipore, Darmstadt,
Germany) or TLR4 inhibitor (10 µM; NBP2-26244; Novus Biologicals,
LLC, Littleton, CO, USA). To evaluate the role of nuclear factor
(NF)-κB, cells were pre-incubated for 1 h at 37°C with an NF-κB
activation inhibitor (150 nM; NF-κB activation inhibitor IV; Merck
Millipore) prior to the addition of AGE or LPS. To investigate the
role of reactive oxygen species (ROS), J774 macrophages were
pre-incubated for 1 h at 37°C in the absence or presence of
N-acetyl-L-cysteine (1 mM; NAC; Wako Pure Chemical
Industries, Ltd. Osaka, Japan) for 1 h and subsequently treated
with either AGE or LPS for 24 h at 37°C.
To investigate the effect of resveratrol, J774
macrophages were pretreated with ethanol as a control solvent of
resveratrol, or resveratrol (2, 20, or 50 µM; Wako Pure Chemical
Industries, Ltd.) for 1 h at 37°C and subsequently stimulated with
AGE or LPS. To evaluate the role of AMPK and SIRT1 on resveratrol
function, cells were pretreated with resveratrol in the absence or
presence of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR;
10 µM, Sigma-Aldrich), which is an AMPK activator, or SIRT1
inhibitor Ex-527 (20 µM, Sigma-Aldrich), respectively, for 1 h at
37°C and subsequently stimulated with LPS.
Determination of cytokines
After treatment as described above, supernatants
were collected in 1.5 ml tubes and stored at −20°C. Levels of IL-6
and IL-1β were determined using a mouse ELISA kit (DY406 and DY401;
R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. A total of 4 repeats were conducted for
each group.
RT-qPCR
Total RNA was prepared using ISOGEN (Nippon Gene
Co., Ltd.) according to the manufacturer's protocol. RNA extraction
and cDNA production were performed a commercial kit (ReverTra Ace;
Toyobo Co., Ltd., Osaka, Japan) as described previously (26). RT-qPCR was performed using the CFX
Connect Real Time PCR cycler (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and a commercial kit (Thunderbird SYBR qPCR Mix; Toyobo
Co., Ltd.) to detect the expressions of IL-6, IL-1β, and GAPDH
mRNA. The following antisense and sense primers were used: IL-6,
forward 5′-ACAACCACGGCCTTCCCTACTT-3′ and reverse
5′-CACGATTTCCCAGAGAACATGTG-3′; IL-1β, forward
5′-TGAAGTTGACGGACCCCAAA-3′ and reverse 5′-TGATGTGCTGCTGTGAGATT-3′;
and GAPDH forward 5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse
5′-TTGCTGTTGAAGTCGCAGGAG-3′. RT-qPCR was performed in duplicate
with a final reaction volume of 20 µl containing 10 µl SYBR-Green,
7.8 µl distilled water, 0.1 µl 100 µM forward and reverse primers,
and 2 µl of cDNA template. The amplification program consisted of a
5 min denaturation at 95°C followed by 40 cycles of amplification
(95°C for 15 sec, 60°C for 30 sec, and 72°C for 20 sec). Expression
levels of each target gene were normalized to the corresponding
GAPDH threshold cycle values using the 2−∆∆Cq
comparative method (27).
Immunocytochemistry
To evaluate the expression of RAGE and TLR4, J774
macrophages were washed twice with PBS and blocked with 5% BSA in
PBS for 1 h at room temperature. Cells were subsequently incubated
with RAGE (1:1,000; ab3611; Abcam, Cambridge, MA, USA) and TLR4
antibodies (1:500; ab22048; Abcam) for 90 min at room temperature
followed by incubation for 1 h at room temperature with a secondary
Alexa 488-conjugated antibody (1:1,000; cat. no. 4412; Cell
Signaling Technology, Inc., Danvers, MA, USA). The cells were
covered with Vectershield and stained with
4′6-diamidino-2-phenylindole (Vector Laboratories, Inc.,
Burlingame, CA, USA). The stained sections were examined using a
fluorescence microscope (DMI6000B; Leica Microsystems Inc., Buffalo
Grove, IL, USA) and LAS AF software (version 3; Leica Microsystems
Inc.).
Western blot analysis
Lysates from the cell culture were prepared using
radioimmunoprecipitation assay buffer (RIPA buffer; Wako Pure
Chemical Industries, Ltd.). Cells were subsequently washed with
cold PBS and incubated with RIPA buffer for 15 min on ice. Cell
lysates were subsequently transferred into 1.5 ml tubes and
centrifuged at 12,000 × g for 20 min at 4°C. Supernatants
were transferred to a fresh tube and stored at −80°C before
analysis. A total of 10 µg protein was loaded per lane and
separated by 10% SDS-PAGE. The expression of NF-κB p65 and β-actin
were analyzed using SDS-PAGE. Lysates were transferred onto
polyvinylidene fluoride membranes and blocked for 1 h at room
temperature using Immunoblock (DS Pharma Biomedical Co., Ltd.,
Osaka, Japan). Membranes were washed with TBST wash buffer and
incubated for 24 h at 4°C with anti-NF-κB p65 subunit antibody
(1:1,000; cat. no. MAB3026; Merck Millipore), or anti-β-actin
antibody (1:10,000; AC-74; Sigma-Aldrich), followed by incubation
for 1 h at room temperature with a horseradish peroxidase
(HRP)-conjugated secondary antibody (1:1,000; NA934; GE Healthcare
Life Sciences, Chalfont UK). Immunoreactive bands were visualized
with Western BLoT Quant HRP Substrate (GE Healthcare Life Sciences)
using ImageQuant LAS 4000 (GE Healthcare Life Sciences). Western
blotting was performed in duplicate.
Statistical analysis
All data are expressed as mean ± standard error of
the mean. Differences between treatment groups were identified
using unpaired t-tests. Multiple group comparisons were made using
one-way analysis of variance followed by Bonferroni's multiple
comparison tests using Statview version 5.0 (SAS Institute, Cary,
NC, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of AGE and LPS on IL-6 and
IL-1β in J774 macrophages
The effects of AGE or LPS on inflammatory responses
including IL-6 secretion and production from J774 macrophages were
examined (Fig. 1A). IL-6 secretion
was significantly upregulated by treatment with ≥200 µg/ml AGE
(P<0.05) or LPS (P<0.01), whereas the BSA vehicle had no
significant effect (Fig. 1A).
Similarly, AGE and LPS treatments also increased IL-6 mRNA
expression of J774 macrophages (AGE, P<0.05; LPS, P<0.01;
Fig. 1B). Treatment with AGE or LPS
had no significant effect on IL-1β secretion (Fig. 1C), whereas the expression of IL-1β
mRNA was significantly upregulated by AGE (P<0.05) and LPS
(P<0.01; Fig. 1D).
Role of RAGE and TLR4 on AGE- or
LPS-induced IL-6 secretion
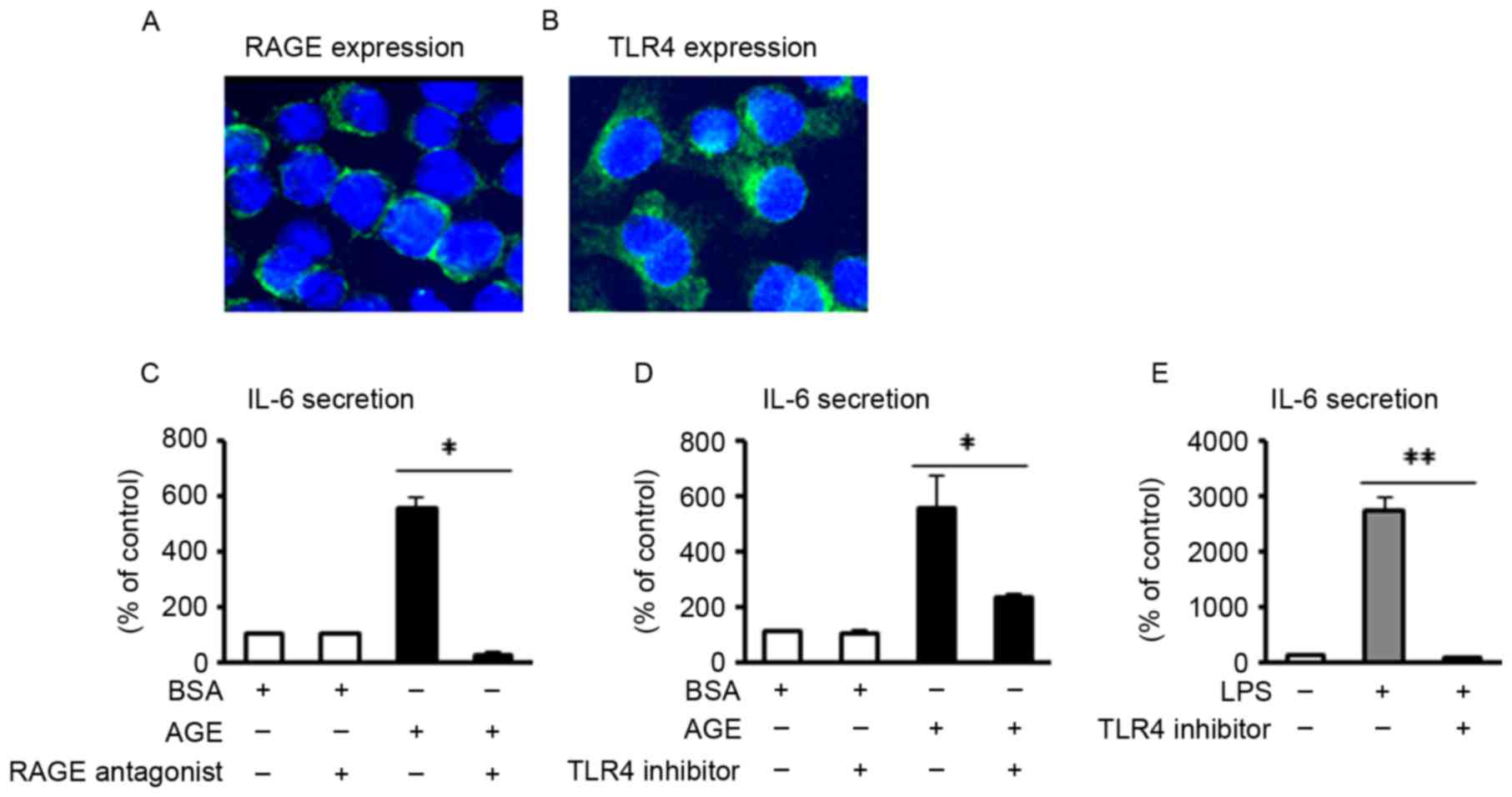
RAGE and TLR4 are two of the major receptors for AGE
and LPS, respectively (17,18). In the present study, it was first
confirmed via immunofluorescence that these two receptors were
expressed in J774 macrophages (Fig. 2A
and B). To investigate the receptor involved in AGE- and
LPS-induced IL-6 secretion, blocking reagents (RAGE antagonist or
TLR4 inhibitor) were added to the J774 macrophages, followed by the
addition of either AGE or LPS, at 1 h following treatment with the
blocking reagent. The results revealed that AGE-induced IL-6
secretion was significantly inhibited by pretreatment with either
the RAGE antagonist or TLR4 inhibitor (P<0.05; Fig. 2C and D). Furthermore, LPS-induced
IL-6 secretion was significantly blocked by pretreatment with TLR4
inhibitor (P<0.01; Fig. 2E).
Role of NF-κB and ROS on AGE- or
LPS-induced IL-6 secretion
NF-κB is a key transcription factor for the
induction of inflammatory cytokines, such as IL-6 (28). Treatment with AGE or LPS induced a
notable upregulation of the NF-κB p65 protein content in J774
macrophages (Fig. 3A). Treatment
with an NF-κB inhibitor significantly reduced the secretion of IL-6
induced by AGE (P<0.05; Fig. 3B)
or LPS (P<0.01; Fig. 3C),
suggesting that both AGE and LPS stimulate IL-6 secretion in an
NF-κB-dependent manner.
It has previously been demonstrated that AGE and LPS
stimulate ROS production (16,29,30).
Therefore, IL-6 secretion induced by treatment with AGE or LPS
suggests that ROS production involves AGE- and LPS-induced
inflammation. To investigate the importance of ROS in AGE- and
LPS-induced inflammation, the effects of the antioxidant NAC on
J774 macrophages were investigated. Pretreatment with NAC
significantly inhibited IL-6 secretion from J774 macrophages
treated with either AGE (P<0.05) or LPS (P<0.01; Fig. 3D and E). These data indicate that ROS
production is a key factor in danger signal-induced IL-6 secretion
in murine macrophages.
Role of resveratrol in AGE- or
LPS-induced IL-6 secretion
J774 macrophages were treated with various
concentrations of resveratrol to check the inhibitory function for
an inflammatory response. IL-6 secretion was significantly elevated
in the supernatants of LPS-treated J774 macrophages compared with
the control group (P<0.01; Fig.
4A). Cells were treated with different doses of resveratrol,
with ethanol as a vehicle control, and resveratrol was found to
significantly decrease LPS-induced IL-6 secretion in a
dose-dependent manner (P<0.05; Fig.
4A). Furthermore, AGE-induced IL-6 secretion was significantly
inhibited by treatment with resveratrol (P<0.05; Fig. 4B). To further investigate the
function of resveratrol, LPS was chosen as a stimulus due to the
greater inflammatory response to LPS compared with AGE. LPS
treatment was also found to markedly increase the level of
intracellular IL-6 and mRNA expression of IL-6 in J774 macrophages
(Fig. 4C and D). Resveratrol
treatment significantly attenuated this increase (P<0.01;
Fig. 4C and D), suggesting that
resveratrol attenuates LPS-induced IL-6 transcription, production,
and secretion in J774 macrophages.
 | Figure 4.Role of resveratrol on AGE- or
LPS-induced IL-6 secretion. (A-D) J774 macrophages were
pre-incubated using EtOH or resveratrol for 1 h; cells were further
incubated for 24 h with or without AGE or LPS. IL-6 levels in (A
and B) the supernatant and (C) intracellular were determined using
ELISA. (D) The IL-6 mRNA levels was assessed by reverse
transcription-quantitative polymerase chain reaction. (E) J774
macrophages were pre-incubated using EtOH or resveratrol with or
without Ex527; cells were further incubated for 24 h with or
without LPS. (F) J774 macrophages were pre-incubated using EtOH,
resveratrol, or AICAR for 1 h; cells were further incubated for 24
h with or without LPS. IL-6 levels in supernatant were determined
using ELISA. Data are expressed as the mean ± standard error of the
mean. n=4 for each experiment. *P<0.05, **P<0.01. AGE,
advanced glycation end-products; LPS, lipopolysaccharides; IL,
interleukin; EtOH, ethanol; AICAR, 5-aminoimidazole-4-carboxamide
ribonucleotide; Resv, resveratrol; BSA, bovine serum albumin. |
It has been reported that resveratrol activates
SIRT1 and AMPK in cell culture and in vivo (22,25). In
the present study, the functional role of resveratrol was
investigated using Ex-527 and AICAR in LPS-induced IL-6 secretion.
It was also investigated whether the effect of resveratrol on
LPS-induced IL-6 secretion was dependent on SIRT1. Treatment with
Ex-527+ethanol had no significant effect on LPS-induced IL-6
secretion from macrophages (Fig.
4E). Although resveratrol treatment suppressed LPS-induced IL-6
secretion, Ex-527 treatment significantly reversed this effect
(P<0.05; Fig. 4E). Pretreatment
with AICAR had a similar effect to treatment with resveratrol,
significantly inhibiting LPS-induced IL-6 secretion from
macrophages (P<0.05; Fig. 4F).
These data suggest that SIRT1 and AMPK serve a role in mediating
the benefits of resveratrol.
Discussion
There is increasing evidence that suggests both AGE
and LPS are associated with inflammation. Numerous studies have
indicated that AGE and LPS promote pro-inflammatory mediator
production, such as IL-6, IL-1β, and TNFα, from a variety of cell
types including macrophages, placenta, chondrocytes, and
endothelial cells (16,17,31–36). In
the present study, it was demonstrated that AGE and LPS increased
IL-6 secretion following the induction of IL-6 mRNA expression in
J774 murine macrophage cell lines. It is well known that LPS
regulates macrophage polarization toward M1-type inflammatory
activity. Jin et al (37)
reported that AGE significantly promotes inflammatory cytokines and
a surface marker of M1-type macrophages, and does not affect
M2-type macrophage markers in mouse bone-marrow-derived
macrophages. Therefore, both AGE and LPS enhance macrophage
differentiation into pro-inflammatory M1 phenotype.
IL-1β is one of the major pro-inflammatory cytokines
produced by immune cells (38,39).
Initially, a stimulus is required to produce the precursor of IL-1β
(pro-IL-1β) in the cells. IL-1β secretion is subsequently regulated
by caspase-1 (also known as an IL-1β-converting enzyme) due to
inflammasome activation, including that of NLRP3 inflammasomes
(38,39). Although treatment with AGE and LPS
significantly upregulated IL-1β mRNA expression, these stimuli had
no effect on IL-1β secretion in J774 macrophages. This suggests
that AGE and LPS have roles in pro-IL-1β production but not in
inflammasome activation.
AGE and/or LPS bind to and signal through
multivalent receptors such as RAGE and/or TLRs (TLR2 and TLR4)
(40,41). In the present study, the expression
of two major receptors, RAGE and TLR4, was confirmed in J774
macrophages. These receptors were then blocked in turn to evaluate
which receptor was involved in the inflammatory effect. Blocking
RAGE or TLR4 abolished AGE-induced inflammation, and the TLR-4
inhibitor abolished LPS-induced IL-6 secretion in J774 macrophages.
Similarly, using neutralization antibody or small interfering RNA
techniques, it was previously demonstrated that AGE stimulated IL-6
secretion via RAGE and/or TLR4, whereas LPS enhanced it via TLR4
(16,34,37,42).
NF-κB is a key component in inflammatory cytokine
production (28). AGE and LPS have
been demonstrated to significantly increase the release of IL-6,
depending on NF-κB activation in various types of cells in
vitro. Liu et al (33)
demonstrated that stimulation with both AGE and LPS induces nuclear
translocation of NF-κB. In the present study it was confirmed that
when NF-κB was inhibited, AGE and LPS increased IL-6 secretion
depending on NF-κB activation. These findings suggest that both AGE
and LPS directly induce inflammation responses via the RAGE and/or
TLR4-NF-κB activation pathway.
ROS are commonly considered as harmful mediators of
acute inflammation (43,44). It is well understood that both AGE
and LPS significantly stimulate ROS production in many types of
cells (16,29,45,46), and
the present authors have previously demonstrated this (43,44). In
the present study, the experiments using ROS inhibitors confirmed
that ROS is essential for the induction of IL-6 secretion by either
AGE or LPS in J774 macrophages. In human THP-1 macrophages and
umbilical vein endothelial cells, AGE induces ROS generation via
the RAGE/NF-κB pathway (45,46) and LPS induces a TLR4-dependent
upregulation in ROS production (47). These previous results and the results
of the present study indicate that ROS may be harmful mediators of
inflammation.
Resveratrol has attracted wide attention due to its
anti-inflammatory, anti-aging and antioxidant effects in animal
models (22,24). It was previously reported that
resveratrol inhibits LPS-induced NF-κB translocation and
inflammatory cytokine secretion in the murine Raw264.7 macrophage
cell line (48). In the present
study, resveratrol was demonstrated to significantly inhibit LPS-
and AGE-induced IL-6 secretion in J774 macrophages. Furthermore,
resveratrol significantly suppressed intracellular IL-6 production
and IL-6 mRNA expression, indicating that the beneficial role of
resveratrol in inflammatory responses is achieved via inhibitory
control at transcriptional levels, resulting in an attenuation of
pro-inflammatory cytokine production.
Resveratrol is known to stimulate SIRT1 and AMPK,
which have been shown to serve many similar functions as energy
sensors and in regulating the nutrient status and stress response
(22,25). SIRT1 activation by resveratrol
improves mitochondrial function and mitochondrial biogenesis, and
increases ATP production (25).
Furthermore, in a study using SIRT1 knockout mice and SIRT1
transgenic mice, Price et al (25) demonstrated that SIRT1 is essential
for AMPK activation and resveratrol function. In the present study,
treatment with Ex527 (a SIRT1 inhibitor) canceled the
anti-inflammatory effect of resveratrol in J774 macrophages. A
previous study found that downregulation of SIRT1 expression
resulted in the anti-inflammatory role of resveratrol being
eliminated in human amnion cells (49), and furthermore, a reduction in SIRT1
activity has been demonstrated to induce hyperacetylation of NF-κB
(50). These data suggest that
resveratrol-induced SIRT1 has a pivotal role in regulating NF-κB
dependent pro-inflammatory mediator production.
AMPK is a metabolic regulator that promotes insulin
sensitivity and fatty acid oxidation (22), and resveratrol activates AMPK in cell
culture and in vivo (25).
Using AMPK knockout mice, Yi et al (48) reported that AMPK is required for many
of the effects of resveratrol on metabolic function. The present
study demonstrated the importance of AMPK activation via the
inhibitory effect of treatment with AICAR (AMPK activator) on IL-6
secretion in J774 macrophages. Yi et al (48) reported that AICAR suppresses
LPS-induced TNF-α secretion in the Raw264.7 macrophage cell line,
and that treatment with an AMPK inhibitor (compound C) also
decreases TNF-α secretion in LPS-treated Raw264.7 macrophages.
Similarly, a previous study demonstrated that compound C decreases
IL-6, IL-1β, and TNF-α secretion in microglia (51). Therefore, Yi et al (48) speculated the importance of the dual
roles of AMPK in macrophage-derived inflammation. Further studies
are required for a detailed clarification of the functions of AMPK
in macrophages.
A limitation of the present study is that it was not
possible to examine whether AGE and LPS induce excessive
inflammatory responses resulting in pregnancy complications in
vivo, and whether resveratrol improves these pregnancy
complications. With respect to this, Furuya et al (52) reported that resveratrol treatment
suppresses inflammatory cytokine production induced by LPS in mice,
resulting in protection against preterm birth. Also, resveratrol
significantly reduces accumulation and inflammation of macrophages
induced by LPS, resulting in the improvement of kidney function
in vivo (53). Therefore, in
future studies, the role of AGE, LPS, and resveratrol in
inflammatory responses associated with pathophysiology of pregnancy
should be examined in vivo. Another limitation is that the
effects of AGE and LPS on cells associated with reproduction, such
as trophoblast/placental cells or uterus cells, were not studied,
nor were the detailed interaction of these cells with macrophages.
Further investigations are required to clarify these
mechanisms.
In conclusion, the results of the present study
demonstrated that exposure to AGE and LPS results in IL-6
production, which is dependent on NF-κB activation and ROS
production through RAGE and/or TLR4, in a murine J774 macrophage
cell line. Additionally, resveratrol is effective in regulating
inflammatory responses associated with SIRT1 and AMPK activation in
macrophages.
Acknowledgements
The present study was supported by the Japan Society
for the Promotion of Science through Scientific Research (grant no.
15K07783) and the Strategic Research Project from Tokyo University
of Agriculture. The present authors would like to thank Enago
(Crimson Interactive K.K., Tokyo, Japan) for the English language
review.
References
|
1
|
Laresgoiti-Servitje E: A leading role for
the immune system in the pathophysiology of preeclampsia. J Leukoc
Biol. 94:247–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faas MM, Spaans F and De Vos P: Monocytes
and macrophages in pregnancy and pre-eclampsia. Front Immunol.
5:2982014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Care AS, Diener KR, Jasper MJ, Brown HM,
Ingman WV and Robertson SA: Macrophages regulate corpus luteum
development during embryo implantation in mice. J Clin Invest.
123:3472–3487. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sacks GP, Studena K, Sargent K and Redman
CW: Normal pregnancy and preeclampsia both produce inflammatory
changes in peripheral blood leukocytes akin to those of sepsis. Am
J Obstet Gynecol. 179:80–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melgert BN, Spaans F, Borghuis T, Klok PA,
Groen B, Bolt A, de Vos P, van Pampus MG, Wong TY, van Goor H, et
al: Pregnancy and preeclampsia affect monocyte subsets in humans
and rats. PLoS One. 7:e452292012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau SY, Guild SJ, Barrett CJ, Chen Q,
McCowan L, Jordan V and Chamley LW: Tumor necrosis factor-alpha,
interleukin-6, and interleukin-10 levels are altered in
preeclampsia: A systematic review and meta-analysis. Am J Reprod
Immunol. 70:412–427. 2013.PubMed/NCBI
|
|
7
|
Robertson SA, Skinner RJ and Care AS:
Essential role for IL-10 in resistance to
lipopolysaccharide-induced preterm labor in mice. J Immunol.
177:4888–4896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romero R, Sirtori M, Oyarzun E, Avila C,
Mazor M, Callahan R, Sabo V, Athanassiadis AP and Hobbins JC:
Infection and labor. V. Prevalence, microbiology, and clinical
significance of intraamniotic infection in women with preterm labor
and intact membranes. Am J Obstet Gynecol. 161:817–824. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meis PJ, Goldenberg RL, Mercer B, Moawad
A, Das A, McNellis D, Johnson F, Iams JD, Thom E and Andrews WW:
The preterm prediction study: Significance of vaginal infections.
National Institute of Child Health and Human Development
Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol.
173:1231–1235. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bo QL, Chen YH, Yu Z, Fu L, Zhou Y, Zhang
GB, Wang H, Zhang ZH and Xu DX: Rosiglitazone pretreatment protects
against lipopolysaccharide-induced fetal demise through inhibiting
placental inflammation. Mol Cell Endocrinol. 423:51–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John WG and Lamb EJ: The Maillard or
browning reaction in diabetes. Eye (Lond). 7:230–237. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chekir C, Nakatsuka M, Noguchi S, Konishi
H, Kamada Y, Sasaki A, Hao L and Hiramatsu Y: Accumulation of
advanced glycation end products in women with preeclampsia:
Possible involvement of placental oxidative and nitrative stress.
Placenta. 27:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naruse K, Sado T, Noguchi T, Tsunemi T,
Yoshida S, Akasaka J, Koike N, Oi H and Kobayashi H: Peripheral
RAGE (receptor for advanced glycation endproducts)-ligands in
normal pregnancy and preeclampsia: Novel markers of inflammatory
response. J Reprod Immunol. 93:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao L, Noguchi S, Kamada Y, Sasaki A,
Matsuda M, Shimizu K, Hiramatsu Y and Nakatsuka M: Adverse effects
of advanced glycation end products on embryonal development. Acta
Med Okayama. 62:93–99. 2008.PubMed/NCBI
|
|
16
|
Huang QT, Zhang M, Zhong M, Yu YH, Liang
WZ, Hang LL, Gao YF, Huang LP and Wang ZJ: Advanced glycation end
products as an upstream molecule triggers ROS-induced sFlt-1
production in extravillous trophoblasts: A novel bridge between
oxidative stress and preeclampsia. Placenta. 34:1177–1182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ibrahim ZA, Armour CL, Phipps S and Sukkar
MB: RAGE and TLRs: Relatives, friends or neighbours? Mol Immunol.
56:739–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szmitko PE and Verma S: Cardiology patient
pages. Red wine and your heart. Circulation. 111:e10–e11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponzo V, Soldati L and Bo S: Resveratrol:
A supplementation for men or for mice? J Transl Med. 12:1582014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Tian X, Zhang L, He C, Ji P, Li Y,
Tan D and Liu G: Beneficial effect of resveratrol on bovine oocyte
maturation and subsequent embryonic development after in vitro
fertilization. Fertil Steril. 101:577–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valenzano DR, Terzibasi E, Genade T,
Cattaneo A, Domenici L and Cellerino A: Resveratrol prolongs
lifespan and retards the onset of age-related markers in a
short-lived vertebrate. Curr Biol. 16:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawasaki Y, Aoki Y, Magata F, Miyamoto A,
Kawashima C, Hojo T, Okuda K, Shirasuna K and Shimizu T: The effect
of single nucleotide polymorphisms in the tumor necrosis factor-α
gene on reproductive performance and immune function in dairy
cattle. J Reprod Dev. 60:173–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brasier AR: The nuclear
factor-kappaB-interleukin-6 signalling pathway mediating vascular
inflammation. Cardiovasc Res. 86:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin QQ, Dong CF, Dong SQ, Dong XL, Hong Y,
Hou XY, Luo DZ, Pei JJ and Liu XP: AGEs induce cell death via
oxidative and endoplasmic reticulum stresses in both human SH-SY5Y
neuroblastoma cells and rat cortical neurons. Cell Mol Neurobiol.
32:1299–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin JK and Tsai SH: Chemoprevention of
cancer and cardiovascular disease by resveratrol. Proc Natl Sci
Counc Repub China B. 23:99–106. 1999.PubMed/NCBI
|
|
31
|
Berbaum K, Shanmugam K, Stuchbury G, Wiede
F, Körner H and Munch G: Induction of novel cytokines and
chemokines by advanced glycation endproducts determined with a
cytometric bead array. Cytokine. 41:198–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lappas M, Permezel M and Rice GE: Advanced
glycation endproducts mediate pro-inflammatory actions in human
gestational tissues via nuclear factor-kappaB and extracellular
signal-regulated kinase 1/2. J Endocrinol. 193:269–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu J, Zhao S, Tang J, Li Z, Zhong T, Liu
Y, Chen D, Zhao M, Li Y, Gong X, et al: Advanced glycation end
products and lipopolysaccharide synergistically stimulate
proinflammatory cytokine/chemokine production in endothelial cells
via activation of both mitogen-activated protein kinases and
nuclear factor-kappaB. FEBS J. 276:4598–4606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen YJ, Sheu ML, Tsai KS, Yang RS and Liu
SH: Advanced glycation end products induce peroxisome
proliferator-activated receptor γ down-regulation-related
inflammatory signals in human chondrocytes via Toll-like receptor-4
and receptor for advanced glycation end products. PLoS One.
8:e666112013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shim E and Babu JP: Glycated albumin
produced in diabetic hyperglycemia promotes monocyte secretion of
inflammatory cytokines and bacterial adherence to epithelial cells.
J Periodontal Res. 50:197–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dragone T, Cianciulli A, Calvello R, Porro
C, Trotta T and Panaro MA: Resveratrol counteracts
lipopolysaccharide-mediated microglial inflammation by modulating a
SOCS-1 dependent signaling pathway. Toxicol In Vitro. 28:1126–1135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin X, Yao T, Zhou Z, Zhu J, Zhang S, Hu W
and Shen C: Advanced Glycation End Products Enhance Macrophages
Polarization into M1 Phenotype through Activating RAGE/NF-κB
Pathway. Biomed Res Int. 2015:7324502015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroder K, Zhou R and Tschopp J: The
NLRP3 inflammasome: A sensor for metabolic danger? Science.
327:296–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takahashi M: Role of the inflammasome in
myocardial infarction. Trends Cardiovasc Med. 21:37–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ott C, Jacobs K, Haucke E, Santos
Navarrete A, Grune T and Simm A: Role of advanced glycation end
products in cellular signaling. Redox Biol. 2:411–429. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vénéreau E, Ceriotti C and Bianchi ME:
DAMPs from cell death to new life. Front Immunol. 6:4222015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng A, Dong Y, Zhu F, Liu Y, Hou FF and
Nie J: AGE-LDL activates Toll like receptor 4 pathway and promotes
inflammatory cytokines production in renal tubular epithelial
cells. Int J Biol Sci. 9:94–107e. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shirasuna K, Seno K, Ohtsu A, Shiratsuki
S, Ohkuchi A, Suzuki H, Matsubara S, Nagayama S, Iwata H and
Kuwayama T: AGEs and HMGB1 increase inflammatory cytokine
production from human placental cells, resulting in an enhancement
of monocyte migration. Am J Reprod Immunol. 75:557–568. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shirasuna K, Takano H, Seno K, Ohtsu A,
Karasawa T, Takahashi M, Ohkuchi A, Suzuki H, Matsubara S, Iwata H
and Kuwayama T: Palmitic acid induces interleukin-1β secretion via
NLRP3 inflammasomes and inflammatory responses through ROS
production in human placental cells. J Reprod Immunol. 116:104–112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Rosuvastatin blocks advanced glycation end
products-elicited reduction of macrophage cholesterol efflux by
suppressing NADPH oxidase activity via inhibition of
geranylgeranylation of Rac-1. Horm Metab Res. 43:619–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lorne E, Dupont H and Abraham E: Toll-like
receptors 2 and 4: Initiators of non-septic inflammation in
critical care medicine? Intensive Care Med. 36:1826–1835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yi CO, Jeon BT, Shin HJ, Jeong EA, Chang
KC, Lee JE, Lee DH, Kim HJ, Kang SS, Cho GJ, et al: Resveratrol
activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2
activation in RAW 264.7 macrophage cells. Anat Cell Biol.
44:194–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lappas M, Mitton A, Lim R, Barker G, Riley
C and Permezel M: SIRT1 is a novel regulator of key pathways of
human labor. Biol Reprod. 84:167–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakamaru Y, Vuppusetty C, Wada H, Milne
JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, et
al: A protein deacetylase SIRT1 is a negative regulator of
metalloproteinase-9. FASEB J. 23:2810–2819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Labuzek K, Liber S, Gabryel B, Bułdak L
and Okopień B: Ambivalent effects of compound C (dorsomorphin) on
inflammatory response in LPS-stimulated rat primary microglial
cultures. Naunyn Schmiedebergs Arch Pharmacol. 381:41–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Furuya H, Taguchi A, Kawana K, Yamashita
A, Inoue E, Yoshida M, Nakamura H, Fujimoto A, Inoue T, Sato M, et
al: Resveratrol protects against pathological preterm birth by
suppression of macrophage-mediated inflammation. Reprod Sci.
22:1561–1568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen L, Yang S, Zumbrun EE, Guan H,
Nagarkatti PS and Nagarkatti M: Resveratrol attenuates
lipopolysaccharide-induced acute kidney injury by suppressing
inflammation driven by macrophages. Mol Nutr Food Res. 59:853–864.
2015. View Article : Google Scholar : PubMed/NCBI
|