Introduction
Recent clinical trials with the aim of developing
tumor antigen (TA)-specific cancer vaccines against a number of
malignancies have focused on the identification of TAs presented by
tumor cells and recognized by T cells (1,2).
Although cancer vaccines that aim to enhance antitumor immune
responses in cancer patients are an attractive approach, current
vaccines remain ineffective in achieving tumor regression (3). Cancer vaccines using TA-derived
peptides are a current therapeutic strategy; however, their
applications are limited due to difficulties with human leukocyte
antigen (HLA) restriction, HLA-peptide binding affinity,
immunogenicity and antigenicity. To develop effective and broadly
applicable vaccines, a number of approaches are currently being
used to design modified peptides with enhanced functional
activities, relative to the parental peptide, including multiple
epitope peptides or longer peptides covered with multiple epitopes
(4). The function of these may be
further enhanced by administration with professional
antigen-presenting cells (5,6). Cancer vaccines may also be developed
through the use of whole recombinant TA proteins as antigens. Using
this method, various epitopes are simultaneously presented to T
cells, as whole TA proteins contain multiple HLA class I and class
II epitopes that are recognized by cluster of differentiation
(CD)-4+ and CD8+ T cells, respectively.
Furthermore, target patients are not restricted by the type of HLA
allele. Many systems now exist for the production of recombinant
proteins, and each of these systems have advantages and
disadvantages with regard to time, cost and risk of endotoxin
contamination (7). A recombinant
protein production system using transgenic (TG) silkworms has
recently been documented by our group, whereby a yeast
transcription activator protein (GAL4) and upstream activating
sequence for GAL4 (UAS) system was observed to be an effective
technique for transgenic gene expression (8). Using this GAL4/UAS system, it was
demonstrated that functional human µ-opioid receptor was expressed
in the TG silkworm (9,10). As silk thread produced by silkworms
may be used as a surgical suture material, the risk of
contamination from endotoxin and allergic reactions is unlikely in
patients. Therefore, recombinant TA protein synthesis by a TG
silkworm system may be useful in the development of cancer
vaccines.
The present study aimed to determine whether
proteins obtained from TG silkworms may be used in the development
of cancer vaccines. Among the TAs available, melanoma antigen
family A4 (MAGE-A4) was selected in the current preliminary study.
MAGE-A4 is typically expressed in a number of malignancies,
including melanoma, head and neck cancer and lung cancer, while
only being expressed in the testis and placenta of normal adult
tissues (11–13). In addition, cancer vaccine clinical
trials using MAGE-A4 peptides and proteins have been documented
(14,15). In the present study, the TA MAGE-A4
protein was produced using a TG silkworm system. Using in
vitro stimulation (IVS), it was subsequently determined whether
MAGE-A4 protein induced MAGE-A4-specific T cells from peripheral
blood mononuclear cells (PBMCs) of healthy donors. Results
suggested that TA proteins produced by a TG silkworm system may be
useful in the development of cancer vaccines.
Materials and methods
Construction of expression
vectors
Plasmids expressing TG silkworm constructs
containing the MAGE-A4 gene were prepared as follows: The MAGE-A4
gene (GenBank accession no. AB464618) was amplified from a Flexi
ORF Clone pF1KB9825 plasmid (Kazusa DNA Research Institute, Chiba,
Japan) using the primers BsmBI_MAGE_U (forward,
5′-GCGTCTCCAGCTATGTCTTCTGAGCAGAAG-3′) and BsmBI_MAGE_His_L
(reverse, 5′-GCGTCTCCCTAGTGATGATGATGGTGATGGACTCCCTCTTCCTCC-3′) in
order to insert a histidine tag sequence for protein purification
and restriction enzyme BsmBI sites (underlined) for gene
construction. The MAGE-A4 gene fragment amplified by polymerase
chain reaction [1 unit of KOD plus polymerase (Toyobo Co., Ltd.,
Osaka, Japan)], 5 µM forward and reverse primers, 1 mM
MgCl2, 1 mM dNTPs, 0.1 µg template plasmid, and 1X
buffer supplied by the manufacturer; Toyobo Co., Ltd., and a
temperature program of 94°C for 30 sec, 55°C for 30 sec and 72°C
for 90 sec for 15 cycles) using a thermal cycler (C-1000; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The resultant DNA fragment
was digested with BsmBI (New England BioLabs, Inc., Ipswich, MA,
USA) at 37°C for 2 h. The fragment was then ligated into the BsmBI
site of the pBac[SerUAS_Ser1intron_hr5/3×P3-AmCyan_A3-Bla] plasmid
(16) by using ligation high version
2 (Toyobo Co., Ltd.) at 16°C for 10 h. The resultant plasmid,
pBac[UAS_MAGEA4/3×P3-AmCyan], carrying a MAGE-A4 gene under a UAS
promoter and an AmCyan gene as a selection marker, was used to
generate TG silkworms.
Generation of TG silkworms
TG silkworms were generated as described previously
(16–18). Briefly, the silkworm strain w1-pnd,
which is non-diapausing and produces non-pigmented eyes and eggs,
was used to generate TG silkworms. The diapausing strain w-1 was
also used to mate TG silkworm lines. All strains were maintained at
the Transgenic Silkworm Research Unit at National Institute of
Agrobiological Sciences (Ibaraki, Japan). Silkworm larvae were
reared on an artificial diet (Nosan Corporation, Yokohama, Japan)
at 25°C. pBac[UAS_MAGE-A4/3×P3-AmCyan] was injected into embryos at
the pre-blastoderm stage with a helper plasmid, pHA3PIG (our
collection), to induce expression of a piggyBac transposase gene
(17), and resulting generation 0
(G0) adults were mated with other G0 adults. G1 silkworms were
screened during the late embryonic stage for expression of the
AmCyan gene driven by a 3×P3 neuro-specific promoter in the
embryonic compound eyes. The TG silkworm lines obtained were then
mated with adults from a Ser1-GAL4 strain carrying a GAL4 gene
under the control of a middle silk gland (MSG)-specific sericin1
promoter and a 3×P3-DsRed2 marker gene (Addgene, Inc., Cambridge,
MA, USA) (8). F1 embryos harboring
GAL4 and UAS constructs were selected based on fluorescence of
AmCyan and DsRed2 using fluorescence microscopy (Olympus
Corporation, Tokyo, Japan).
Extraction and purification of
recombinant MAGE-A4 from MSGs
A pair of MSG (~300 µg) was isolated from one larvae
on the sixth day of the 5th instar, then immersed in 1 ml of 20 mM
phosphate (pH 7.2) and gently shaken at 4°C for 2 h. The resulting
extract was frozen at −80°C for 3 h, and then thawed at 4°C
overnight before removal of debris from each extract by filtration.
Protein supernatant extracted using a freeze-and-thaw method as
described above (10 µg of protein; centrifuged at 2,280 × g for 10
min at 4°C) from MSGs were separated and analyzed by SDS-PAGE using
4–12% gradient gels (NuPAGE Bis-Tris Gels; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
instructions. The gel was stained with 0.2% Coomassie Brilliant
Blue R-250 (Nacalai Tesque, Kyoto, Japan). For western blotting,
the gel was transferred onto nylon membranes (Hybond P PVDF; no.
10600023; GE Healthcare Life Sciences, Chalfont, UK), and incubated
in blocking buffer (Blocking One; no. 03953-95; Nacalai Tesque,
Kyoto, Japan) at room temperature for 1 h. Membrane was incubated
with an anti-histidine tag primary antibody (A190-114A, 1:1,000;
Bethyl Laboratories, Montgomery, TX, USA) at 4°C overnight.
Subsequently, the membrane was washed three times with PBS with
Tween-20 (PBST) [8 mM Na2HPO4, 2 mM
KH2PO4 (pH 7.4), 150 mM NaCl, 3 mM KCl, 0.05%
Tween-20] and incubated with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin (Ig)-G secondary antibody (NA934,
1:20,000; GE Healthcare Life Sciences) at room temperature for 1 h.
Then, the membrane was washed two times with PBST. Immunoreactive
protein bands were detected using ECL Prime reagent (GE Healthcare
Life Sciences) and an LAS-3000 Image Analyzer (Fujifilm Image
Reader LAS-3000, version 2; Fujifilm Corporation, Tokyo,
Japan).
Protein lysates from MSGs were also loaded onto a
nickel affinity column (5 ml; GE Healthcare Life Sciences)
equilibrated with 20 mM phosphate (pH 7.4) and 500 mM NaCl for
purification of recombinant MAGE-A4. After sample loading, the
column was washed with 45 ml of 20 mM phosphate (pH 7.4) and 50 mM
imidazole, and recombinant MAGE-A4 was eluted with 20 mM phosphate
(pH 7.4) and 500 mM imidazole. Each fraction from the column was
evaluated using 12.5% SDS-PAGE.
Cell lines
The present study used two human squamous cell
carcinoma of the head and neck (SCCHN) cell lines, namely Kuma-1
(MAGE-A4+) and HSC-4 (MAGE-A4−), according to
a previously described method (19).
Kuma-1 was provided by Dr. Kyogo Itoh at the Kurume University
School of Medicine (Kurume, Japan). HSC-4 was purchased from the
Japanese Cancer Research Bank (Tokyo, Japan). The SCCHN cell lines
were cultured at 37°C in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum, L-glutamine (2 mM) and
antibiotics (50 U/ml penicillin and 50 µg/ml streptomycin; all from
Gibco; Thermo Fisher Scientific, Inc.). Tumor cells were harvested,
re-suspended in AIM-V medium (Invitrogen; Thermo Fisher Scientific,
Inc.) at 5×106/ml, and lysed by three freeze-thaw
cycles. A freeze-thaw cycle consisted of 5 min in liquid nitrogen
followed by 5 min at 37°C. Tumor cell lysates of 5×105
cell equivalents/ml were used as a source of antigen in interferon
(IFN)-γ ELISA assay, as previously described (20).
Induction of anti-MAGE-A4-specific T
cells by IVS
During January 2015 to July 2015, PBMCs were
isolated from 5-healthy donors by density gradient centrifugation
using a Ficoll-Paque PLUS media (GE Healthcare Life Sciences).
Donor cells were obtained in accordance with the regulations by an
approved protocol and consent forms of the Institutional Review
Board of Gunma University (Maebashi, Japan). Dendritic cells (DC)
were generated from PBMCs, as described previously (21). CD4+ and CD8+
T-cells were isolated from non-adherent PBMCs using immunomagnetic
beads (CD8 MicroBeads; Miltenyi Biotech GmbH, Bergisch Gladbach,
Germany). CD4+ or CD8+ T-cells
(5×104) and DCs (1×104) treated by X-ray
irradiation (30 Gy) were co-cultured at 37°C for 7 days in the
presence of recombinant MAGE-A4 protein (10 µg/ml) in 96-well
round-bottomed plates (BD Biosciences, Franklin Lakes, NJ, USA) in
a final volume of 0.2 ml/well AIM-V medium supplemented with 5%
(v/v) human AB serum (Access Cell Culture LLC, Vista, CA, USA) and
5 ng/ml interleukin (IL)-7 (R&D Systems, Inc., Minneapolis, MN,
USA). This solution formed the culture medium (CM). On day 7,
responder CD4+ or CD8+ T-cells were
re-stimulated with irradiated autologous DCs (1×104) in
the presence of MAGE-A4 protein (10 µg/ml) and grown in CM. On day
9, half of the medium volume was replenished with CM containing 10
IU/ml IL-2. Responding cells were screened on day 14 for the
production of IFN-γ in the presence of MAGE-A4 protein using an
IFN-γ ELISA kit (EHIFNG; Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Positive cells were
selected and transferred to 24-well plates and cultured at 37°C in
CM supplemented with 10 IU/ml IL-2. Cells in culture were subjected
to a weekly MAGE-A4 protein re-stimulation (10 µg/ml) with
irradiated DCs (1×105) or PBMCs (1×106).
Evaluation of MAGE-A4-specific T-cell
responses
Responder T cells (3×104/well) were
co-cultured with irradiated DCs (1×104/well) at 37°C in
the presence of MAGE-A4 protein (10 µg/ml) in 96-well flat-bottomed
plates in a final volume of 200 µl AIM-V medium containing 5% (v/v)
human AB serum. Culture supernatants were harvested after 24 h to
measure antigen-induced IFN-γ production. The harvested
supernatants of the responder T cell medium were frozen and stored
at −80°C until IFN-γ concentration was measured. An IFN-γ ELISA kit
(EHIFNG; Pierce; Thermo Fisher Scientific, Inc.) was used to
quantify the concentration of IFN-γ within the supernatants,
according to the manufacturer's instructions, using a detection
limit of 2 pg/ml. An antibody blocking assay was also performed to
determine the effect on IFN-γ production, whereby irradiated DCs
were pre-incubated with anti-HLA-class I antibody (10 µg/ml, no.
560187) or anti-HLA-class II antibody (10 µg/ml; no. 555556) (both
from BD Biosciences) at 37°C for 30 min prior to co-culture with
responder T cells.
Statistical analysis
Data were expressed as mean ± standard error and
analyzed using a Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS 22.0 software (IBM SPSS, Armonk,
NY, USA).
Results
Generation of TG silkworms expressing
MAGE-A4
To generate TG silkworm strains expressing MAGE-A4
protein in MSGs, the plasmid pBac[UAS_MAGEA4/3×P3-AmCyan], encoding
the MAGE-A4 gene under control of a UAS promoter, was injected into
370 eggs. A total of 171 eggs hatched, and these G0 adults were
mated with other G0 adults to generate G1 offspring. A total of 8
broods expressing fluorescent AmCyan were selected after
fluorescence screening of G1 offspring, and 4 TG silkworm lines
were ultimately established (Table
I). All 4 TG silkworm lines were mated with a Ser1-GAL4 strain
expressing an MSG-specific GAL4 gene. To confirm the expression of
MAGE-A4 protein in selected TG silkworms, extract from the MSGs of
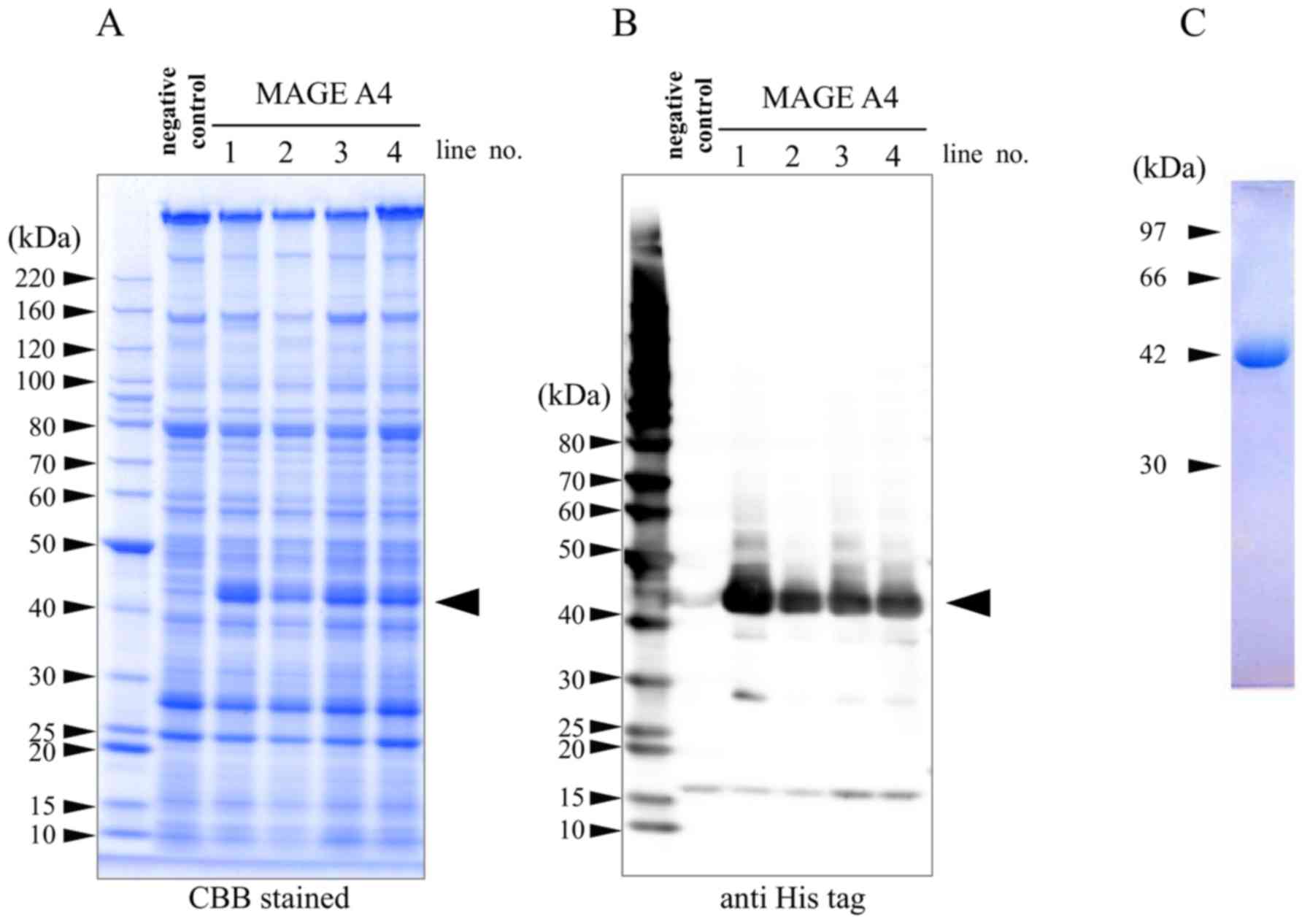
silkworms was evaluated by SDS-PAGE (Fig. 1A) and western blot analysis (Fig. 1B). On coomassie brilliant
blue-stained gels and immunoblots, specific bands at ~42 kDa were
observed in all four lanes of the MAGE-A4 TG lines, but not in the
negative control lane. A number of low-molecular-weight bands were
also detected on the immunoblots, though were likely derived from
degradation products. Purified recombinant MAGE-A4 was confirmed as
a single band at ~42 kDa by nickel affinity chromatography and
SDS-PAGE analysis (Fig. 1C). The
MAGE-A4 fractions were dialyzed with 20 mM phosphate (pH 7.4). A
total of 170 µg of MAGE-A4 protein was obtained per TG silkworm
after purification.
 | Table I.Efficiency of transgenic silkworms
production. |
Table I.
Efficiency of transgenic silkworms
production.
| Strain | Injected eggs | Hatched eggs | G1 broods | G1 broods with
positive larvae | Established
lines |
|---|
| MAGE-A4 | 370 | 171 | 56 | 8 | 4 |
Induction of MAGE-A4-specific
CD4+ T cell responses
MAGE-A4-specific CD4+ T cells were
generated from the PBMCs of healthy donors. After four rounds of
IVS, outgrowing CD4+ T cells were assessed for the
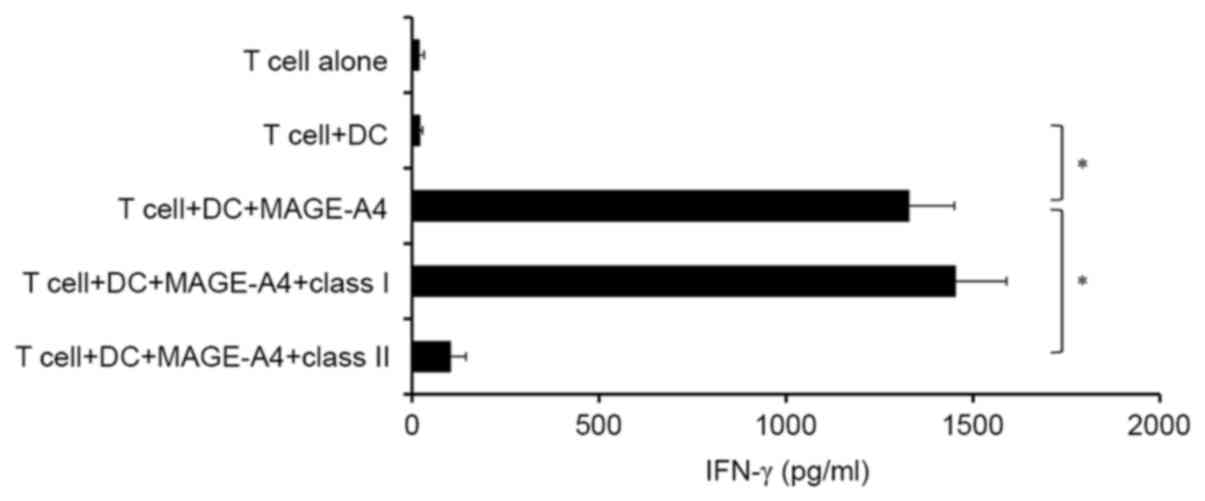
production of IFN-γ using ELISA. It was observed that
CD4+ effector T cells produced IFN-γ in response to
MAGE-A4 protein (Fig. 2). In turn,
production of IFN-γ in response to MAGE-A4 protein was
significantly blocked by anti-HLA class II antibody, but not
anti-HLA class I antibody (P<0.05; Fig. 2). However, MAGE-A4-specific
CD8+ T cells were not induced to produce IFN-γ using the
same culture system (data not shown). Therefore, subsequent
experiments were performed using MAGE-A4-specific CD4+ T
cells.
To determine the ability of induced MAGE-A4-specific
CD4+ T cells to recognize MAGE-A4+ tumor
cells, tumor lysates were used as a source of antigen instead of
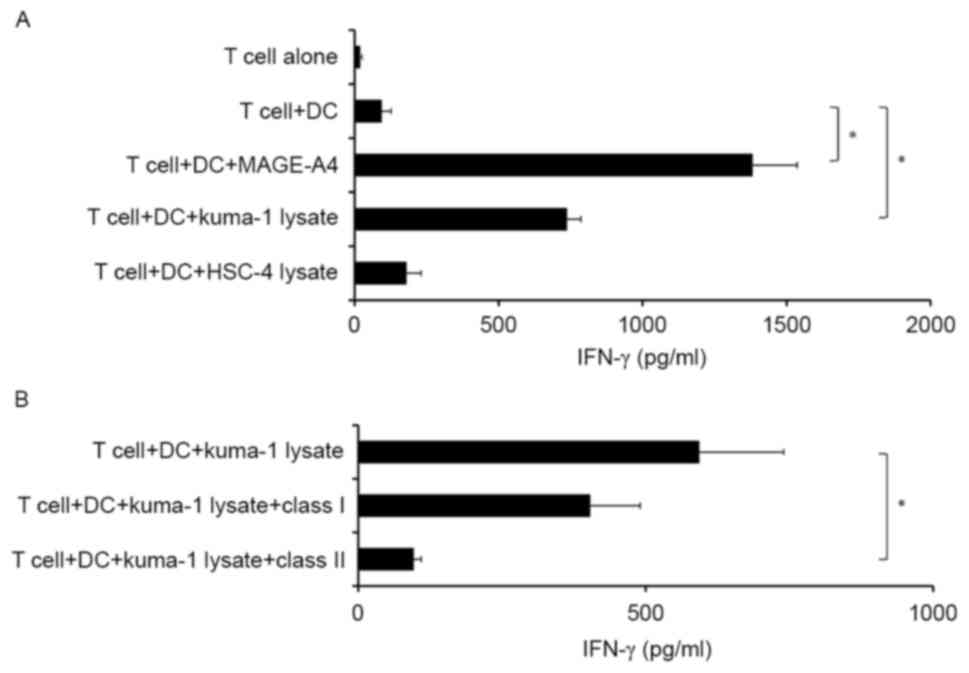
recombinant MAGE-A4 protein. Notably, MAGE-A4-specific
CD4+ T cells produced IFN-γ in response to autologous
DCs incubated with MAGE-A4+ tumor lysates, but not
MAGE-A4-tumor lysates (Fig. 3A).
Furthermore, T cell reactivity was significantly inhibited by
anti-HLA-class II antibody, but not anti-HLA-class I antibody
(P<0.05; Fig. 3B). These results
suggest that induced MAGE-A4-specific CD4+ T cells may
recognize tumor-derived MAGE-A4 antigen when presented by HLA class
II molecules on antigen-presenting DCs.
Discussion
In the present study, TA with immunogenic properties
was successfully produced using a TG silkworm system. Cancer
immunotherapy aims to activate and upregulate host immune responses
against tumor cells, and a large number of agents and strategies
are currently under development or in clinical trials and practice
(22). Regarding cancer vaccines,
the majority of clinical trials have used peptide-based vaccines;
however, they have shown only limited success despite the induction
of TA-specific T cell responses in cancer patients (3). Although protein-based vaccines are a
high-cost approach when compared to peptide-based vaccines,
full-length proteins contain all the potential epitopes capable of
stimulating CD4+ and CD8+ T cells, and may be
used regardless of the patients' HLA alleles. Many host systems,
including bacteria, yeast and insect cells, are now used in the
production of recombinant proteins, and the preferred method of
production varies according to the purpose of use (7). The TG silkworm system has been used for
the expression of recombinant proteins since 2000, and the
expression of a number of recombinant proteins in TG silkworms,
including green fluorescent protein, human serum albumin, membrane
receptors and monoclonal antibodies has been documented (23–25). The
TG silkworm system has a number of advantages, including its lower
cost, mass breeding capacity, availability of multiple tissues for
expression, suitability for large scale production and reduced
endotoxin contamination. Regarding the extraction of recombinant
proteins from tissues, recombinant MAGE-A4 protein has been
extracted from MSGs and silkworm cocoons in our preliminary
experiments. As protein extraction from MSGs was more efficient and
achieves a higher yield than that from silkworm cocoons, we used
MSGs as the source of recombinant MAGE-A4 protein for in
vitro study. The MAGE-A4 protein produced using the TG silkworm
system successfully induced MAGE-A4-specific CD4+ T cell
responses, suggesting that the MAGE-A4 protein is engulfed,
processed and presented to CD4+ T cells by
antigen-presenting cells in association with HLA class II
molecules. Notably, the induced MAGE-A4-specific CD4+ T
cells also recognized antigen-presenting cells incubated with a
MAGE-A4+ tumor cell lysate. This result indicates that
common immunological epitopes exist between the recombinant MAGE-A4
protein produced and MAGE-A4 expressed in tumor cells. However,
MAGE-A4-specific CD8+ T cells were not produced in the
current experimental system. DCs are capable of presenting
exogenous antigens in the context of HLA class I through
cross-priming; however, cross-presentation is influenced by many
factors, including the DC maturation status, type of DC subset and
type of antigen (26,27). Studies are currently ongoing to
determine the activities of CD8+ T cells under various
conditions including the types of cytokines used and DC activation
status.
The MAGE-A4 protein produced in the current study is
among a number of cancer-testis antigens that have been identified
as cancer therapeutic targets based on their unique expression
patterns (28,29). Cesson et al (30) observed that naturally acquired T-cell
responses were activated against MAGE-A4 in patients with SCCHN,
indicating that vaccines that boost pre-existing MAGE-A4-specific
T-cell responses in cancer patients may be a useful therapeutic
strategy. A number of clinical trials employing MAGE-A4 as a
vaccine in cancer patients have been documented. For instance,
Takahashi et al (14) treated
patients with colon cancer with pulmonary metastasis with an
artificially synthesized long peptide of MAGE-A4 that acted as a
helper/killer-hybrid cell epitope. The artificially synthesized
long peptide induced MAGE-A4-specific Th1 and Tc1 cells and
complement-fixing IgG antibodies. In addition, tumor growth and
levels of carcinoembryonic antigen tumor marker were significantly
decreased in the final diagnosis (14). More recently, a cancer vaccine
clinical trial with the MAGE-A4 protein, derived from M15
Escherichia coli, was conducted in patients with advanced
esophageal, stomach or lung cancer (15). Although protein synthesis was not
discussed, it was suggested that recombinant proteins manufactured
in E. coli may contain residual endotoxins that are harmful
to the host (15). Apart from the
method of protein synthesis, results of this trial indicated that
vaccination with MAGE-A4 protein vaccine was safe and induced
CD4+ and/or CD8+ T cell responses in a number
of patients. In addition, overall survival rate was longer in
patients with tumor cells expressing high levels of MAGE-A4 or HLA
class I and exhibiting MAGE-A4-specific immune responses after
vaccination (15). Therefore,
MAGE-A4 is a potential target for cancer vaccines, and recombinant
MAGE-A4 protein may be a potent immunogen, capable of inducing
broad T-cell responses in a larger population of cancer patients. A
major factor in the design of cancer vaccines is the selection of
tumor antigens. Although Cheever et al (31) has documented a prioritized list of
cancer vaccine antigens based on predefined and pre-weighted
objective criteria, a single antigen cancer vaccine is unlikely to
be effective, due to the heterogeneity of antigen expression in
tumors and the emergence of antigen loss variants. Therefore,
studies aiming to produce other tumor antigens, including wild-type
p53 and Wilms tumor 1 (31), using
the TG silkworm system are currently ongoing.
Numerous clinical trials using immune checkpoint
inhibitors have been conducted and therapeutic benefits were
achieved in certain patient populations (32,33).
However, as immune checkpoint inhibitors are not intended to target
tumor cells themselves, these drugs augment non-specific immune
responses against not only tumor cells, but also normal cells, and
thus, cause various autoimmune reactions, including colitis,
pneumonitis and endocrine disorders as side effects (32,33). In
the future, strategies combining the induction and activation of
TA-specific T cells with cancer protein-based vaccines and the
activation and proliferation of TA-specific T cells with immune
checkpoint inhibitors may lead to safer and more effective
immunotherapy for the treatment of cancer.
In conclusion, MAGE-A4 produced by the TG silkworm
system successfully induced MAGE-A4-specific CD4+ T cell
responses. Furthermore, these CD4+ T cells could
recognize antigen-presenting cells pulsed with a
MAGE-A4+ tumor cell lysate. Thus, recombinant tumor
antigen production using the TG silkworm system may be a novel tool
in the preparation of cancer vaccines.
Acknowledgements
The present study was supported by the Gunma
University Medical Innovation Project to Kazuaki Chikamatsu and
Shigeki Takeda and Grants-in-Aid for Scientific Research to Kazuaki
Chikamatsu (grant no. 26670736).
References
|
1
|
Coulie PG, Van den Eynde BJ, van der
Bruggen P and Boon T: Tumor antigens recognized by T lymphocytes:
At the core of cancer immunotherapy. Nat Rev Cancer. 14:135–146.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoos A, Parmiani G, Hege K, Sznol M,
Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U,
et al: Cancer vaccine clinical trial working group. A clinical
development paradigm for cancer vaccines and related biologics. J
Immunother. 30:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pol J, Bloy N, Buqué A, Eggermont A,
Cremer I, Sautes-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer
G and Galluzzi L: Trial watch: Peptide-based anticancer vaccines.
Oncoimmunology. 4:e9744112015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parmiani G, Castelli C, Dalerba P,
Mortarini R, Rivoltini L, Marincola FM and Anichini A: Cancer
immunotherapy with peptide-based vaccines: What have we achieved?
Where are we going? J Natl Cancer Inst. 94:805–818. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tagliamonte M, Petrizzo A, Tornesello ML,
Buonaguro FM and Buonaguro L: Antigen-specific vaccines for cancer
treatment. Hum Vaccin Immunother. 10:3332–3346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palomares LA, Estrada-Mondaca S and
Ramírez OT: Production of recombinant proteins: Challenges and
solutions: Methods. Mol Biol. 267:1–52. 2004.
|
|
8
|
Tatematsu K, Kobayashi I, Uchino K,
Sezutsu H, Iizuka T, Yonemura N and Tamura T: Construction of a
binary transgenic gene expression system for recombinant protein
production in the middle silk gland of the silkworm Bombyx mori.
Transgenic Res. 19:473–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tateno M, Toyooka M, Shikano Y, Takeda S,
Kuwabara N, Sezutsu H and Tamura T: Production and characterization
of the recombinant human mu-opioid receptor from transgenic
silkworms. J Biochme. 145:37–42. 2009. View Article : Google Scholar
|
|
10
|
Nikaido Y, Kurosawa A, Saikawa H, Kuroiwa
S, Suzuki C, Kuwabara N, Hoshino H, Obata H, Saito S, Saito T, et
al: In vivo and in vitro evaluation of novel μ-opioid receptor
agonist compounds. Eur J Pharmacol. 767:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barrow C, Browning J, MacGregor D, Davis
ID, Sturrock S, Jungbluth AA and Cebon J: Tumor antigen expression
in melanoma varies according to antigen and stage. Clin Cancer Res.
12:764–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montoro JR, Mamede RC, Neder Serafini L,
Saggioro FP, Figueiredo DL, Silva WA Jr, Jungbluth AA, Spagnoli GC
and Zago MA: Expression of cancer-testis antigens MAGE-A4 and
MAGE-C1 in oral squamous cell carcinoma. Head Neck. 34:1123–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shigematsu Y, Hanagiri T, Shiota H, Kuroda
K, Baba T, Mizukami M, So T, Ichiki Y, Yasuda M, So T, et al:
Clinical significance of cancer/testis antigens expression in
patients with non-small cell lung cancer. Lung Cancer. 68:105–110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi N, Ohkuri T, Homma S, Ohtake J,
Wakita D, Togashi Y, Kitamura H, Todo S and Nishimura T: First
clinical trial of cancer vaccine therapy with artificially
synthesized helper/killer-hybrid epitope long peptide of MAGE-A4
cancer antigen. Cancer Sci. 103:150–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito T, Wada H, Yamasaki M, Miyata H,
Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M and Doki Y: High
expression of MAGE-A4 and MHC class I antigens in tumor cells and
induction of MAGE-A4 immune responses are prognostic markers of
CHP-MAGE-A4 cancer vaccine. Vaccine. 32:5901–5907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tada M, Tatematsu K, Ishii-Watabe A,
Harazono A, Takakura D, Hashii N, Sezutsu H and Kawasaki N:
Characterization of anti-CD20 monoclonal antibody produced by
transgenic silkworms (Bombyx mori). MAbs. 7:1138–1150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamura T, Thibert C, Royer C, Kanda T,
Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, et
al: Germline transformation of the silkworm Bombyx mori L. Using a
piggyBac transposon derived vector. Nat Biotechnol. 18:81–84. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tatematsu K, Uchino K, Sezutsu H and
Tamura T: Effect of ATG initiation codon context motifs on the
efficiency of translation of mRNA derived from exogenous genes in
the transgenic silkworm, Bombyx mori. SpringerPlus. 3:1362014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eura M, Ogi K, Chikamatsu K, Lee KD,
Nakano K, Masuyama K, Itoh K and Ishikawa T: Expression of the MAGE
gene family in human head-and-neck squamous-cell carcinomas. Int J
Cancer. 64:304–308. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi S, Kumai T, Matsuda Y, Aoki N,
Sato K, Kimura S, Kitada M, Tateno M, Celis E and Kobayashi H:
Six-transmembrane epithelial antigen of the prostate and enhancer
of zeste homolog 2 as immunotherapeutic targets for lung cancer. J
Transl Med. 9:1912011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chikamatsu K, Nakano K, Storkus WJ,
Appella E, Lotze MT, Whiteside TL and DeLeo AB: Generation of
anti-p53 cytotoxic T lymphocytes from human peripheral blood using
autologous dendritic cells. Clin Cancer Res. 5:1281–1288.
1999.PubMed/NCBI
|
|
22
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imamura M, Nakai J, Inoue S, Quan GX,
Kanda T and Tamura T: Targeted gene expression using the GAL4/UAS
system in the silkworm Bombyx mori. Genetics. 165:1329–1340.
2003.PubMed/NCBI
|
|
24
|
Ogawa S, Tomita M, Shimizu K and Yoshizato
K: Generation of a transgenic silkworm that secretes recombinant
proteins in the sericin layer of cocoon: Production of recombinant
human serum albumin. J Biotechnol. 128:531–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iizuka M, Ogawa S, Takeuchi A, Nakakita S,
Kubo Y, Miyawaki Y, Hirabayashi J and Tomita M: Production of a
recombinant mouse monoclonal antibody in transgenic silkworm
cocoons. FEBS J. 276:5806–5820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fehres CM, Unger WWJ, Garcia-Vallejo JJ
and van Kooyk Y: Understanding the biology of antigen
cross-presentation for the design of vaccines against cancer. Front
Immunol. 5:1492014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gutiérrez-Martínez E, Planès R, Anselmi G,
Reynolds M, Menezes S, Adiko AC, Saveanu L and Guermonprez P:
Cross-presentation of cell-associated antigens by MHC class I in
dendritic cell subsets. Front Immunol. 6:3632015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fratta E, Coral S, Covre A, Parisi G,
Colizzi F, Danielli R, Nicolay HJ, Sigalotti L and Maio M: The
biology of cancer testis antigens: Putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gjerstorff MF, Andersen MH and Ditzel HJ:
Oncogenic cancer/testis antigens: Prime candidates immunotherapy.
Oncotarget. 6:15772–15787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cesson V, Rivals JP, Escher A, Piotet E,
Thielemans K, Posevitz V, Dojcinovic D, Monnier P, Speiser D, Bron
L and Romero P: MAGE-A3 and MAGE-A4 specific CD4(+) T cells in head
and neck cancer patients: Detection of naturally acquired responses
and identification of new epitopes. Cancer Immunol Immunother.
60:23–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheever MA, Allison JP, Ferris AS, Finn
OJ, Hasting BM, Hecht TT, Mellman I, Prindiville SA, Viner JL,
Weiner LM and Matrisian LM: The prioritization of cancer antigens:
A national cancer institute pilot project for the acceleration of
translational research. Clin Cancer Res. 15:5323–5337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|