Introduction
Licorice root (Glycyrrhiza uralensis Fisch)
is a traditional Chinese herbal medicine widely used in China and
other Asian countries as a tonic herbal medicine, which is used to
invigorate systems or promote general health (1). Glycyrrhizic acid is a water-soluble
extract of the dried licorice rhizome that possesses
anti-inflammatory, anti-oxidative and liver-protective effects
(2–4)
and has two stereoisomers (18α and 18β). Magnesium
isoglycyrrhizinate (MgIG) is a magnesium salt of 18α-glycyrrhizic
acid, which exhibits important pharmacological activities. Previous
studies have demonstrated that MgIG may inhibit inflammatory
responses in through the phospholipase A2/arachidonic
acid and signal transducer and activator of transcription 3
pathways thus protecting liver function (5,6).
Furthermore, MgIG protects liver cells from hypoxia-reoxygenation,
ischemia/reperfusion and free fatty acid-induced injury (7–9). It has
been suggested that MgIG exhibits potential hepatoprotective
activity against hepatotoxicity induced by anticancer drugs
(10). However, the effects and
potential mechanisms of action of MgIG on the liver and heart
remain unknown. Therefore, the current study aimed to identify the
protective effects of MgIG on impairment in the liver and heart
induced by doxorubicin (DOX).
DOX was introduced in the 1970s and has since become
one of the most commonly used anthracycline antibiotics to treat
hematological and solid tumors (11,12).
However, even at clinically relevant doses, the therapeutic
application of DOX is limited by systemic toxicity, including
cardiotoxicity, hepatotoxicity and nephrotoxicity (13,14). DOX
promotes reactive oxygen species (ROS), which mediates these organ
toxicities (15–17). Cardiomyocytes are more vulnerable to
attacks by oxygen free radicals since they are susceptible to ROS
and contain lower levels of antioxidants, including glutathione
peroxidase (GSH-Px) and superoxide dismutase (SOD) than other
organs (16,18). The liver is another organ that is
particularly sensitive to DOX, due to its metabolism and
detoxification activities. It is estimated that ~40% of patients
treated with DOX experience liver injury, since large amounts of
DOX accumulate and are metabolized in the liver (15,19).
Furthermore, the concurrent administration of DOX, paclitaxel and
docetaxel enhances oxidative stress in the liver (20).
The present study investigated the effects and
potential mechanisms of MgIG in DOX-treated mice to determine
whether MgIG exhibits hepatoprotective and cardioprotective
effects, in order to discern whether treatment with MgIG may be an
effective method of limiting the toxicity induced by DOX.
Materials and methods
Reagents
MgIG was supplied by Chia Tai Tianqing
Pharmaceutical Group Co., Ltd. (Lianyungang, China; drug approval
number, H20051942). DOX hydrochloride was purchased from Zhejiang
Hisun Pharmaceutical Co. Ltd. (Zhejiang, China; drug approval
number, H33021980).
Animals
A total of 50 male Kunming mice weighing 20.0±2.0 g
(4–5 weeks old) were purchased from the Laboratory Animal Center of
Hebei Medical University (Shijiazhuang, China). The mice were
housed in rust-free cages at 20–22°C and 45–55% relative humidity
on a 12 h light-dark cycle, and given a normal pelleted diet and
tap water ad libitum. All mice were handled according to the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (21). All experiments were
approved by the Ethics Committee for Animal Experiments of Hebei
Medical University (approval number, HEBMU-2015-01; approval date,
January 22, 2015).
Experimental design
Following a 1-week adaptation period, mice were
randomly and divided into 5 equal groups (n=10/group): A control
group [control, saline (normal saline 0.01 ml/g/day),
intraperitoneal (i.p.)]; a model group (DOX, 30 mg/kg, i.p.); and
treatment groups that received low-dose MgIG (L-MgIG, 10 mg/kg/day,
i.p.), middle-dose MgIG (M-MgIG, 20 mg/kg/day, i.p.) and high-dose
MgIG (H-MgIG, 40 mg/kg/day, i.p.). Mice in the treatment groups
were administered MgIG for 1 week; then mice in the treatment and
model groups were administered with 30 mg/kg DOX once. Following 48
h, all mice were weighed and anesthetized with sodium pentobarbital
(50 mg/kg, i.p.). When the mice were anesthetized, blood was
sampled from the eyes of the mice. The blood stood in the room
temperature 30–60 min, and then the serum was separated
(centrifuged at 2,200 × g for 10 min at room temperature) for
biochemical analysis and liver and heart samples were quickly
excised and snap frozen in liquid nitrogen or fixed in a 4%
paraformaldehyde solution. The samples were then excised and
examined as described below.
Histopathological analysis
Hepatic and cardiac tissue samples were fixed in 4%
paraformaldehyde solution for ≥1 week at room temperature and
embedded in paraffin. Paraffin-embedded samples were cut to
4-µm-thick sections, mounted on glass slides and stained with
hematoxylin and eosin (H&E) for 50 min at room temperature for
histopathological analysis, according to the conventional staining
steps. To analyze the staining results, micrographs were scanned at
×400 magnification with a digital light microscope (Leica DM750;
Leica Microsystems GmbH, Wetzlar, Germany).
Serum biochemical analysis
Changes in the activity of the cardiac-specific
enzymes creatine kinase (CK) (22),
CK-MB and lactate dehydrogenase (LDH) in the serum were determined.
Furthermore, levels of the two hepatic-specific enzymes aspartate
aminotransferase (AST) (23) and
alanine aminotransferase (ALT), the oxidative stress markers SOD
and GSH-Px, and methane dicarboxylic aldehyde (MDA) were
measured.
The activity of CK (cat. no. A032), CK-MB (cat. no.
E006) and LDH (cat. no. A020-1) in the serum was determined at 37°C
following the guidelines of the relevant assay kits (Nanjing
Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China).
Serum levels of AST and ALT were detected by
spectrophotometry-based methods following the guidelines of the AST
(cat. no. C010-1) and ALT (cat. no. C009-1) assay kits (Nanjing
Jiancheng Bioengineering Institute Co., Ltd.). SOD (cat. no.
A001-1) and GSH-Px (cat. no. A005) activity, and MDA (cat. no.
A003-1) serum levels were measured following the manuals of the
corresponding assay kits (Nanjing Jiancheng Bioengineering
Institute Co., Ltd.). All procedures were conducted in strict
accordance with the manufacturers protocols.
Western blot analysis
Total proteins were obtained from frozen liver and
heart tissue homogenates following the manufacturers protocol. The
samples were homogenized and lysed with RIPA lysis buffer
containing 50 mM Tris-HCl, 125 mM sodium chloride, 5 mM sodium
pyrophosphate, 50 mM sodium fluoride, 1 mM EDTA, 1 mM
dithiothreitol, 0.1% SDS (w/v), 1% TritonX-100 (v/v) and protease
inhibitor cocktail (all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). After incubation for 20 min on ice, the lysis buffer solution
was centrifuged at 4°C at 7,800 × g for 20 min. The protein
concentration was measured using a Bradford Protein Assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). ~50 µg of
total proteins were loaded and separated on 10% SDS-PAGE, and then
transferred to a nitrocellulose membrane. Membranes were blocked
with blocking buffer (20 mM Tris-buffered saline and 0.1% Tween-20)
containing 5% (w/v) non-fat milk for 2 h at room temperature and
then incubated with the membrane was incubated with the primary
antibody overnight at 4°C. The primary antibodies were are follows:
Rabbit anti-caspase-3 (dilution 1:1,000; cat. no., sc-7148; Santa
Cruz Biotechnology, Inc.), rabbit anti-B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax; dilution 1:300; cat. no.,
BA0315; Wuhan Boster Biological Technology, Ltd., Wuhan, China),
rabbit anti-Bcl-2 (dilution 1:1,000; cat. no., BS1031; Bioworld
Technology, Inc., St. Louis Park, MN, USA), rabbit anti-nuclear
factor (NF)-κBp65 (dilution 1:1,000; cat. no., BS1253; Bioworld
Technology, Inc.) and mouse anti-β-actin (dilution 1:2,000; cat.
no., TA-09; ZSGB-BIO; OriGene Technologies, Inc., Rockville, MD,
USA) antibodies. Horseradish peroxidase conjugated goat anti-rabbit
immunoglobulin (Ig)G (dilution 1:3,000; cat. no., ZB2305; ZSGB-BIO;
OriGene Technologies, Inc.) or goat anti-mouse IgG (dilution,
1:3,000; cat. no., ZB2301; ZSGB-BIO; OriGene Technologies, Inc.)
were used as the secondary antibodies. The membrane was incubated
with the secondary antibodies at room temperature for 1 h. The
bound antibodies were visualized using Amersham ECL Western
Blotting Detection Reagent (GE Healthcare Life Sciences, Little
Chalfont, UK) and quantified by densitometry using an image
analyzer. The gray values of the hybridized bands were analyzed
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA). β-actin was used as an internal protein
control for normalization.
Terminal deoxynucleotidyl transferase
mediated dUTP nick end labeling (TUNEL) assay
TUNEL staining was performed using an in situ
cell death detection kit (Roche Applied Science, Mannheim, Germany)
following the manufacturers protocol. Briefly, liver and the heart
sections were deparaffinized, dehydrated using a series of
increasing concentrations of alcohol, washed in distilled water
followed by PBS and deproteinized using proteinase K (20 µg/ml) for
30 min at 37°C. Subsequently, sections were rinsed and incubated
with the TUNEL reagent at 37°C for 1 h. Following rinsing, the
sections were visualized using a peroxidase-conjugated
anti-fluorescein antibody (in the TUNEL kit) with 0.02%
3,3-diaminobenzidine (Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) and then counterstained with 0.5% hematoxylin
at room temperature for 10–30 sec. Neutral balsam (Shanghai Guchen
Biotechnology Co., Ltd., Shanghai, China) was used to bond the
slides and cover glass together. To analyze the staining results,
micrographs were scanned at ×400 magnification with a digital light
microscope system (Leica DM750). An image analysis system
(Image-Pro Plus software; version 6.0) was used to analyze 20
randomly selected fields per slide at magnification ×400 to
determine the area of positive staining.
Data analysis
All data are provided as the mean ± standard error
of the mean. Differences among the groups were assessed by one-way
analysis of variance followed by Kruskal-Wallis and Tukeys tests.
The statistical analysis software, Origin (version 7.5; OriginLab,
Northampton, MA, USA) and SPSS (version 22.0; IBM Corp., Armonk,
NY, USA) were used. P<0.05 was considered to indicate a
statistically significant difference.
Results
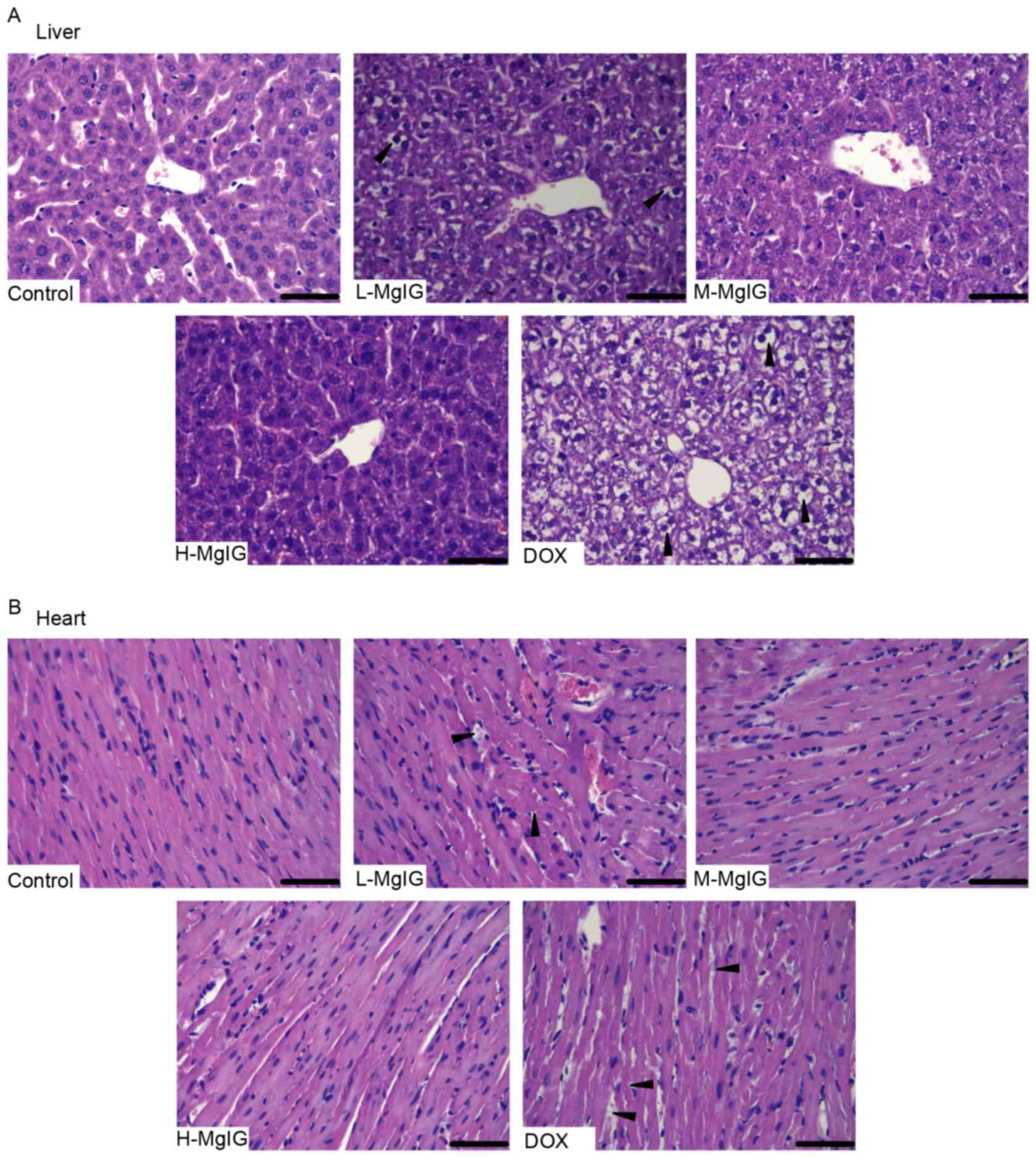
Amelioration of morphological changes
by MgIG
H&E staining was used to observe the
histological changes of the liver and heart induced by DOX and the
therapeutic effects of MgIG. Liver and heart tissue taken from the
control group exhibited normal architecture (Fig. 1). However, in the DOX group, the
hepatocytes became fatty, the nuclei were swollen and the liver
cells dissolved due to putrescence, as indicated by the black
triangles (Fig. 1A). Cardiomyocytes
in the DOX group exhibited a disordered arrangement, breaks and
necrosis, as indicated by the black triangles (Fig. 1B). However, in the MgIG pre-treatment
groups, there was a decrease in the number of lesions in the liver
and heart (Fig. 1A and B). These
effects were greater in the medium-dose and high-dose MgIG
groups.
Amelioration of biochemical index
changes by MgIG
The changes in activity of the two hepatic-specific
enzymes AST and ALT and the three cardiac-specific enzymes CK,
CK-MB and LDH were measured in the serum to determine the
protective effects of MgIG against DOX-induced damage in the liver
and heart. As presented in Table I,
AST and ALT activities in the DOX group (258.61±9.85 and
146.62±4.52 IU/l, respectively) increased ~2 to 3-fold compared
with the control group (121.79±6.24 and 56.68±2.45 IU/l,
respectively). However, serum ALT and AST activities in the MgIG
pre-treatment groups were all significantly lower than in the DOX
group (P<0.01). CK, CK-MB and LDH activity increased by ~2-fold
in the DOX group compared with the control group. However, serum
CK, CK-MB, and LDH activities in the MgIG pre-treatment groups were
significantly lower than in the DOX group (P<0.01). The effects
of MgIG in the liver and heart were dose-dependent, with greater
decreases in serum ALT, AST, CK, CK-MB and LDH activity occurring
in the groups treated with higher doses of MgIG.
 | Table I.Effects of MgIG injection on
biochemical indexes changes in serum. |
Table I.
Effects of MgIG injection on
biochemical indexes changes in serum.
| Groups | AST (IU/l) | ALT (IU/l) | CK (IU/l) | CK-MB (IU/l) | LDH (IU/l) |
|---|
| Control |
121.79±6.24 |
56.68±2.45 |
494.01±18.37 |
208.55±9.78 |
300.90±13.14 |
| L-MgIG |
180.48±7.23b |
94.49±3.69b |
645.93±26.01b |
298.37±11.92b |
357.58±13.97b |
| M-MgIG |
172.37±6.36b |
87.78±3.44b |
630.50±23.62b |
296.63±9.98b |
337.94±13.27b |
| H-MgIG |
151.59±6.32b |
79.64±2.42b |
628.47±28.60b |
268.44±12.39b |
326.35±12.65b |
| DOX |
258.61±9.85a |
146.62±4.52a |
894.92±31.65a |
376.91±12.71a |
512.81±16.23a |
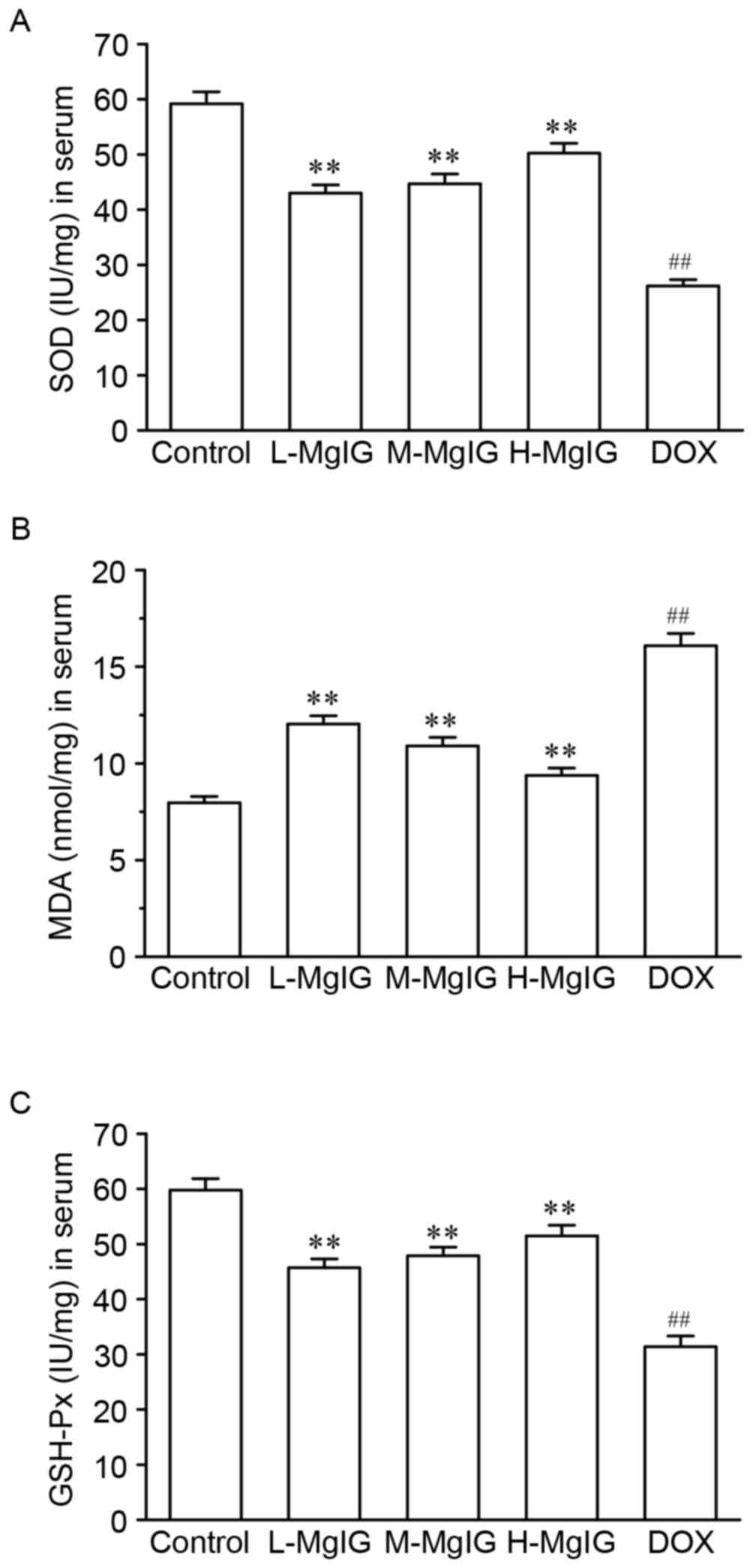
Inhibition of oxidative stress by
MgIG
Levels of the three oxidative stress markers SOD,
GSH-Px and MDA in the serum were measured to assess the oxidative
stress induced by DOX (Fig. 2).
Compared with the control group, SOD and GSH-Px levels were
significantly reduced (SOD: Control, 59.18±2.17 IU/mg vs. DOX,
26.18±1.14 IU/mg; GSH-Px: Control, 59.78±2.08 IU/mg vs. DOX,
31.39±1.94 IU/mg), whereas MDA levels were significantly increased
in the DOX group (MDA: Control, 7.96±0.33 nmol/mg vs. DOX,
16.08±0.64 nmol/mg). However, pre-treatment with MgIG significantly
increased SOD and GSH-Px and reduced MDA levels compared with the
DOX group in a dose-dependent manner (P<0.01).
Suppression of apoptosis by MgIG
Levels of several apoptotic markers were measured by
western blot analysis in the liver and heart tissues (Fig. 3). Compared with the control group,
the expression of Bax/Bcl-2, caspase-3 and NF-κBp65 were
significantly upregulated in the DOX groups (all P<0.01).
However, in the MgIG pre-treatment groups, the expression of
Bax/Bcl-2, caspase-3 and NF-κBp65 were significantly reduced in a
dose-dependent manner compared with the DOX group (all P<0.01).
This indicates that MgIG treatment increases the anti-apoptotic
abilities of hepatocytes and cardiomyocytes.
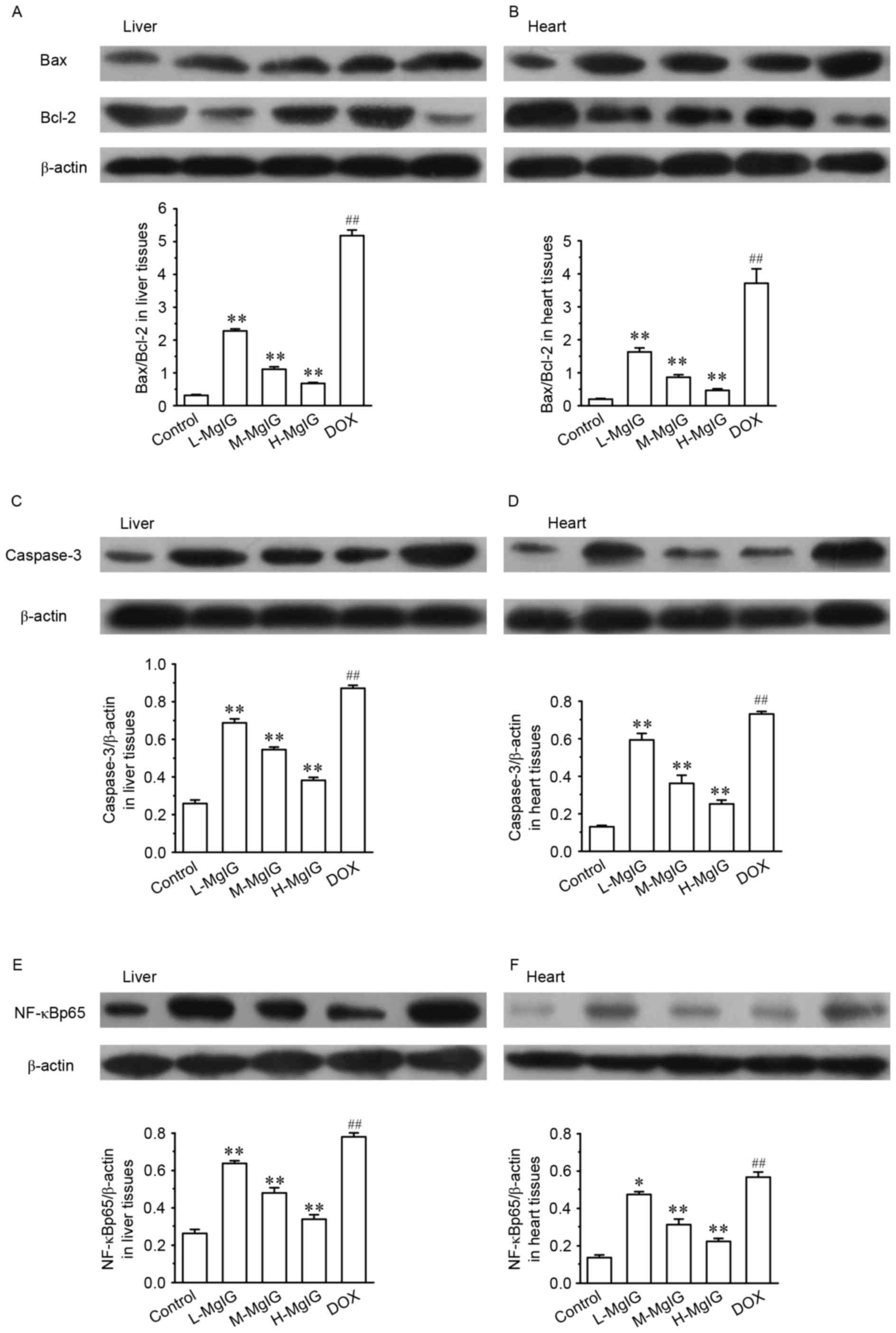 | Figure 3.Effects of MgIG treatment on the
expression of (A and B) Bax, Bcl-2, (C and D) caspase-3 and (E and
F) NF-κBp65 in liver and heart tissues, measured by western blot
analysis. The calculated relative intensities of Bax/Bcl-2,
caspase-3/β-actin, and NF-κBp65/β-actin are presented under the
representative immunoblots for each group. Data are presented the
mean ± standard error of the mean. *P<0.05 and **P<0.01 vs.
the DOX group; ##P<0.01 vs. the control group. MgIG,
magnesium isoglycyrrhizinate; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; NF-κBp65, nuclear factor κBp65; DOX,
doxorubicin; L-MgIG, low-dose MgIG; M-MgIG, medium-dose MgIG;
H-MgIG, high-dose MgIG. |
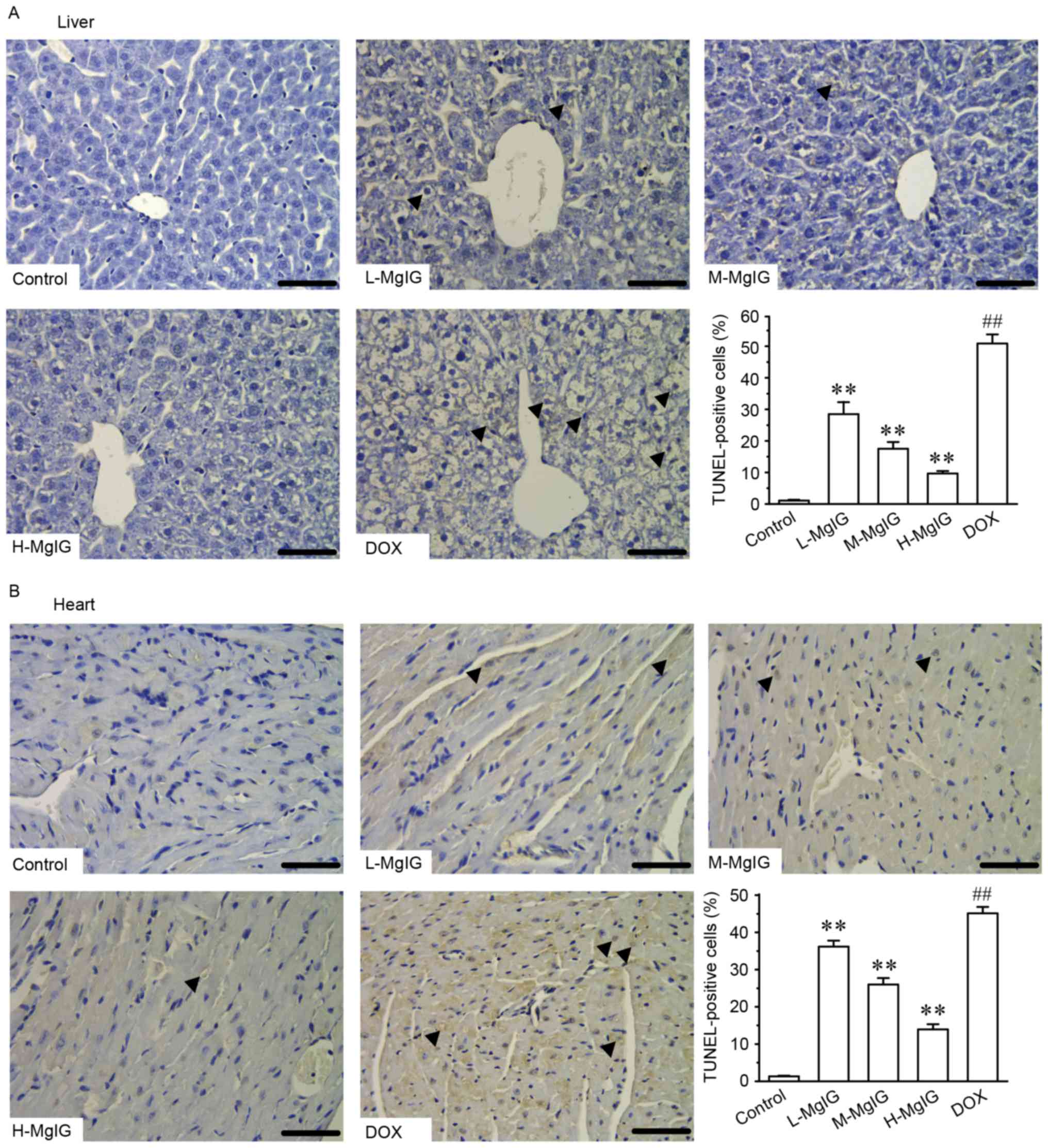
Additionally, TUNEL staining was used to assess
apoptosis in cardiac and hepatic cells. Apoptosis in hepatic and
cardiac cells remained at consistently low levels in the control
group (Fig. 4). However, the number
of TUNEL-positive cardiac and hepatic cells was significantly
increased in the DOX group compared with the control group
(P<0.01). However, treatment with MgIG significantly decreased
the extent of apoptosis compared with the DOX group in a
dose-dependent manner (P<0.01).
Discussion
Licorice is described in one of the oldest
materia medica texts, ‘Shennongs Classic Materia Medica’ and
has been used to treat various ailments in China for centuries due
to its beneficial properties (24).
Glycyrrhizic acid is one of its primary active components and
exhibits antiviral and antimicrobial activities (24). As an important stereoisomer of
glycyrrhizic acid, MgIG is commonly used as a hepatoprotective
medicine, which induces fewer side-effects than other similar
treatments (25). Furthermore, it
has been demonstrated that MgIG exhibits hepatoprotective activity
against the hepatotoxicity induced by anticancer drugs (10), in a liver injury model induced by
hepatectomy (6) and in a
hypoxia/reoxygenation injury (ischemia/reperfusion) model in liver
cells (8). The results of the
present indicated that MgIG exhibits hepato- and cardioprotective
effects. The results of H&E staining obtained from liver and
heart tissues demonstrate that the extent of injuries induced by
DOX was decreased in mice that received MgIG pre-treatment,
indicating that MgIG exhibits hepato- and cardioprotective
effects.
Transaminase is an indicator of liver function and
is indispensable as a catalyst in the process of human metabolism.
The serum levels of AST and ALT increase rapidly when liver cells
are damaged by inflammation, necrosis or poisoning (26). It was demonstrated that serum levels
of AST and ALT in the MgIG-treatment groups were significantly
lower than in the DOX group, indicating that MgIG exerts its
protective effects in the liver by regulating the activity of AST
and ALT. To examine the cardioprotective effects, the serum levels
of CK, CK-MB and LDH, which are usually used in the evaluation of
myocardial injury (27–29), were measured in the present study.
MgIG reversed the increase in the activities of myocardial enzymes
induced by DOX. This indicates that MgIG may ameliorate heart
injury by regulating the activity of AST and ALT.
Over the past few decades, the number of cancer
cases has increased. More patients require treatment for cancer and
experience severe side-effects following treatment with various
anti-cancer drugs (30,31). DOX is used clinically as an
anti-cancer drug, however its therapeutic application is been
limited due to the serious adverse reactions experienced by
patients, which affect the heart, liver and other organs (13,14). In
the current study, a high dose of DOX was used to induce
cardiotoxicity and hepatotoxicity in mice. The results revealed
that MgIG may, at least in part, alleviate the acute toxicity
induced by DOX. Its underlying mechanism of action may be
associated with the antioxidant stress and the anti-apoptotic
activities of MgIG.
Following acute stimulus with high doses of DOX, the
body may generate excessive free radicals, thus triggering massive
peroxidation and resulting in antioxidant depletion (32–34). SOD
and GSH-Px belong to the ROS scavenger enzymatic system and may
neutralize certain reactive molecules and counterbalance the
oxidative destruction induced by free radicals (35,36). MDA
is a representative aldehydic lipid peroxidative product that is
poisonous to cells (37). The
present study indicated that serum levels of SOD and GSH-Px were
reduced and that concentrations of MDA were elevated in DOX-treated
mice, demonstrating that peroxidation was induced by DOX. In all
groups receiving treatment with MgIG, SOD and GSH-Px levels
increased, whereas those of MDA decreased. The results of previous
studies indicated that oxidative stress is responsible for the
cardiotoxicity and hepatotoxicity induced by DOX and is associated
with decreased activity of the antioxidant system (20,38,39). As
a consequence, it was hypothesized that MgIG may improve the bodys
antioxidant capacity, which may be an important mechanism of MgIG
in resisting damage to the liver and heart induced by DOX.
Elevated ROS may induce apoptotic cell death and
damage to tissues and organs (40–42).
NF-κBp65 is predominant in the apoptotic regulation process by
regulating the expression of downstream Bcl-2 genes. Bax
participates in apoptotic responses via intrinsic
mitochondrial-dependent signaling by inducing the release of
cytochrome c (23,43–45).
Another cell death protease is caspase-3, which serves a role in
cell apoptosis. The activation of apoptotic pathways finally
results in the activation of caspase-3 (46). In the present study, the expression
of Bax/Bcl-2, caspase-3 and NF-κBp65 were all significantly
increased in liver and heart tissues of the group exposed to DOX.
These observations reveal that exposure to high-doses of DOX
enhances apoptosis. However, treatment with MgIG significantly
reduced expression of the apoptotic factors Bax/Bcl-2, caspase-3
and NF-κBp65 and decreased the number of TUNEL-positive cells in
the liver and heart tissues. Furthermore, the effects of MgIG in
the high-dose group were greater compared with the other groups,
indicating that MgIG acts in a dose-dependent manner. This may be
another important molecular mechanism by which MgIG protects
against the hepatotoxicity and cardiotoxicity induced by DOX.
In conclusion, the results of the present study
demonstrate that MgIG may help to ameliorate DOX-induced liver and
heart injuries in mice. One of its mechanisms of action may be its
anti-lipid peroxidation and anti-apoptotic effects in hepatocytes
and cardiomyocytes. These observations indicate that MgIG may be
used as a novel treatment to protect the liver and heart from
adverse reactions induced by anti-cancer drugs.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81573669)
and the Heibei Province Key Research Project of Medical Science of
2016 of Health and Family Planning Commission of Hebei (grant no.
20160181).
References
|
1
|
Andersen-Parrado P: Licorice: Not just
candy, but a tonic herb with myriad healing properties. Better
Nutrition. 59:33–34. 1997.
|
|
2
|
Cosmetic Ingredient Review Expert Panel, .
Final report on the safety assessment of glycyrrhetinic acid,
potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate,
glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl
glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate,
dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium
glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate.
Int J Toxicol. 26 Suppl 2:S79–S112. 2007. View Article : Google Scholar
|
|
3
|
Ming LJ and Yin AC: Therapeutic effects of
glycyrrhizic acid. Nat Prod Commun. 8:415–418. 2013.PubMed/NCBI
|
|
4
|
van Rossum TG, Vulto AG, de Man RA,
Brouwer JT and Schalm SW: Review article: Glycyrrhizin as a
potential treatment for chronic hepatitis C. Aliment Pharmacol
Ther. 12:199–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie C, Li X, Wu J, Liang Z, Deng F, Xie W,
Zhu M, Zhu J, Zhu W, Geng S, et al: Anti-inflammatory activity of
magnesium isoglycyrrhizinate through inhibition of phospholipase
a2/arachidonic acid pathway. Inflammation. 38:1639–1648. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang GH, Yang HY, Zhang JC, Ren JJ, Sang
XT, Lu X, Zhong SX and Mao YL: Magnesium isoglycyrrhizinate
inhibits inflammatory response through STAT3 pathway to protect
remnant liver function. World J Gastroenterol. 21:12370–12380.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng J, Wu G, Hu GX, Peng YZ and Xiong
XJ: Protective effects against and potential mechanisms underlying
the effect of magnesium isoglycyrrhizinate in hypoxia-reoxygenation
injury in rat liver cells. Genet Mol Res. 14:15453–15461. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang X, Qin J and Lu S: Magnesium
isoglycyrrhizinate protects hepatic L02 cells from
ischemia/reperfusion induced injury. Int J Clin Exp Pathol.
7:4755–4764. 2014.PubMed/NCBI
|
|
9
|
Cheng Y, Zhang J, Shang J and Zhang L:
Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2
cells by magnesium isoglycyrrhizinate in vitro. Pharmacology.
84:183–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vincenzi B, Armento G, Spalato Ceruso M,
Catania G, Leakos M, Santini D, Minotti G and Tonini G:
Drug-induced hepatotoxicity in cancer patients-implication for
treatment. Expert Opin Drug Saf. 15:1219–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalyanaraman B: Teaching the basics of
redox biology to medical and graduate students: Oxidants,
antioxidants and disease mechanisms. Redox Biol. 1:244–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aleisa AM, Al-Rejaie SS, Bakheet SA,
Al-Bekari AM, Al-Shabanah OA, Al-Majed A, Al-Yahya AA and Qureshi
S: Effect of metformin on clastogenic and biochemical changes
induced by adriamycin in Swiss albino mice. Mutat Res. 634:93–100.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panda S and Kar A:
Periplogenin-3-O-D-glucopyranosyl
-(1->6)-D-glucopyaranosyl-(1->4) -D-cymaropyranoside,
isolated from Aegle marmelos protects doxorubicin induced
cardiovascular problems and hepatotoxicity in rats. Cardiovasc
Ther. 27:108–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zordoky BN, Anwar-Mohamed A, Aboutabl ME
and El-Kadi AO: Acute doxorubicin toxicity differentially alters
cytochrome P450 expression and arachidonic acid metabolism in rat
kidney and liver. Drug Metab Dispos. 39:1440–1450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Mei X, Yuan J, Lu W, Li B and Xu
D: Taurine zinc solid dispersions attenuate doxorubicin-induced
hepatotoxicity and cardiotoxicity in rats. Toxicol Appl Pharmacol.
289:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Q, Chen L, Lu Q, Sharma S, Li L,
Morimoto S and Wang G: Quercetin attenuates doxorubicin
cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol.
171:4440–4454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Indu R, Azhar TS, Nair A and Nair CK:
Amelioration of doxorubicin induced cardio-and hepato-toxicity by
carotenoids. J Cancer Res Ther. 10:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalyanaraman B, Joseph J, Kalivendi S,
Wang S, Konorev E and Kotamraju S: Doxorubicin-induced apoptosis:
Implications in cardiotoxicity. Mol Cell Biochem 234–235. 1–124.
2002.
|
|
19
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pieniazek A, Czepas J, Piasecka-Zelga J,
Gwoździński K and Koceva-Chyła A: Oxidative stress induced in rat
liver by anticancer drugs doxorubicin, paclitaxel and docetaxel.
Adv Med Sci. 58:104–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Institute of Health, . Guide for
the Care and Use of Laboratory Animals. The National Academies
Press; Washington, DC: 1996
|
|
22
|
Arola OJ, Saraste A, Pulkki K, Kallajoki
M, Parvinen M and Voipio-Pulkki LM: Acute doxorubicin
cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res.
60:1789–1792. 2000.PubMed/NCBI
|
|
23
|
Wang L, Yang R, Yuan B, Liu Y and Liu C:
The antiviral and antimicrobial activities of licorice, a
widely-used Chinese herb. Acta Pharm Sin B. 5:310–315. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Q, Wang J, Liu R, Wang Z, Li Y, Zhang
Y, Hao X, Huang Y, Xie W and Wei H: Amelioration of concanavalin
A-induced autoimmune hepatitis by magnesium isoglycyrrhizinate
through inhibition of CD4(+)CD25(−)CD69(+) subset proliferation.
Drug Des Devel Ther. 10:443–453. 2016.PubMed/NCBI
|
|
25
|
Navarro VJ and Senior JR: Drug-related
hepatotoxicity. N Engl J Med. 354:731–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang JP, Zhang YY, Zhang Y, Gao YG, Ma
JJ, Wang N, Wang JY, Xie Y, Zhang FH and Chu L: Salvia miltiorrhiza
(Danshen) injection ameliorates iron overload-induced cardiac
damage in mice. Planta Med. 79:744–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dawson EA, Shave R, George K, Whyte G,
Ball D, Gaze D and Collinson P: Cardiac drift during prolonged
exercise with echocardiographic evidence of reduced diastolic
function of the heart. Eur J Appl Physiol. 94:305–309. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan EM, Thomas MJ, Bandy B and Tibbits
GF: Effects of doxorubicin, 4-epirubicin, and antioxidant enzymes
on the contractility of isolated cardiomyocytes. Can J Physiol
Pharmacol. 74:904–910. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shirakami Y, Sakai H and Shimizu M:
Retinoid roles in blocking hepatocellular carcinoma. Hepatobiliary
Surg Nutr. 4:222–228. 2015.PubMed/NCBI
|
|
30
|
Shrum B, Costello P, McDonald W, Howlett
C, Donnelly M and McAlister VC: In vitro three dimensional culture
of hepatocellular carcinoma to measure prognosis and responsiveness
to chemotherapeutic agents. Hepatobiliary Surg Nutr. 5:204–208.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
González-Vallinas M and Breuhahn K:
MicroRNAs are key regulators of hepatocellular carcinoma (HCC) cell
dissemination-what we learned from microRNA-494. Hepatobiliary Surg
Nutr. 5:372–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conklin KA: Chemotherapy-associated
oxidative stress: Impact on chemotherapeutic effectiveness. Integr
Cancer Ther. 3:294–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Wang H, Cui L, Zhang Y, Liu Y,
Chu X, Liu Z, Zhang J and Chu L: Continuing treatment with Salvia
miltiorrhiza injection attenuates myocardial fibrosis in chronic
iron-overloaded mice. PLoS One. 10:e01240612015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fridovich I: Oxygen radicals from
acetaldehyde. Free Radic Biol Med. 7:557–558. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forman HJ and Dickinson DA: Introduction
to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule.
Free Radic Biol Med. 37:594–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gutteridge JM: Lipid peroxidation and
antioxidants as biomarkers of tissue damage. Clin Chem.
41:1819–1828. 1995.PubMed/NCBI
|
|
38
|
Zhou S, Palmeira CM and Wallace KB:
Doxorubicin-induced persistent oxidative stress to cardiac
myocytes. Toxicol Lett. 121:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalender Y, Yel M and Kalender S:
Doxorubicin hepatotoxicity and hepatic free radical metabolism in
rats. The effects of vitamin E and catechin. Toxicology. 209:39–45.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robinson P, Kasembeli M, Bharadwaj U,
Engineer N, Eckols KT and Tweardy DJ: Substance P receptor
signaling mediates doxorubicin-induced cardiomyocyte apoptosis and
triple-negative breast cancer chemoresistance. Biomed Res Int.
2016:19592702016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krishnamurthy B, Rani N, Bharti S,
Golechha M, Bhatia J, Nag TC, Ray R, Arava S and Arya DS:
Febuxostat ameliorates doxorubicin-induced cardiotoxicity in rats.
Chem Biol Interact. 237:96–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beere HM, Wolf BB, Cain K, Mosser DD,
Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM and Green DR:
Heat-shock protein 70 inhibits apoptosis by preventing recruitment
of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng B, Wu L, Ma L, Liu S, Li L, Xie W
and Li X: Telekin induces apoptosis associated with the
mitochondria-mediated pathway in human hepatocellular carcinoma
cells. Biol Pharm Bull. 36:1118–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagai K, Oda A and Konishi H: Theanine
prevents doxorubicin-induced acute hepatotoxicity by reducing
intrinsic apoptotic response. Food Chem Toxicol. 78:147–152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Muller I, Niethammer D and Bruchelt G:
Anthracycline-derived chemotherapeutics in apoptosis and free
radical cytotoxicity (Review). Int J Mol Med. 1:491–494.
1998.PubMed/NCBI
|