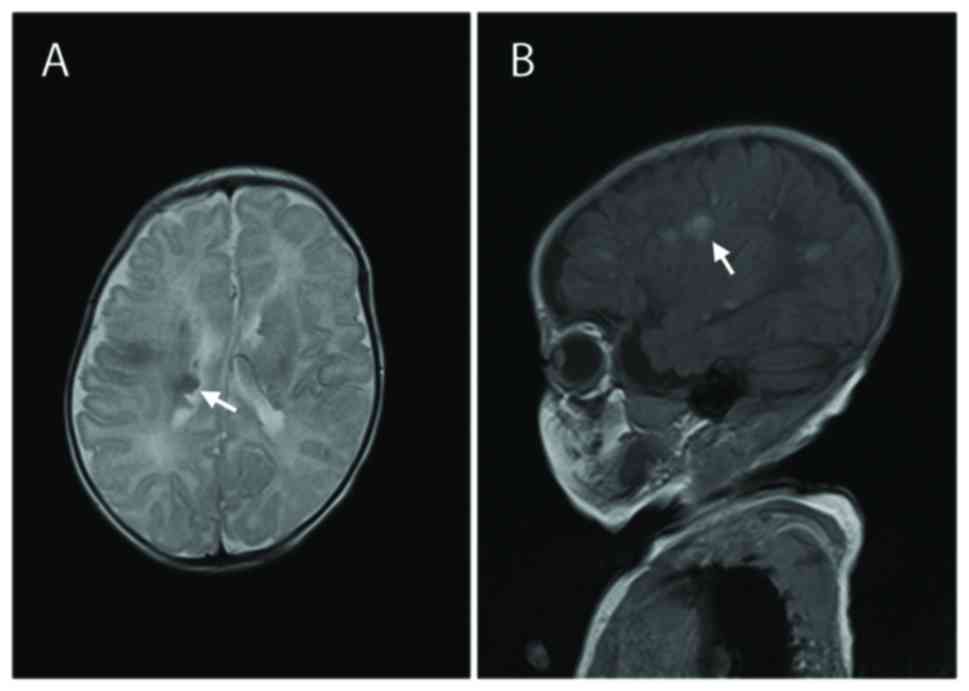
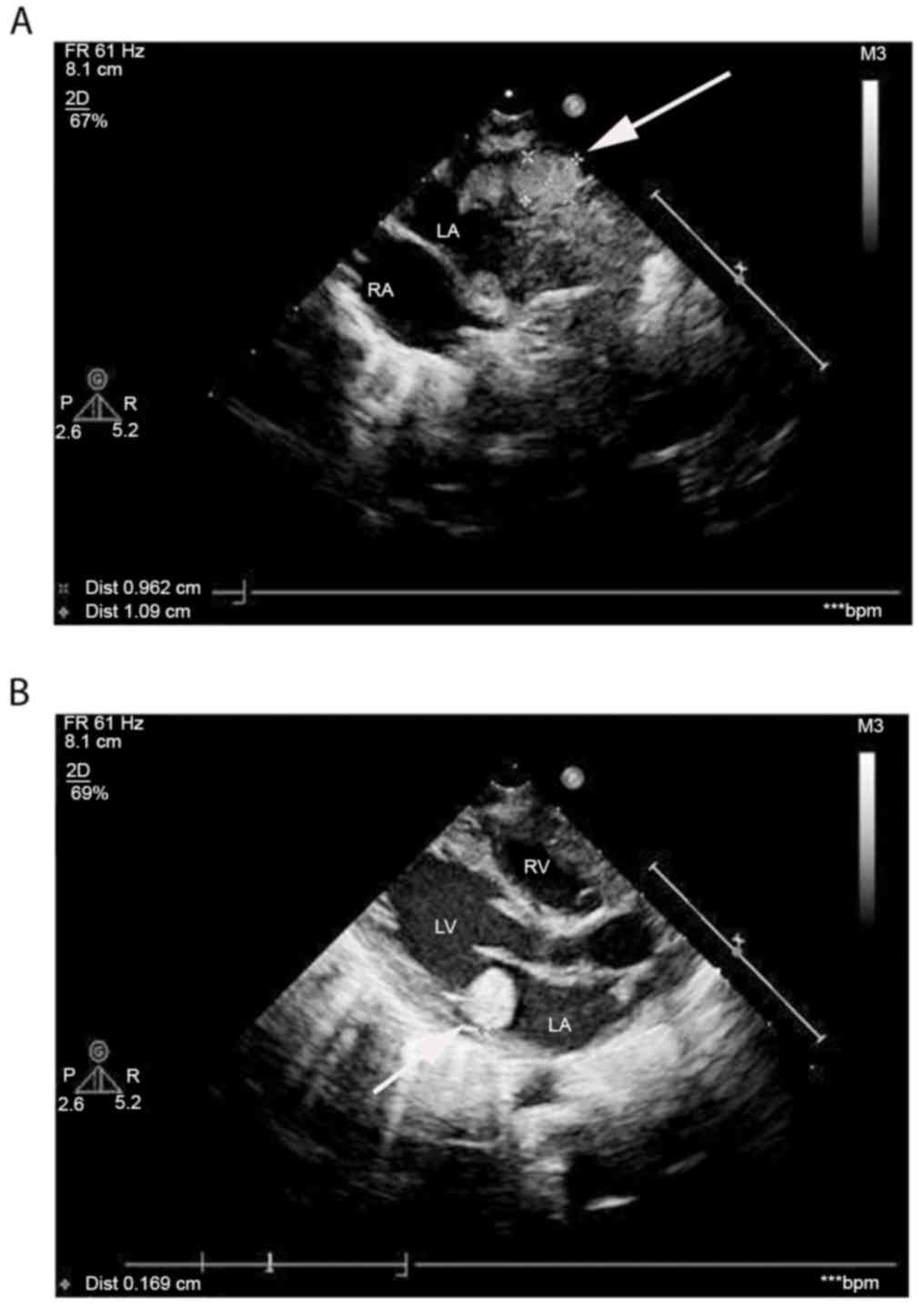
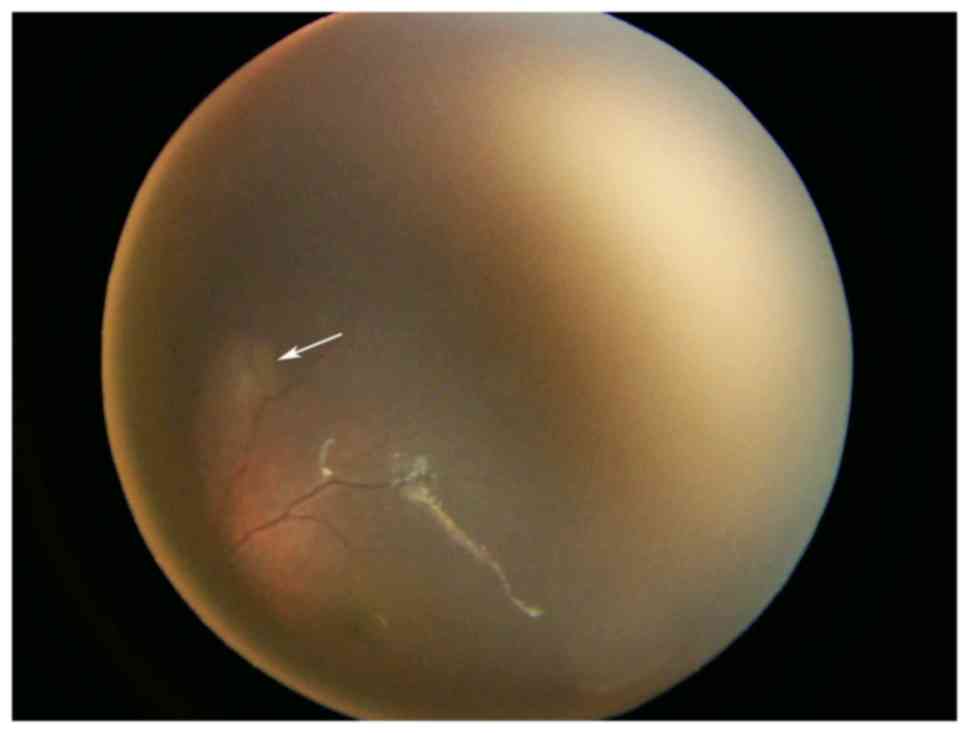
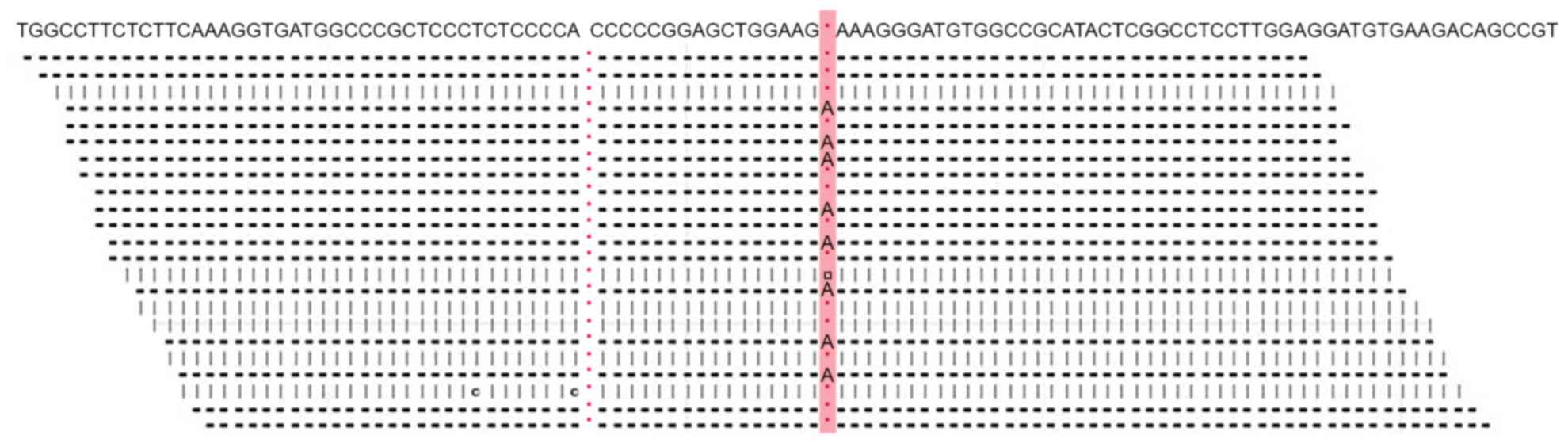
|
1
|
Curatolo P and Maria BL: Tuberous
sclerosis. Handb Clin Neurol. 111:323–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crino PB, Nathanson KL and Henske EP: The
tuberous sclerosis complex. N Engl J Med. 355:1345–1356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wortmann SB, Reimer A, Creemers JW and
Mullaart RA: Prenatal diagnosis of cerebral lesions in Tuberous
sclerosis complex (TSC). Case report and review of the literature.
Eur J Paediatr Neurol. 12:123–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee KA, Won HS, Shim JY, Lee PR and Kim A:
Molecular genetic, cardiac and neurodevelopmental findings in cases
of prenatally diagnosed rhabdomyoma associated with tuberous
sclerosis complex. Ultrasound Obstet Gynecol. 41:306–311. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepherd CW, Gomez MR, Lie JT and Crowson
CS: Causes of death in patients with tuberous sclerosis. Mayo Clin
Proc. 66:792–796. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saxena A and Sampson JR: Epilepsy in
Tuberous Sclerosis: Phenotypes, mechanisms and treatments. Semin
Neurol. 35:269–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pascual-Castroviejo I: Neurosurgical
treatment of tuberous sclerosis complex lesions. Childs Nerv Syst.
27:1211–1219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krueger DA and Northrup H: International
Tuberous Sclerosis Complex Consensus Group: Tuberous sclerosis
complex surveillance and management: Recommendations of the 2012
International Tuberous Sclerosis Complex Consensus Conference.
Pediatr Neurol. 49:255–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Go CY, Mackay MT, Weiss SK, Stephens D,
Adams-Webber T, Ashwal S and Snead OC III: Child Neurology Society;
American Academy of Neurology: Evidence-based guideline update:
Medical treatment of infantile spasms. Report of the Guideline
Development Subcommittee of the American Academy of Neurology and
the Practice Committee of the Child Neurology Society. Neurology.
78:1974–1980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Press Announcements-FDA approves Afinitor
for non-cancerous kidney tumors caused by rare genetic disease.
https://elbiruniblogspotcom.blogspot.ca/2012/04/press-announcements-fda-approves_27.htmlApril
27–2012
|
|
11
|
French JA, Lawson JA, Yapici Z, Ikeda H,
Polster T, Nabbout R, Curatolo P, de Vries PJ, Dlugos DJ, Berkowitz
N, et al: Adjunctive everolimus therapy for treatment-resistant
focal-onset seizures associated with tuberous sclerosis (EXIST-3):
A phase 3, randomised, double-blind, placebo-controlled study. The
Lancet. 388:2153–2163. 2016. View Article : Google Scholar
|
|
12
|
AG Novartis International: Novartis drug
Votubia® receives EU approval to treat refractory
partial-onset seizures in patients with TSC. https://www.novartis.com/news/media-releases/novartis-drug-votubiar-receives-eu-approval-treat-refractory-partial-onsetJanuary
31–2017
|
|
13
|
Canpolat M, Per H, Gumus H, Yikilmaz A,
Unal E, Patiroglu T, Cinar L, Kurtsoy A and Kumandas S: Rapamycin
has a beneficial effect on controlling epilepsy in children with
tuberous sclerosis complex: Results of 7 children from a cohort of
86. Childs Nerv Syst. 30:227–240. 2014. View Article : Google Scholar
|
|
14
|
Gipson TT, Gerner G, Srivastava S, Poretti
A, Vaurio R, Hartman A and Johnston MV: Early Neurodevelopmental
Screening in TSC: A Potential Window of Opportunity. Pediatr
Neurol. 51:398–402. 2014. View Article : Google Scholar
|
|
15
|
Isaacs H Jr: Fetal and neonatal cardiac
tumors. Pediatr Cardiol. 25:252–273. 2004. View Article : Google Scholar
|
|
16
|
Bader RS, Chitayat D, Kelly E, Ryan G,
Smallhorn JF, Toi A and Hornberger LK: Fetal rhabdomyoma: Prenatal
diagnosis, clinical outcome, and incidence of associated tuberous
sclerosis complex. J Pediatr. 143:620–624. 2003. View Article : Google Scholar
|
|
17
|
Verhaaren HA, Vanakker O, De Wolf D, Suys
B, Francois K and Matthys D: Left ventricular outflow obstruction
in rhabdomyoma of infancy: Meta-analysis of the literature. J
Pediatr. 143:258–263. 2003. View Article : Google Scholar
|
|
18
|
Stiller B, Hetzer R, Meyer R, Dittrich S,
Pees C, Alexi-Meskishvili V and Lange PE: Primary cardiac tumours:
When is surgery necessary? Eur J Cardiothorac Surg. 20:1002–1006.
2001. View Article : Google Scholar
|
|
19
|
Napolioni V, Moavero R and Curatolo P:
Recent advances in neurobiology of Tuberous Sclerosis Complex.
Brain Dev. 31:104–113. 2009. View Article : Google Scholar
|
|
20
|
Chu-Shore CJ, Major P, Camposano S,
Muzykewicz D and Thiele EA: The natural history of epilepsy in
tuberous sclerosis complex. Epilepsia. 51:1236–1241. 2010.
View Article : Google Scholar
|
|
21
|
Curatolo P, Aronica E, Jansen A, Jansen F,
Kotulska K, Lagae L, Moavero R and Jozwiak S: Early onset epileptic
encephalopathy or genetically determined encephalopathy with early
onset epilepsy? Lessons learned from TSC. Eur J Paediatr Neurol.
20:203–211. 2016. View Article : Google Scholar
|
|
22
|
Jozwiak S, Kotulska K, Domańska-Pakieła D,
Lojszczyk B, Syczewska M, Chmielewski D, Dunin-Wasowicz D, Kmieć T,
Szymkiewicz-Dangel J, Kornacka M, et al: Antiepileptic treatment
before the onset of seizures reduces epilepsy severity and risk of
mental retardation in infants with tuberous sclerosis complex. Eur
J Paediatr Neurol. 15:424–431. 2011. View Article : Google Scholar
|
|
23
|
Cusmai R, Moavero R, Bombardieri R,
Vigevano F and Curatolo P: Long-term neurological outcome in
children with early-onset epilepsy associated with tuberous
sclerosis. Epilepsy Behav. 22:735–739. 2011. View Article : Google Scholar
|
|
24
|
Mizuguchi M and Takashima S:
Neuropathology of tuberous sclerosis. Brain Dev. 23:508–515. 2001.
View Article : Google Scholar
|
|
25
|
Cardis MA and DeKlotz CM: Cutaneous
manifestations of tuberous sclerosis complex and the
paediatrician's role. Arch Dis Child. 102:858–863. 2017. View Article : Google Scholar
|
|
26
|
Hake S: Cutaneous manifestations of
tuberous sclerosis. Ochsner J. 10:200–204. 2010.
|
|
27
|
Schwartz RA, Fernández G, Kotulska K and
Jóźwiak S: Tuberous sclerosis complex: Advances in diagnosis,
genetics and management. J Am Acad Dermatol. 57:189–202. 2007.
View Article : Google Scholar
|
|
28
|
Knopke S, Olze H, Becker ET, Manthey D,
Lindig-Knopke C, Jöhrens K, Stölzel K and Böttcher A: Head and neck
hamartomas: 10 years of experience at the Charité-University
Medical Center Berlin. HNO. 63:552–556. 2015. View Article : Google Scholar
|
|
29
|
Kingswood JC, Bissler JJ, Budde K, Hulbert
J, Guay-Woodford L, Sampson JR, Sauter M, Cox J, Patel U, Elmslie
F, et al: Review of the Tuberous Sclerosis Renal Guidelines from
the 2012 Consensus Conference: Current Data and Future Study.
Nephron. 134:51–58. 2016. View Article : Google Scholar
|
|
30
|
Korula S, Ekbote A, Kumar N, Danda S,
Agarwal I and Chaturvedi S: Renal manifestations of tuberous
sclerosis among children: An Indian experience and review of the
literature. Clin Kidney J. 7:134–137. 2014. View Article : Google Scholar
|
|
31
|
Rouviere O, Nivet H, Grenier N, Zini L and
Lechevallier E: Kidney damage due to tuberous sclerosis complex:
Management recommendations. Diagn Interv Imaging. 94:225–237. 2013.
View Article : Google Scholar
|
|
32
|
Isaacs H: Perinatal (fetal and neonatal)
tuberous sclerosis: A review. Am J Perinatol. 26:755–760. 2009.
View Article : Google Scholar
|
|
33
|
Martignoni G, Bonetti F, Pea M, Tardanico
R, Brunelli M and Eble JN: Renal disease in adults with TSC2/PKD1
contiguous gene syndrome. Am J Surg Pathol. 26:198–205. 2002.
View Article : Google Scholar
|
|
34
|
Ismail NF, Malik Nik Abdul NM, Mohseni J,
Rani AM, Hayati F, Salmi AR, Narazah MY, Zabidi-Hussin ZA, Silawati
AR, Keng WT, et al: Two novel gross deletions of TSC2 in Malaysian
patients with tuberous sclerosis complex and TSC2/PKD1 contiguous
deletion syndrome. Jpn J Clin Oncol. 44:506–511. 2014. View Article : Google Scholar
|
|
35
|
van Slegtenhorst M, de Hoogt R, Hermans C,
Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A,
Halley D, Young J, et al: Identification of the tuberous sclerosis
gene TSC1 on chromosome 9q34. Science. 277:805–808. 1997.
View Article : Google Scholar
|
|
36
|
European Chromosome 16 Tuberous Sclerosis
Consortium: Identification and characterization of the tuberous
sclerosis gene on chromosome 16. Cell. 75:1305–1315. 1993.
View Article : Google Scholar
|
|
37
|
Kwiatkowski DJ: Rhebbing up mTOR: New
insights on TSC1 and TSC2, and the pathogenesis of tuberous
sclerosis. Cancer Biol Ther. 2:471–476. 2003. View Article : Google Scholar
|
|
38
|
Dabora SL, Jozwiak S, Franz DN, Roberts
PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, et
al: Mutational analysis in a cohort of 224 tuberous sclerosis
patients indicates increased severity of TSC2, compared with TSC1,
disease in multiple organs. Am J Hum Genet. 68:64–80. 2001.
View Article : Google Scholar
|
|
39
|
Kim WS: Mammalian target of rapamycin
inhibitors for treatment in tuberous sclerosis. Korean J Pediatr.
54:241–245. 2011. View Article : Google Scholar
|
|
40
|
Franz DN, Leonard J, Tudor C, Chuck G,
Care M, Sethuraman G, Dinopoulos A, Thomas G and Crone KR:
Rapamycin causes regression of astrocytomas in tuberous sclerosis
complex. Ann Neurol. 59:490–498. 2006. View Article : Google Scholar
|
|
41
|
Hofbauer GF, Marcollo-Pini A, Corsenca A,
Kistler AD, French LE, Wuthrich RP and Serra AL: The mTOR inhibitor
rapamycin significantly improves facial angiofibroma lesions in a
patient with tuberous sclerosis. Br J Dermatol. 159:473–475. 2008.
View Article : Google Scholar
|
|
42
|
Micozkadioglu H, Koc Z, Ozelsancak R and
Yildiz I: Rapamycin therapy for renal, brain, and skin lesions in a
tuberous sclerosis patient. Ren Fail. 32:1233–1236. 2010.
View Article : Google Scholar
|
|
43
|
Rapamune: Prescribing information, .
United States Food and Drug Administration. Wyeth Pharmaceuticals,
Inc.; May. 2015, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021083s058,021110s075lbl.pdfMay
28–2016
|