Introduction
Pulmonary alveolar microlithiasis (PAM) is a
long-term disease, which progresses slowly, producing a gradual
decline in lung function. The disease can show a familial
distribution in approximately 38–61% of cases, belonging to the
group of autosomal recessive genetic diseases (1). X-ray energy dispersive spectroscopy of
the lung suggests that the ratio of phosphorus to calcium ions in
the microlithiasis observed in patients with PAM is 1:2, which is
equivalent to the ratio in calcium phosphate and hydroxyapatite,
suggesting that the main component in the microlithiasis is
phosphate. The SLC34A2 gene encodes a type IIb sodium phosphate
co-transporter (NaPi-Ib), involved in the metabolism of inorganic
phosphorus in vivo (2).
A c575C>A homozygous mutation (T192K phenotype)
has been found in all of three patients of a family affected by
PAM, by sequencing all the exon PCR products of the SLC34A2 gene,
additionally, heterozygous mutations were found in the parents and
children of the patients (3). In
order to further confirm that c.575C>A homozygous mutations
cause the functional changes seen in PAM, in this study, we
constructed a eukaryotic expression vector of the SLC34A2 gene and
transfected it into human alveolar epithelial cells. The effects of
the SLC34A2 and normal gene expression, respectively, on the
phosphorylation of extracellular fluid by the alveolar epithelial
cells was compared.
Materials and methods
Tissue and cell sources
Normal lung tissues were obtained from patients
undergoing pulmonary bulla resections; PAM lung tissues were from
patients undergoing CT-guided percutaneous lung biopsies that
belonged to the same family. The human alveolar epithelial cells
A549 were routinely preserved, recovered, passed and counted for
further use in the laboratory. The study was approved by the Ethics
Committee of the Second Hospital of Jilin University and informed
consents were signed by the patients and/or guardians.
Construction of an SLC34A2 gene
eukaryotic expression vector
Total RNA extractions from lung tissue samples were
performed with a total RNA extraction kit (RNAiso Reagent, Takara
Bio Inc., Dalian, China). Reverse transcription synthesis of cDNA
was performed using a reverse transcription kit (D6110A, Takara Bio
Inc.).
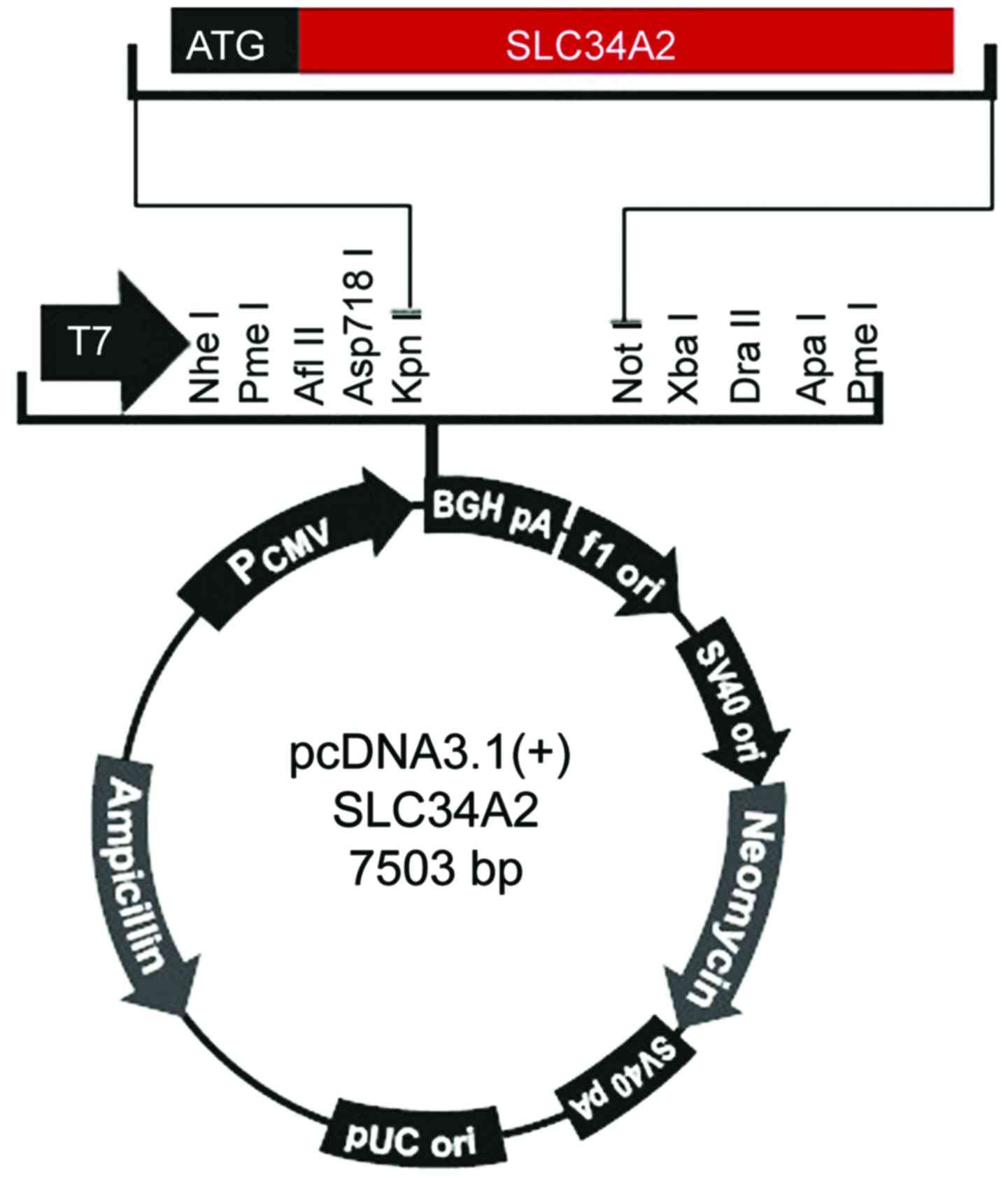
Primers and vectors were constructed according to
the SLC34A2 cDNA sequence (accession no. XM_005248129) included in
Genbank, two primers were designed, vector corresponding
restriction endonuclease recognition sites and protective bases
were added to the 5′ end of the upstream and downstream primers,
respectively. The primers were synthesized and purified by the BGI,
the iterated words were the endonuclease recognition sequences. The
sequence for the upstream primer f was
5′-ACGGTACCTAATGGCTCCTGGCCTGAAT-3′, including a KpnI
endonuclease recognition site and the two protective bases. The
sequence for the downstream primer r was
5′-TAGCGGCCGCCTACAAGGCCGTGCATTCG-3′, containing a NotI
digestion recognition site (Fig. 1).
The size of the target gene fragment was 2073 bp.
For PCR amplification, the reaction system included
4.0 µl cDNA + 4.0 µl dNTP (100 µmol/l) + 1.5 µl of upstream and
downstream primers (50 ng/µl each) + 0.5 µl Premix Ex Taq + 5.0 µl
10X PCR buffer (containing magnesium chloride), adding water to
make the total volume reach 50 µl. The reaction conditions
consisted of 97°C for 5 min, a total of 30 cycles of 94°C for 50
sec, 55°C for 55 sec, and 72°C for 60 sec, and then a final
extension at 72°C for 10 min. The amplified products were analyzed
by 2% agarose gel electrophoresis to determine whether the
electrophoretic bands were consistent with the design.
For extraction of gel purified products, the DNA gel
recovery kit (Takara Bio Inc.) was used. Enzyme digestions were
performed on the pcDNA3.1(+) plasmid (Touching Technology Co.,
Ltd., Shanghai, China) using restriction endonucleases KpnI
and NotI (Takara Bio Inc.). Ligations were done using the
DNA quick connect kit (Takara Bio Inc.). Escherichia coli
DH5α competent cells (iCloning Beijing Biotech Co., Ltd., Beijing,
China) were prepared and used for transformations.
Recombinant plasmids were transformed, screened and
extracted using a plasmid DNA kit (DP103; Tiangen Biochemical
Technology Co., Ltd., Beijing, China). The recombinant plasmid
pcDNA3.1(+)-SLC34A2 was digested with KpnI and NotI
and 2% agarose gel electrophoresis was used to visualize the
resulting fragments. The recombinant plasmid pcDNA3.1(+)-SLC34A2
(normal) and pcDNA3.1(+)-SLC34A2 (PAM) with the target gene was
conserved in glycerol, and sent to Beijing BGI for sequencing.
Cell transfection
For plasmid extractions, 20 µl of bacterial liquid
cultures containing pcDNA3.1(+) plasmid, pcDNA3.1(+)-SLC34A2
(normal) plasmid and pcDNA3.1(+)-SLC34A2 (PAM) plasmid were
inoculated into 100 ml LB medium with ampicillin, respectively, and
incubated overnight for 15 h. The plasmids were extracted according
to the endotoxin free plasmid DNA Maxi kit (DP117; Tiangen
Biochemical Technology Co., Ltd.). Liposome-mediated transfections
of A549 cells were performed in three groups: empty vector plasmid
pcDNA3.1(+) transfection (plasmid control group), normal SLC34A2
gene-linked pcDNA3.1(+) recombinant plasmid transfected (PAM
group), pcDNA3.1(+) plasmid recombined with PAM SLC34A2 gene (PAM
group).
The transfection efficiency was detected by reverse
transcription PCR (RT-PCR). RNA was extracted using the TRIzol
method (Takara Bio Inc.), cDNA was synthesized with reverse
transcription kit, PCR primers, amplification system and the
conditions were the same as above, products were identified by
electrophoresis.
Detection of inorganic phosphorus
concentration in culture medium
After 48 h of co-cultivation, the concentration of
inorganic phosphorus was determined using a Hitachi 3700 automatic
biochemical analyzer (Beijing no. 6 plant) using the phosphorus
molybdenum blue colorimetric detection method. Measurements were
repeated three times and average values were obtained for each
experiment.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (SPSS, IL, USA). The measurement data are expressed as
mean ± standard deviation. Single factor ANOVA analysis was used
for comparisons among the groups, and the LSD-t method was used for
multiple comparisons. A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
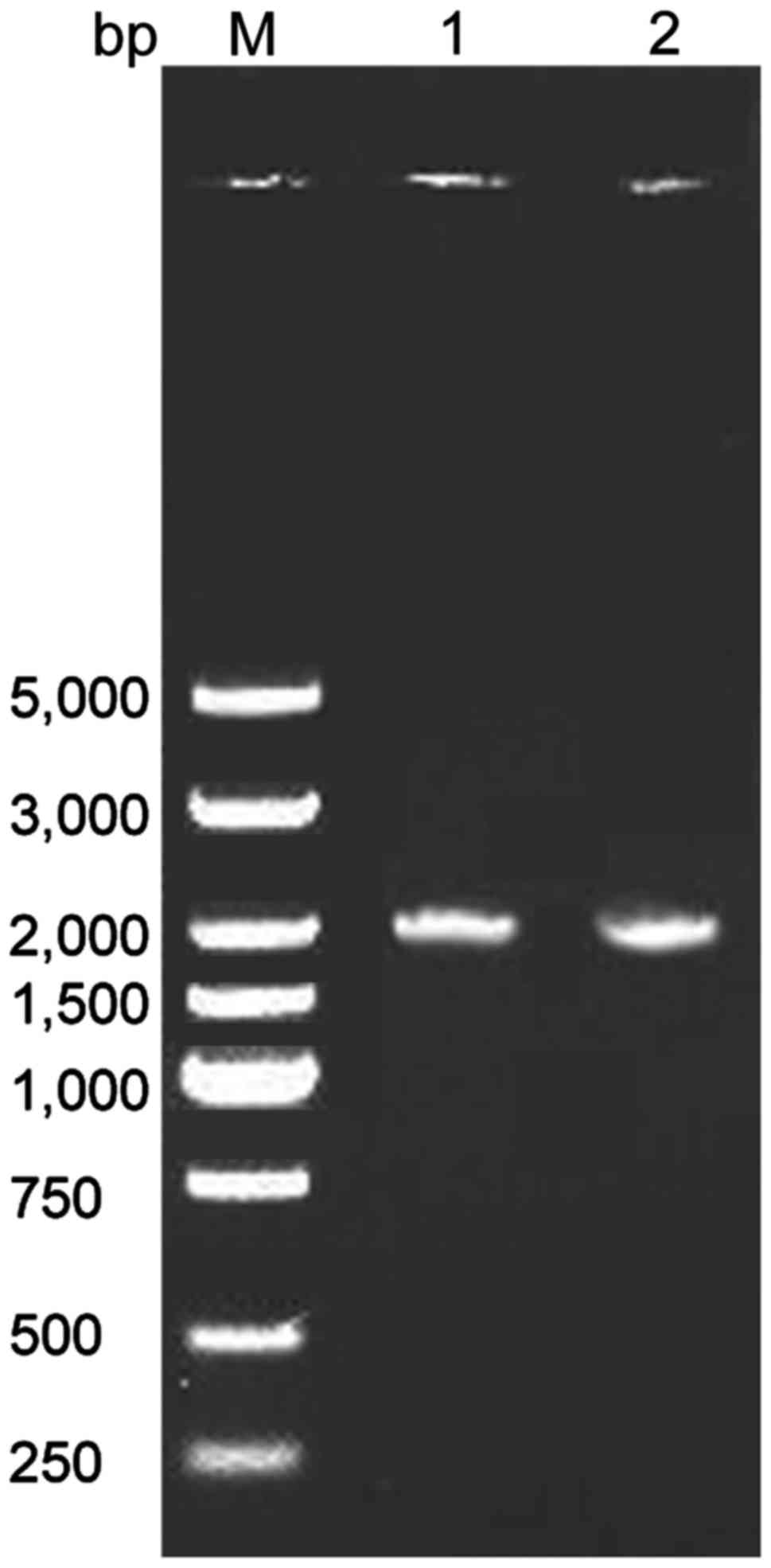
RT-PCR results of SLC34A2 gene
The results showed that the amplified fragments of
SLC34A2 gene in normal and PAM patients were approximately 2000 bp,
in agreement with the expected size for SLC34A2 (Fig. 2).
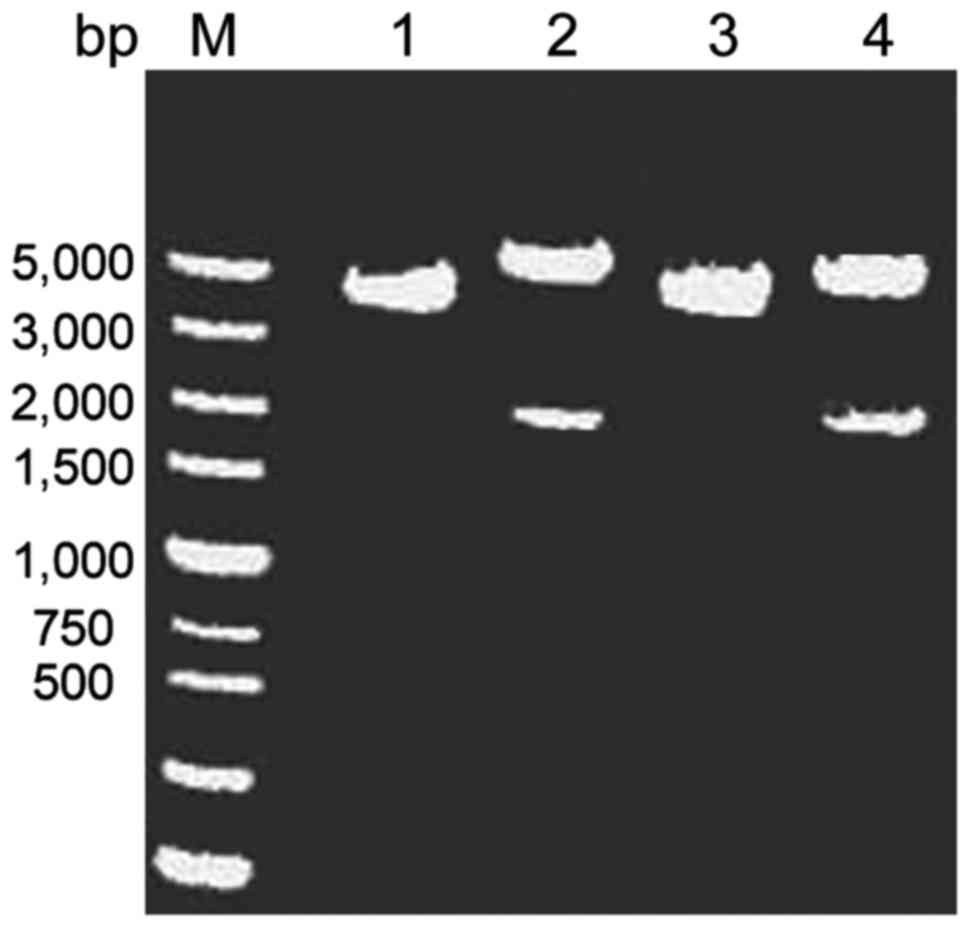
Construction and identification of
recombinant plasmids
The length of the pcDNA3.1(+) plasmid was 5428 bp,
when amplifying SLC34A2 gene, 2 bp of protective bases were added,
the length of the gene was 2075 bp; therefore, the length of the
recombinant plasmid was calculated to be 7503 bp. After double
digestion and electrophoresis, two fragments were generated. The
large fragment was located at approximately 5000 bp, corresponding
to the pcDNA3.1(+) plasmid fragment (5428 bp), and the small
fragment was at approximately 2000 bp, which corresponded to the
PCR product (2075 bp), indicating that the target gene fragment was
successfully attached to the vector pcDNA3.1(+) (Fig. 3).
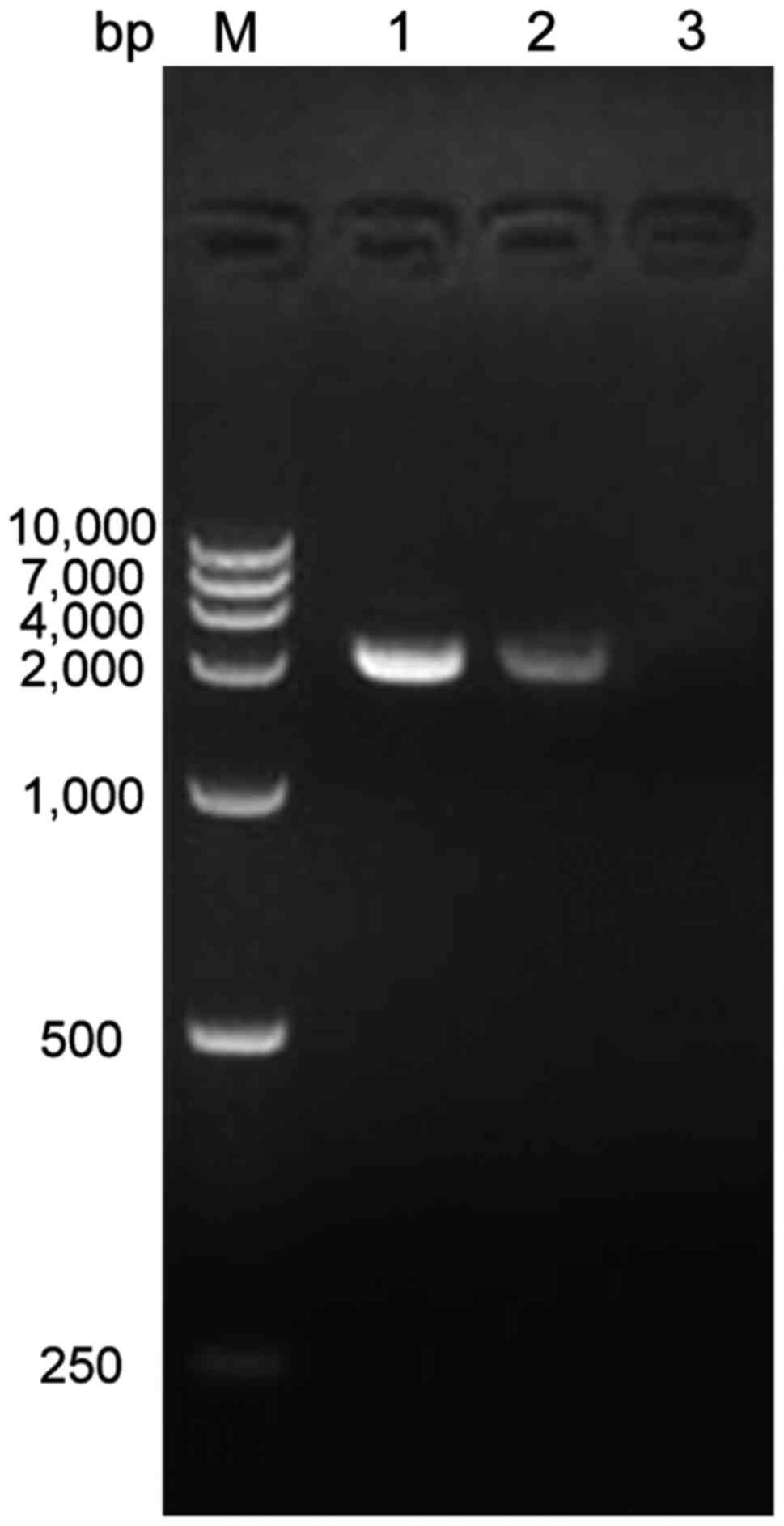
RT-PCR results of recombinant plasmid
transfected cells
The results showed that the amplified fragment of
SLC34A2 gene in the normal control group and PAM group was both at
approximately 2000 bp, which was consistent with the size of
SLC34A2. The plasmid control group did not contain the SLC34A2
gene, therefore no electrophoretic band appeared (Fig. 4).
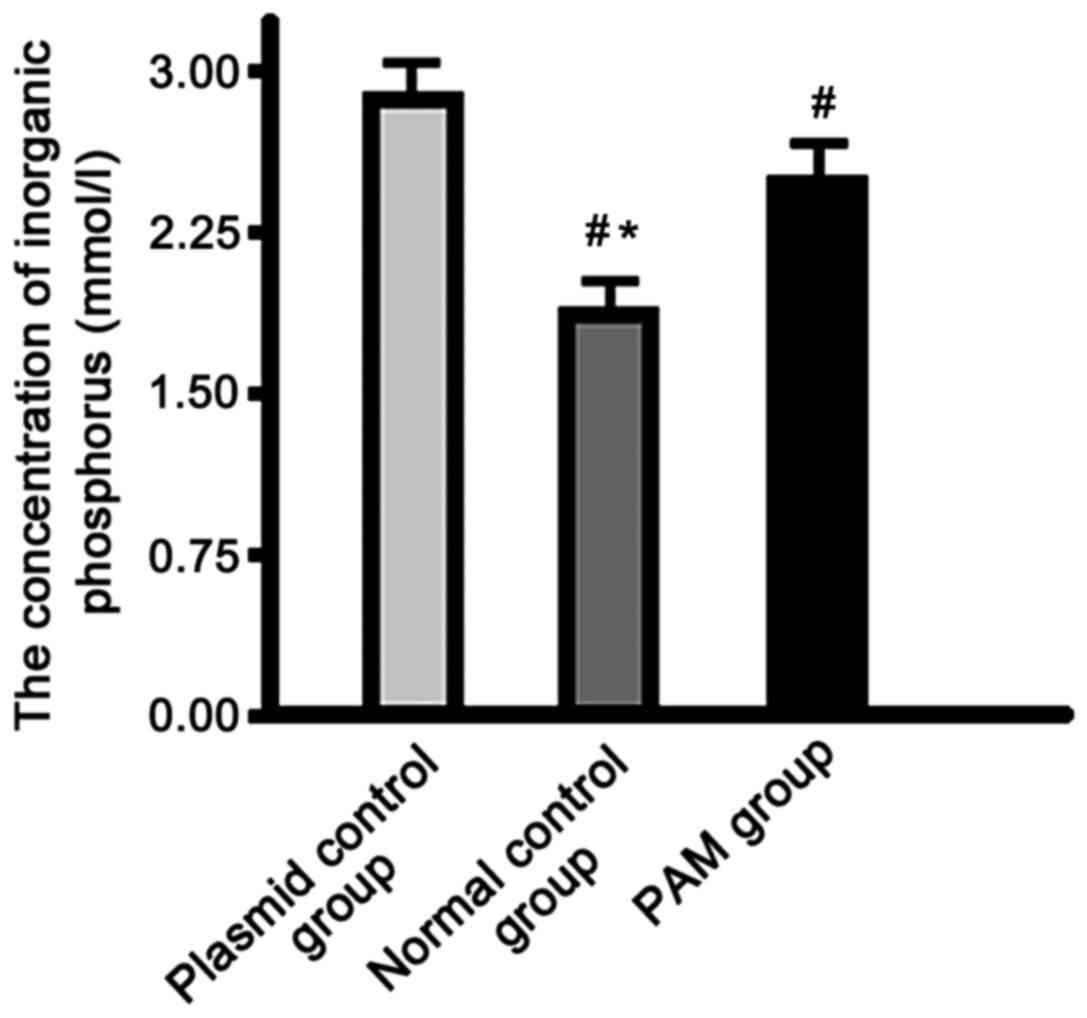
Determination of inorganic phosphorus
concentration
The concentration of inorganic phosphorus in the
supernatant of the normal control group was significantly lower
than that in the plasmid control and PAM groups, and the PAM group
showed significantly lower levels than the control group
(P<0.01) (Fig. 5).
Discussion
The SLC34A2 gene has 12 exons and its total gene
fragment is long (4). In order to
clone the complete coding sequence of the gene, total RNA from lung
tissues was extracted, reverse transcribed with Oligo (dT) and the
amplified with specific primers to obtain the target gene sequence.
In this way, we were able to remove the intron sequences and retain
the complete coding sequence, which was needed to construct the
eukaryotic expression vector in pvcDNA3.1(+). This plasmid contains
a strong promoter of the cytomegalovirus at its 5′ end, the
beginning of the replication site contains also the SV40 promoter,
which expresses exogenous genes efficiently in mammalian cells, the
expression of ampicillin and neomycin resistance genes allowed
screening for the positive recombinant vector after transformation
(4). The identity of our expression
vectors was confirmed by restriction digestion and sequencing. The
human lung adenocarcinoma cell line A549 exhibits characteristics
of lung cancer malignant tumor cells and the phenotype of alveolar
type II epithelial cells. The cell line is commonly used in the
study of the occurrence, differentiation and structure and function
of normal alveolar type II epithelial cells (5). Lipofectamine 2000 cationic
liposome-mediated eukaryotic cell transfections are highly
efficient, and the lipofectamine technology is the most widely used
transfection method employed in research these days.
The concentration of inorganic phosphorus in the
supernatant of the normal control group was significantly lower
than that in the plasmid control and the PAM groups, and the
concentration in the PAM group was significantly lower than that in
the plasmid control group. This suggests that the SLC34A2 gene
mutation can reduce the phosphate transport function in PAM
patients, which may be lead to the characteristic pathological
changes observed in them. The most important part of inorganic
phosphorus metabolism in the human body is intestinal, and
intestinal inorganic phosphorus metabolism mainly relies on
NaPi-Ib. NaPi-Ib protein shows a segmental expression in the small
intestine. It displays its most abundant expression in the ileal
brush edge, and is little or almost undetectable in the duodenum
and jejunum (6). Nicotinamide can
inhibit the expression of NaPi-Ib in the brush edge of the jejunum,
thereby reducing intestinal absorption of phosphorus (7); the antiviral drug foscarnet
co-transports with NaPi-Ib in the small intestinal epithelial
cells, competitively inhibiting phosphorus absorption, therefore
reducing the level of phosphorus (8). The expression of NaPi-Ib has been
detected from the 16.5 days in the rat embryo, and the expression
of NaPi-Ib gets gradually increased during development in the lung
(7). The SLC34A2 gene is expressed
only in type II alveolar epithelium cells in the lungs (8), the main component of pulmonary
surfactant produced by type II alveolar epithelial cells is
dipalmitoyl lecithin. Phosphate is an important component of
phospholipids, phosphate released by degraded phospholipids can be
transported to the cells by NaPi-Ib in the form of
NaH2PO4 in the presence of sodium ions, that
is alveolar phosphorus metabolism depends mainly on the normal
function of NaPi-Ib (9,10).
Our results showed that after transfection with the
normal SLC34A2 gene, the resulting cells could more effectively
transport the phosphate from the culture supernatant, causing the
concentration of inorganic phosphorus in the culture supernatant to
be significantly decreased. Furthermore, the concentrations in the
supernatant were significantly lower than those in the control and
PAM groups. Cells with the PAM mutated SLC34A2 gene expressed from
the recombinant plasmid were still able to transport phosphate,
therefore the concentration of phosphate in the medium decreased
compared with that in the plasmid control group, but increased
compared to that in the normal control group. Due to the SLC34A2
gene exon 6 c.575C>A homozygous mutation in PAM patients, the
original threonine is mutated into a lysine, resulting in a
decreased function of the NaPi-Ib. The mutant transporter results
in an impaired phosphate transport function, the phosphorus ion
cannot be completely cleaned from the alveoli and phosphate is
deposited in large quantities in the alveoli, where it finally
combines with calcium ions to form calcium phosphate
(microlithiasis) (11,12). However, the transporter's function in
the mutant is not completely lost, so PAM patients show no
significant clinical symptoms during childhood and adolescence,
when the microlithiasis is still negligible. During the
asymptomatic stages, the alveolar and lobular septal structure is
complete, gas exchange is not affected, and normal lung function is
maintained (13,14). But with the progress of the disease,
calcium phosphate deposition accumulates, and microlithiasis
gradually ensues. When eventually the entire alveolar cavity is
filled, and the alveolar wall pressure increases, alveolar injury,
inflammatory infiltration, alveolar wall thickening and
interstitial fibrous tissue hyperplasia begins to appear in the
alveoli, resulting in a restrictive ventilatory dysfunction and
decreased diffuse function, leading to gradually deteriorating lung
function (15,16).
The identification of genes involved in the
development of PAM has resulted in early and accurate diagnosis of
sporadic cases and the diagnosis of asymptomatic family members in
the PAM family. However, several traditional therapies developed
according to PAM's molecular biology theory have proved to be
ineffective (15). New effective
treatments are sorely needed. For example, the cystic fibrosis
transmembrane regulation treatment may be used for PAM patients in
the future (16). Furthermore, gene
therapy methods are expected to be developed as more reliable
information becomes available, studies like this one contribute to
the growing body of knowledge that should form the basis of such
novel approaches.
Acknowledgements
The present study was supported by the Norman
Bethune Program of Jilin University (2015417).
References
|
1
|
Samano MN, Waisberg DR, Canzian M, Campos
SV, Pêgo-Fernandes PM and Jatene FB: Lung transplantation for
pulmonary alveolar microlithiasis: A case report. Clinics (Sao
Paulo). 65:233–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jankovic S, Pavlov N, Ivkosic A, Erceg I,
Glavina-Durdov M, Tocilj J, Dragisic-Ivulic S and Primorac D:
Pulmonary alveolar microlithiasis in childhood: Clinical and
radiological follow-up. Pediatr Pulmonol. 34:384–387. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Proesmans M, Boon M, Verbeken E, Ozcelik
U, Kiper N, Van de Casseye W and De Boeck K: Pulmonary alveolar
microlithiasis: A case report and review of the literature. Eur J
Pediatr. 171:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishihara Y, Hagiwara K, Zen K, Huqun,
Hosokawa Y and Natsuhara A: A case of pulmonary alveolar
microlithiasis with an intragenetic deletion in SLC34A2 detected by
a genome-wide SNP study. Thorax. 64:365–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hagiwara K, Johkoh T and Tachibana T:
Molecular basis of lung disease, insight from rare lung
disordersPulmonary Alveolar Microlithiasis. Panos R, Trapnell B and
McCormack F: Humana Press; Totowa, NJ: pp. 154–156. 2009
|
|
6
|
Izumi S Huqun, Miyazawa H, Ishii K,
Uchiyama B and Ishida T: The autozygous segments predicted by a
genome-wide SNP typing revealed mutations in the type IIb sodium
phosphate co-transporter (SLC34A2) causing pulmonary alveolar
microlithiasis. Proc Am Thorac Soc. 3:pp. A1022006;
|
|
7
|
Corut A, Senyigit A, Ugur SA, Altin S,
Ozcelik U, Calisir H, Yildirim Z, Gocmen A and Tolun A: Mutations
in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly
associated with testicular microlithiasis. Am J Hum Genet.
79:650–656. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izumi S Huqun, Miyazawa H, Ishii K,
Uchiyama B, Ishida T, Tanaka S, Tazawa R, Fukuyama S, Tanaka T, et
al: Mutations in the SLC34A2 gene are associated with pulmonary
alveolar microlithiasis. Am J Respir Crit Care Med. 175:263–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Izumi H, Kurai J, Kodani M, Watanabe M,
Yamamoto A, Nanba E, Adachi K, Igishi T and Shimizu E: A novel
SLC34A2 mutation in a patient with pulmonary alveolar
microlithiasis. Hum Genome Var. 4:160472017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poelma DL, Ju MR, Bakker SC, Zimmermann
LJ, Lachmann BF and van Iwaarden JF: A common pathway for the
uptake of surfactant lipids by alveolar cells. Am J Respir Cell Mol
Biol. 30:751–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dogan OT, Ozsahin SL, Gul E, Arslan S,
Koksal B, Berk S, Ozdemir O and Akkurt I: A frame-shift mutation in
the SLC34A2 gene in three patients with pulmonary alveolar
microlithiasis in an inbred family. Intern Med. 49:45–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin X, Wang H, Wu D, Zhao G, Shao J and
Dai Y: SLC34A2 Gene mutation of pulmonary alveolar microlithiasis:
Report of four cases and review of literatures. Respir Med.
107:217–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monabati A, Ghayumi MA and Kumar PV:
Familial pulmonary alveolar microlithiasis diagnosed by
bronchoalveolar lavage. A case report. Acta Cytol. 51:80–82. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong YQ, Hu CP, Cai XD and Nie HP: A
novel mutation of the SLC34A2 gene in a Chinese pedigree with
pulmonary alveolar microlithiasis. Zhonghua Yi Xue Yi Chuan Xue Za
Zhi. 26:365–368. 2009.(In Chinese). PubMed/NCBI
|
|
15
|
Tachibana T, Hagiwara K and Johkoh T:
Pulmonary alveolar microlithiasis: Review and management. Curr Opin
Pulm Med. 15:486–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozcelik U, Yalcin E, Ariyurek M, Ersoz DD,
Cinel G, Gulhan B and Kiper N: Long-term results of disodium
etidronate treatment in pulmonary alveolar microlithiasis. Pediatr
Pulmonol. 45:514–517. 2010.PubMed/NCBI
|