Introduction
Garlic has frequently been used in gastronomy for
centuries. However, accumulating data have demonstrated other
useful elements of garlic, such as its bioactivities on cancer
prevention, and anti-microbial, anti-oxidation, and insecticidal
properties (1–5). In spite of these beneficial effects,
numerous individuals do not enjoy raw garlic due to the pungent
odor released by the compound allicin (1,5–7). Therefore, modified forms of garlic have
been prepared to reduce this unpleasant odor. Black garlic, a
garlic preparation, is formed by incubating raw garlic in an
environment of high temperature and humidity, which initiates a
non-enzymatic browning reaction to transfer the allicin into
water-soluble compounds, such as S-allylcysteine and S-allyl
melcaptocysteine, enabling the efficient removal of the pungent
smell (1,7–9). In
vivo and in vitro experiments have demonstrated that
black garlic is able to retain the original bioactivities of raw
garlic on clinical application (10–12).
Moreover, the anti-oxidation effect of black garlic is even
stronger than that of raw garlic (1). In addition, novel functions of black
garlic have also continued to be reported, including its protective
effects against diabetes, allergies and liver injury (10–12).
The liver is an important metabolic organ with
various complex physiological functions, including nutrient
metabolism (lipids, proteins and carbohydrates) and waste excretion
and detoxification (13–16). Liver injury, initiated by exposure to
high levels of environmental toxins, results in metabolic
dysfunctions and subsequent elevation of inflammation and oxidative
stress (13–16). Moreover, the levels of inflammatory
cytokines, such as IL-6 and IL-8, and reactive oxygen species (ROS)
within the tissue rapidly accumulate, damaging the liver. In order
to prevent further injury, the defense system, specifically the
immune system and anti-oxidative enzymes, including catalase (CAT),
superoxide dismutase (SOD), and glutathione peroxidase (GPx), are
activated to eliminate these harmful factors (14,17–19).
To stimulate cell and tissue damage, tert-Butyl
hydroperoxide (tBHP) is commonly used as a hepatocytotoxic agent
(19,20). In cells, tBHP is metabolized through
two pathways: i) tBHP is metabolized by cytochrome P450 and results
in increases in peroxyl and alkoxyl radicals to initiate
lipoperoxidation of membrane lipids and production of
malondialdehyde (MDA); and ii) tBHP is detoxified to tert-butanol
and results in rapid glutathione (GSH) oxidation (19–21).
Both pathways lead to liver cell injury. Thus, the use of
tBHP-treated cells is a well-recognized experimental model in
laboratory investigation. Moreover, the levels of MDA and GSH are
critical indicators of lipid peroxidation.
In the present study the hepatoprotective effect of
black garlic on tBHP-stimulated rat clone-9 hepatocytes and the
underlying mechanism responsible was determined using antioxidative
enzyme activity analysis, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting. The
contents of MDA and GSH; anti-oxidative enzyme activities of CAT,
SOD, GPx; and mRNA expression levels of IL-6 and IL-8 were
determined to evaluate the level of cell damage. Black garlic
efficiently attenuated tBHP-initiated cell death, lipid
peroxidation, oxidative stress and inflammation in rat clone-9
hepatocytes. Moreover, this attenuation may be accomplished through
c-Jun N-terminal kinase (JNK) signaling. Thus, this study indicated
that black garlic provides a protective effect on injured liver
cells and thus may be applied in adjuvant therapy and health foods
for the management of liver injury.
Materials and methods
Materials
MDA assay kit (LPO-586) was purchased from EMD
Millipore (Billerica, MA, USA). GSH (CS0260), CAT (CAT100), SOD
(19160) and GPx (CGP1) activity assay kits were obtained from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Mouse
monoclonal antibodies against JNK1/2 (sc-7345) and phospho-JNK1/2
(sc-6254) were purchased from Santa Cruz Biotechnology. All other
chemicals were of reagent grade and purchased from Sigma-Aldrich
(Merck Millipore), unless stated otherwise.
Cell cultures
Rat clone-9 hepatocytes were supplied by the Food
Industry Research and Development Institute (Taiwan, China).
Hepatocytes were grown in Dulbecco's modified Eagle's medium
(DMEM), supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (all Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and maintained in an atmosphere containing
5% CO2 at 37°C, using an incubator.
Preparation of black garlic
extract
Unpeeled raw garlic heads were incubated at 70°C in
90% relative humidity for 35 days using a thermohygrostatic
chamber. Black garlic was subsequently combined with deionized
water at a solid:liquid ratio of 1:10. Samples were obtained with
deionized water for 30 min at room temperature using an ultrasonic
bath (Taiwan Supercritical Technology Co., Ltd., Fenyuan, Taiwan).
Extracts were centrifuged (2,500 × g; 10 min; 4°C) and supernatants
were collected. Supernatants were subsequently dried using a
freeze-dryer (Labconco freeze-dry/shell freeze system; Labconco
Corp., Kansas City, MO, USA) and the dried extracts were stored at
−20°C prior to analysis.
Cell viability assay
Cell viability was determined using an MTT assay.
Cells were cultured at a density of ×104
cells/cm2 on 96-well plates. Following stimulation, 0.5
mg/ml of MTT solution was added to each well and the mixture was
incubated at 37°C for 3 h. Formazan crystals were dissolved by
adding dimethyl sulfoxide solution and absorbance was measured at
570 nm using a spectrophotometer.
MDA assay for lipid peroxidation
Cells were cultured in a monolayer on 24-well
plates. Following stimulation, the culture medium was replaced with
0.5 ml cell lysis buffer after three washes with PBS. MDA contents
were then determined using an MDA assay kit, according to the
manufacturer's instructions.
GSH level and CAT/SOD/GPx enzyme
activity assay
Cells were cultured in a monolayer on 24-well
plates. Following stimulation, the levels of GSH, CAT, SOD, and GPx
enzyme activities in rat clone-9 hepatocytes were measured in
triplicate using commercial assay kits, according to manufacturers'
instructions.
Western blot analysis
Cells were collected and lysed with radio
immunoprecipitation assay buffer (1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS and protease/phosphatase inhibitor
cocktail). Total cell lysate concentrations were determined using a
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Proteins (50 µg/ml) were separated by SDS-PAGE (10% running and 4%
stacking). Equal amounts of protein from the control and
experimental groups was suspended in 5X sample buffer and distilled
water and boiled for 10 min, then subjected to SDS-PAGE. The
electrophoresis was carried out for 2 h and the separated proteins
were transferred to a nitrocellulose membrane. Following blocking
with 5% skim milk for 1 h at room temperature, the membrane was
blotted with the JNK-(sc-7345) and phosphor-JNK (sc-6254; both
Santa Cruz Biotechnology) specific primary antibody (diluted in
1:500) overnight at 4°C. The membranes were washed with
Tris-buffered saline with Tween-20 buffer, they were blotted with
anti-mouse secondary antibody (1:3,000; cat. no. 7076; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 2 h at room
temperature. Immunodetection was performed by using a western light
chemiluminescent detection system (Applied Biosystems; Thermo
Fisher Scientific Inc.).
RT-qPCR
RNA was isolated from the rat clone-9 hepatocytes.
The collected samples were homogenized with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) by rotor-stator homogenizer, and
then placed on the benchtop at room temperature for 5 min. Each
sample was added to 0.2 ml chloroform and agitated vigorously for
15 sec to mix completely. The samples were kept on the benchtop at
room temperature for 2–3 min then centrifuged at 12,000 × g for 15
min at 4°C. The upper phase was transferred to new tubes and 0.5 ml
isopropanol was added. The samples were mixed gently and placed on
the benchtop at room temperature for 10 min prior to centrifugation
at 12,000 × g for 10 min at 4°C. The supernatant was discarded and
1 ml 75% ethanol was added per tube. This was then centrifuged at
7,500 × g for 5 min at 4°C. The supernatant was removed completely
and briefly left to air-dry the RNA pellet. The RNA was
re-dissolved in an appropriate volume (15 µl) of RNase-free water.
DNase was added to remove genomic DNA.
The reverse transcription steps were carried out by
using Thermo RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) and BioRad C1000 Thermal Cycler (Bio-Rad
Laboratories, Inc.). Initially, 5 µg total RNA and 1 µl Oligo (dT)
18 primer and complement was added to the RNase-free tubes and the
volume was made up to 12 µl with distilled water. The mixtures were
incubated at 65°C for 5 min then chilled on ice. Each tube was then
further administered 5 µl 5X Reaction Buffer, 1 µl RiboLock RNase
Inhibitor (20 U/µl), 2 µl 10 mM dNTP Mix and 1 µl RevertAid M-MuLV
RT (200 U/µl) to give a final total volume of 20 µl. The mixtures
were incubated at 42°C for 60 min to allow cDNA synthesis and then
increased to 70°C for 10 min to terminate the reaction.
PCR was performed using an ABI Prism 7900HT (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Amplification of specific PCR products was
detected using SYBR-Green PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The designed primers in this study
were as follows: For PAI-1, forward 5′-CATCCCCCATCCTACGTGG-3′,
reverse 5′-CCCCATAGGGTGAGAAAACCA-3′; for PKC, forward
5′-ATTCTATGCGGCAGAGATTTCC-3′, reverse
5′-TCCTTCTGAATCCAACATGACG-3′.
RNA samples were normalized to the levels of GAPDH
and 18S rRNA. All primer pairs had at least 1 primer crossing an
exon-exon boundary. The RT-qPCR was performed in triplicate in a
total reaction volume of 20 ml containing 10 ml of SYBR Green PCR
Master Mix, 300 nM forward and reverse primers, 4 ml of distilled
H2O, and 4 ml of complementary DNA from each sample.
Samples were heated for 10 min to 95°C and amplified for 40 cycles
of 95°C for 15 sec and 60°C for 60 sec. Quantification was
performed using the 2−ΔΔCq method (22), where the Cq value was defined as the
threshold cycle of PCR at which amplified product was detected. The
ΔCq value was obtained by subtracting the Cq value of the
housekeeping gene (GAPDH or 18S rRNA) from the Cq value
of the gene of interest. The present study used the ΔCq
value of controls as the calibrator. The fold change was calculated
according to the formula 2−ΔΔCq, where ΔΔCq
was the difference between the ΔCq value and the ΔCq calibrator
value.
Statistical analysis
Results are expressed as mean ± standard error of
the mean. Statistical analysis was determined via an independent
Student t-test for two groups of data and analysis of variance
followed by Scheffe's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Black garlic extracts attenuate
tBHP-induced cell death of rat clone-9 hepatocytes
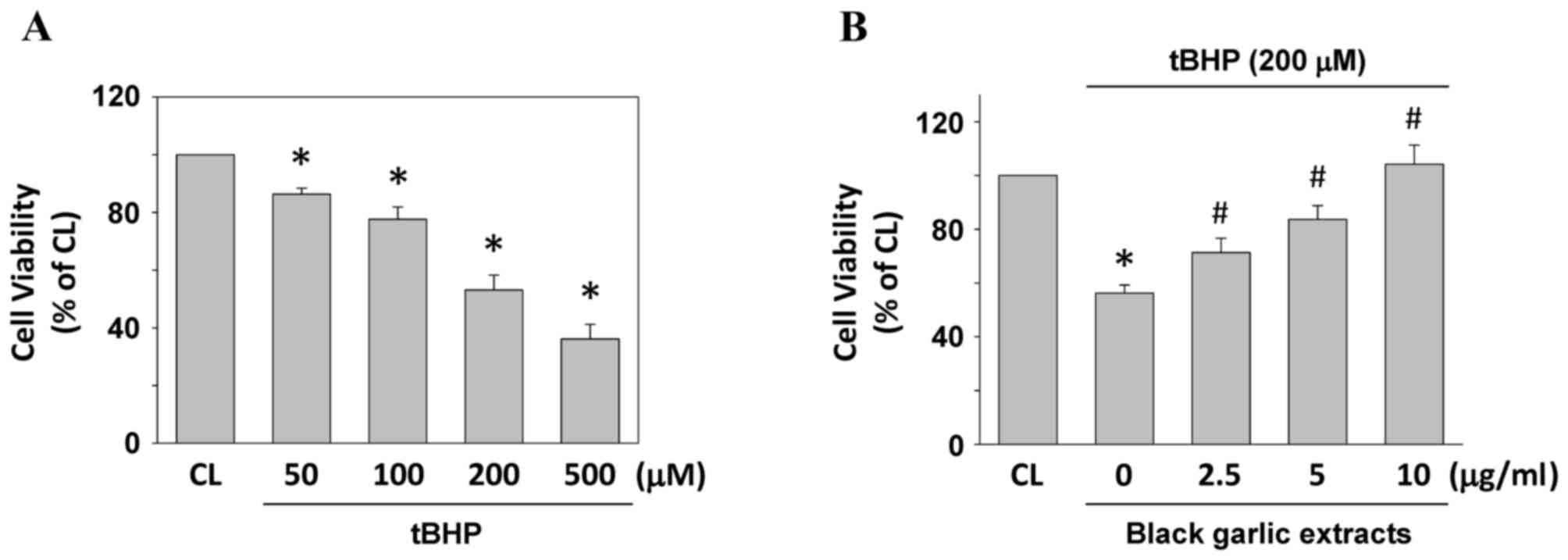
Cells were kept as the control or were treated with
tBHP at 50, 100, 200 and 500 µM for 24 h to determine the cell
viability of rat clone-9 hepatocytes. Treating cells with tBHP
resulted in the significant cell death of rat clone-9 hepatocytes
in a dose-dependent manner, as compared with the untreated control
(P<0.05; Fig. 1A). To investigate
the protective effect of black garlic extracts on tBHP-treated
cells, rat clone-9 cells were kept as the control or were
pretreated with black garlic extracts at 0, 2.5, 5 or 10 µg/ml for
1 h. Black garlic extract-pretreated cells were treated with tBHP
(200 µM) for 24 h. Black garlic extract significantly restored the
cell viability of tBHP-treated rat clone-9 hepatocytes (Fig. 1B, P<0.05).
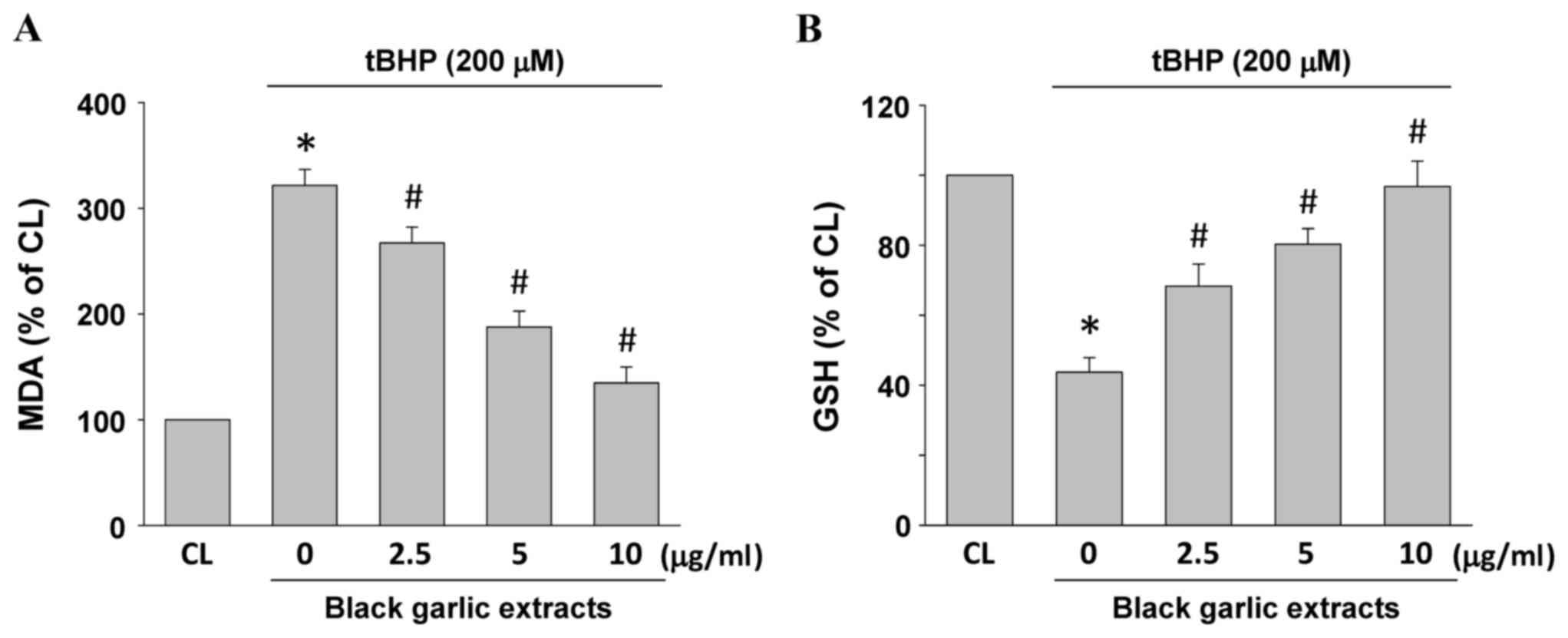
Black garlic extracts inhibit
tBHP-increased MDA accumulation and GSH depletion in rat clone-9
hepatocytes
MDA and GSH levels in the cells were used to
evaluate lipid peroxidation and oxidative stress in rat clone-9
hepatocytes. Rat clone-9 hepatocytes were kept as the control or
were pretreated with black garlic extracts at 0, 2.5, 5 or 10 µg/ml
for 1 h. Black garlic extract-pretreated cells were treated with
tBHP (200 µM) for 24 h. MDA (Fig.
2A) and GSH (Fig. 2B) levels
were significantly increased and diminished, respectively, in the
tBHP-treated cells as compared with the control (P<0.05).
However, black garlic extracts significantly restored the
tBHP-increased MDA levels (Fig. 2A,
P<0.05) and tBHP-diminished GSH levels (Fig. 2B, P<0.05) in a dose-dependent
manner in rat clone-9 hepatocytes.
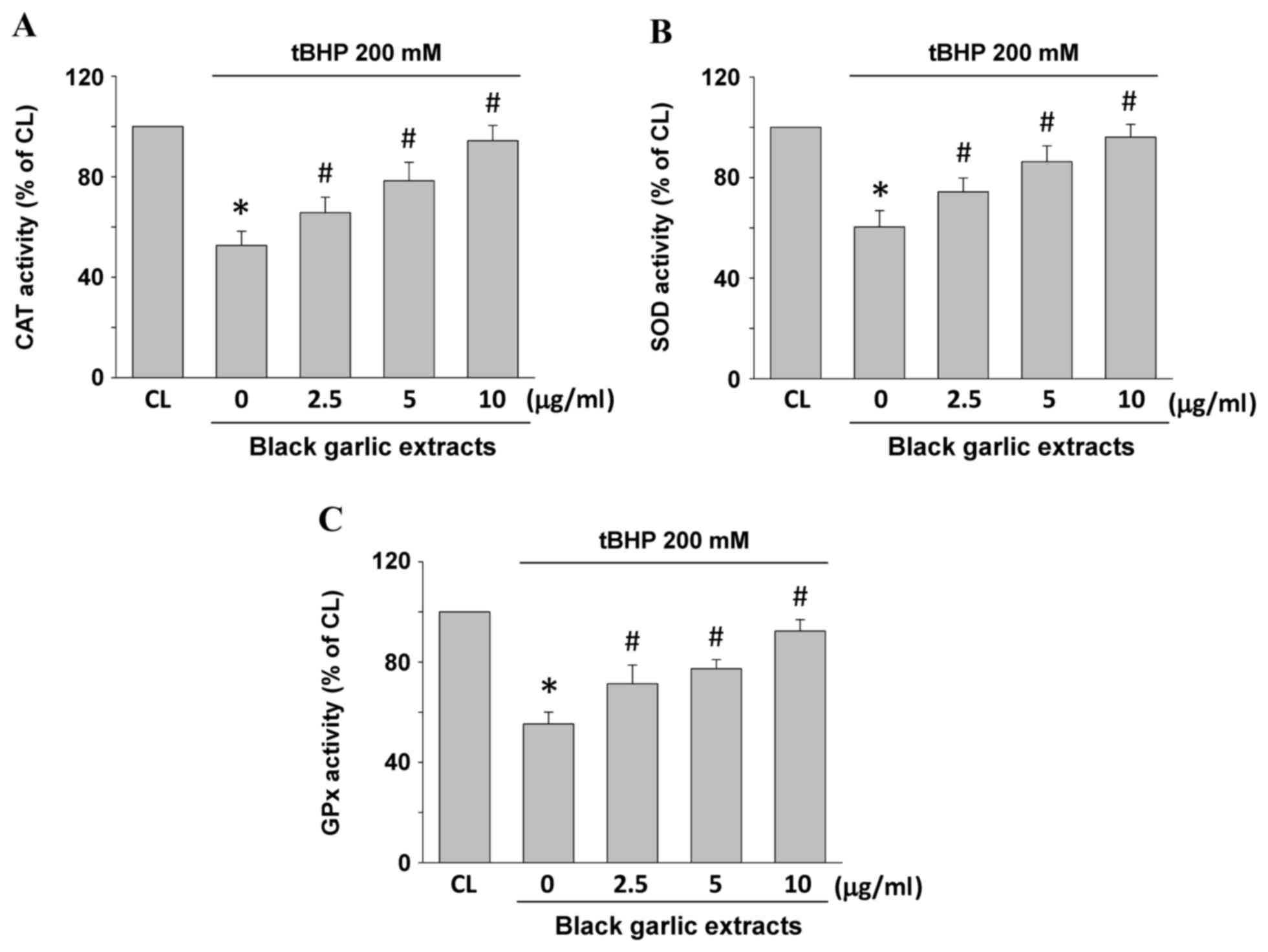
Black garlic extracts recover
tBHP-decreased antioxidant enzyme activity in rat clone-9
hepatocytes
Antioxidative activity of SOD, CAT and GPx may also
be indicators of oxidative stress in cells. Rat clone-9 hepatocytes
were kept as the control or were pretreated with black garlic
extracts at 0, 2.5, 5 and 10 µg/ml, respectively, for 1 h.
Subsequently, black garlic extract-pretreated cells were treated
with tBHP (200 µM) for 24 h. CAT (Fig.
3A), SOD (Fig. 3B) and GPx
(Fig. 3C) enzyme activities
significantly decreased in the tBHP-treated cells as compared with
the control (P<0.05). However, black garlic extracts
significantly restored the tBHP-decrease of all three types of
enzyme activities in a dose-dependent manner in rat clone-9
hepatocytes (P<0.05).
Black garlic extracts inhibit
tBHP-induced IL-6 and IL-8 mRNA expression levels in rat clone-9
hepatocytes
To determine whether black garlic extracts elicit an
anti-inflammatory effect on hepatocytes, the mRNA expression levels
of inflammatory markers, IL-6 and IL-8, were determined (Fig. 4). Rat clone-9 hepatocytes were kept
as the control or were pretreated with black garlic extracts at 0,
2.5, 5 and 10 µg/ml for 1 h and black garlic extract-pretreated
cells were treated with tBHP (200 µM) for 24 h. As compared with
the control, tBHP significantly induced IL-6 and IL-8 mRNA
expression levels in the rat clone-9 hepatocytes (P<0.05).
However, black garlic extracts significantly inhibited tBHP-induced
IL-6 and IL-8 mRNA expression levels in a dose-dependent manner in
rat clone-9 hepatocytes (P<0.05).
Black garlic extract inhibits IL-6 and
IL-8 mRNA expression levels of tBHP induction via JNK signaling in
rat clone-9 hepatocytes
Subsequently, whether the MAPK pathway (ERK1/2, JNK,
and/or p38 kinases) mediates the inflammatory effects of tBHP and
the antagonized effect of black garlic extracts in hepatocytes was
investigated. Rat clone-9 hepatocytes were kept as the control or
were pretreated with DMSO or MAPK inhibitors (ERK/PD98059,
JNK/SP600125 or p38/SB203580) for 1 h. DMSO- and
MAPK-inhibitor-pretreated cells were treated with tBHP (200 µM) for
24 h. tBHP was identified to significantly induce IL-6 and IL-8
mRNA expression levels in the DMSO-pretreated rat clone-9
hepatocytes when compared with the control (P<0.05). However,
the inhibition activity of JNK, but not of ERK and p38, blocked
these tBHP effects (Fig. 5A).
Moreover, tBHP (200 µM) also induced transient JNK phosphorylation
within 5–10 min in rat clone-9 hepatocytes (Fig. 5B). Rat clone-9 hepatocytes were kept
as the control or were pretreated with black garlic extracts at 0,
5 and 10 µg/ml for 1 h. Subsequently, black garlic
extract-pretreated cells were treated with tBHP (200 µM) for 10
min. tBHP induced JNK phosphorylation effectively in the rat
clone-9 hepatocytes when compared with the control. However, black
garlic extracts inhibited the JNK phosphorylation of tBHP induction
in a dose-dependent manner in rat clone-9 hepatocytes (Fig. 5C).
 | Figure 5.Black garlic extract inhibits IL-6 and
IL-8 mRNA expression levels of tBHP induction through JNK signaling
in rat clone-9 hepatocytes. (A) Cells were kept as CL or were
treated with tBHP at 200 µM for 24 h. Prior to stimulation with
tBHP, cells were pretreated with DMSO or MAPK inhibitors
(ERK/PD98059, JNK/SP600125 or p38/SB203580) for 1 h. mRNA
expression levels of IL-6 and IL-8 were determined by reverse
transcription-quantitative polymerase chain reaction. (B) Cells
were kept as CL or were treated with tBHP at 200 µM for 5, 10 and
30 min, and 1 and 2 h, respectively. JNK phosphorylation was
determined by western blot analysis. (C) Cells were kept as CL or
were treated with tBHP at 200 µM for 24 h. Prior to stimulation
with tBHP, cells were pretreated with 0–10 µg/ml black garlic
extract for 1 h. JNK phosphorylation was determined by western blot
analysis. Data in (A) are presented as the mean ± standard error of
the mean from three independent experiments. *P<0.05 vs. CL
cells; #P<0.05 vs. cells treated with DMSO/tBHP.
Results in (B) and (C) are representative of three independent
experiments with similar results. tBHP, tert-Butyl hydroperoxide;
CL, control; IL, interleukin; JNK, c-Jun N-terminal protein kinase;
MAPK, mitogen-activated protein kinase; DMSO, dimethyl sulfoxide;
ERK, extracellular signal-regulated kinase. |
Discussion
The present study revealed that black garlic was
able to attenuate liver injury induced by tBHP through the
inhibition of JNK activation in the rat clone-9 hepatocytes.
Firstly, this work showed that black garlic restored tBHP-induced
cell death of rat clone-9 hepatocytes. Secondly, black garlic
decreased tBHP-increased lipid peroxidation, oxidative stress and
inflammation in rat clone-9 hepatocytes. Finally, the inhibitory
effect of black garlic on tBHP-induced liver cell injury was
associated with the downregulation of JNK phosphorylation. Thus,
these results contribute to a novel notion about the
hepatoprotective potential of black garlic in injured liver cells
and elucidate a possible molecular mechanism.
Previous animal studies have investigated the effect
of black garlic on hepatoprotection (5,10–12). The
present study further established that black garlic contributed to
the hindering of tBHP-induced liver cell death, lipid peroxidation,
oxidative stress and inflammation. Our results were supported by
the physicochemical analysis of black garlic in previous studies,
showing that antioxidant components of black garlic, including
polyphenol and flavonoid, increased significantly during the
preparation process when compared with raw garlic (1,23–25).
Natural foods and herbs have always been considered as important
and interesting sources in developing healthy foods and drugs as
they have fewer side effects, superior acceptability and are
inexpensive. Data from previous studies and our studies have
demonstrated that black garlic possesses multiple biological
functions to antagonize distinctive disease development, including
liver disease, cancer and hyperlipidemia among others (10–12,26–29). In
addition, it has also been reported that the content of
cysteine-containing compounds, the main components responsible for
the pungent odor in black garlic, decreased significantly when
compared to that in raw garlic after the preparation process
(1). Consequently, black garlic is
becoming one of a number of popular natural products gradually
being introduced into health food and clinical disease treatment in
Asian countries, including Taiwan, China and Korea (1).
Abnormal inductions of lipid peroxidation and
oxidative stress are common destructive mechanisms in injured liver
cells and typically accompany the alteration of the intracellular
redox balance (19,29). During these processes, free radicals
and other highly reactive substances are significantly produced to
induce peroxisomal and microsomal oxidation of fatty acids and
mitochondrial dysfunction. Therefore, these reactions seem to have
crucial roles in leading to the pathogenesis of liver tissue,
including steatohepatitis and fatty liver (30–33). In
the present study, MDA accumulation and GSH depletion in rat
clone-9 hepatocytes demonstrated that tBHP was metabolized in cells
and therefore induced the imbalance of redox and the occurrence of
lipid peroxidation. Moreover, the downregulation of the activities
of antioxidative enzymes, such as CAT, SOD and GPx, also
demonstrated that intracellular oxidative stress increased under
tBHP stimulation. These situations may therefore disrupt the array
and composition of membrane lipids and subsequently result in
hepatic inflammation and cell death. Efficient neutralized activity
of black garlic in tBHP-induced rat clone-9 hepatocytes in the
current study suggested that the hepatoprotective effects of black
garlic may occur through restoring the intracellular redox, by
increasing the antioxidant (GSH) level and antioxidative enzyme
(CAT/SOD/GPx) activities.
The present study has indicated that black garlic
has the potential to block tBHP-induced liver cell injury,
including cell death, lipid peroxidation, oxidative stress and
inflammation in rat clone-9 cells. Moreover, JNK signaling may
regulate the hindering effects of black garlic. As a result, the
findings of the present study propose that black garlic may have an
important role in liver protection. Therefore, this study suggests
that a novel perspective on the application of black garlic on the
physiological and pathophysiological management of the liver is
developing, and warrants further exploration.
Acknowledgements
The present study was supported by Chang Gung
Memorial Hospital-Kaohsiung Medical Center, Chang Gung Memorial
Hospital (grant nos. CZRPG880253, CMRPF6A0073, CMRPF6C0032 and
CMRPG8C1261), Chang Gung University of Science and Technology and
by the National Science Council, Taiwan (grant nos.
NSC101-2622-B-255-001-CC3, NSC102-2313-B-255-002, MOST
103-2313-B-255-001 and MOST103-2622-B-255-001-CC3).
References
|
1
|
Choi IS, Cha HS and Lee YS:
Physicochemical and antioxidant properties of black garlic.
Molecules. 19:16811–16823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banerjee SK and Maulik SK: Effect of
garlic on cardiovascular disorders: A review. Nutr J. 1:42002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahman K and Lowe GM: Garlic and
cardiovascular disease: A critical review. J Nutr. 136(3 Suppl):
736S–740S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha AW, Ying T and Kim WK: The effects of
black garlic (Allium satvium) extracts on lipid metabolism in rats
fed a high fat diet. Nutr Res Pract. 9:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin JH, Lee CW, Oh SJ, Yun J, Kang MR,
Han SB, Park H, Jung JC, Chung YH and Kang JS: Hepatoprotective
effect of aged black garlic extract in rodents. Toxicol Res.
30:49–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McRae MP: A review of studies of garlic
(Allium sativum) on serum lipids and blood pressure before and
after 1994: Does the amount of allicin released from garlic powder
tablets play a role? J Chiropr Med. 4:182–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichikawa M, Ryu K, Yoshida J, Ide N,
Yoshida S, Sasaoka T and Sumi S: Antioxidant effects of
tetrahydro-beta-carboline derivatives identified in aged garlic
extract. BioFactors. 16:57–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corzo-Martínez M, Corzo N and Villamiel M:
Biological properties of onions and garlic. Trends Food Sci
Technol. 18:609–625. 2007. View Article : Google Scholar
|
|
9
|
Jang EK, Seo JH and Lee SP: Physiological
activity and antioxidative effects of aged black garlic (Allium
sativum L.) extract. Korean J Food Sci Technol. 40:443–448.
2008.
|
|
10
|
Kim JH, Nam SH, Rico CW and Kang MY: A
comparative study on the antioxidative and anti-allergic activities
of fresh and aged black garlic extracts. Int J Food Sci Technol.
47:1176–1182. 2012. View Article : Google Scholar
|
|
11
|
Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ,
Kim MJ and Kim JI: Antioxidant effect of garlic and aged black
garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract.
3:156–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MH, Kim MJ, Lee JH, Han LJ, Kim JH,
Sok DE and Kim MR: Hepatoproective effect of aged black garlic on
chronic alcohol-induced liver injury in rats. J Med Food.
14:732–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A: A review on hepatoprotective
herbal drugs. Int J Res Pharm and Chem. 2:92–102. 2012.
|
|
14
|
Senthilkumar R, Chandran R and
Parimelazhagan T: Hepatoprotective effect of Rhodiola imbricata
rhizome against paracetamol-induced liver toxicity in rats. Saudi J
Biol Sci. 21:409–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tacke F, Luedde T and Trautwein C:
Inflammatory pathways in liver homeostasis and liver injury. Clin
Rev Allergy Immunol. 36:4–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sagar R, Bhaiji A, Toppo FA, Rath B and
Sahoo HB: A comprehensive review on herbal drugs for
hepatoprotection of 21st Century. Int J Nutr Pharmacol Neurol Dis.
4:191–197. 2014. View Article : Google Scholar
|
|
17
|
Lacour S, Gautier JC, Pallardy M and
Roberts R: Cytokines as potential biomarkers of liver toxicity.
Cancer Biomark. 1:29–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hinson JA, Reid AB, McCullough SS and
James LP: Acetaminophen-induced hepatotoxicity: Role of metabolic
activation, reactive oxygen/nitrogen species, and mitochondrial
permeability transition. Drug Metab Rev. 36:805–822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kučera O, Endlicher R, Roušar T, Lotková
H, Garnol T, Drahota Z and Cervinková Z: The effect of tert-butyl
hydroperoxide-induced oxidative stress on lean and steatotic rat
hepatocytes in vitro. Oxid Med Cell Longev. 2014:7525062014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellomo G, Thor H and Orrenius S: Increase
in cytosolic Ca2+ concentration during t-butyl hydroperoxide
metabolism by isolated hepatocytes involves NADPH oxidation and
mobilization of intracellular Ca2+ stores. FEBS Lett. 168:38–42.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crane D, Häussinger D, Graf P and Sies H:
Decreased flux through pyruvate dehydrogenase by thiol oxidation
during t-butyl hydroperoxide metabolism in perfused rat liver.
Hoppe Seylers Z Physiol Chem. 364:977–987. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Block E: The chemistry of garlic and
onions. Sci Am. 252:114–119. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amagase H, Petsch BL, Matsuura H, Kasuga K
and Itakura Y: Intake of garlic and its bioactive components. J
Nutr. 131(3 Suppl): 955S–962S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin JH, Choi DJ, Chung MJ, Kang MJ and
Sung NJ: Changes of physicochemical components and antioxidant of
aged garlic at different temperatures. J Korean Soc Food Sci Nutr.
37:1174–1181. 2008. View Article : Google Scholar
|
|
26
|
Lee EN, Cho YW, Kim HK, Park JK, Kim HJ,
Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS and Yoon S: Chloroform
extract of aged black garlic attenuates TNF-α-induced ROS
generation, VCAM-1 expression, NF-κB activation and adhesiveness
for monocytes in human umbilical vein endothelial cells. Phytother
Res. 25:92–100. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HK, Choi YW, Lee EN, Park JK, Kim SG,
Park DJ, Kim BS, Lim YT and Yoon S: 5-Hydroxymethylfurfural from
black garlic extract prevents TNFα-induced monocytic cell adhesion
to HUVECs by suppression of vascular cell adhesion molecule-1
expression, reactive oxygen species generation and NF-κB
activation. Phytother Res. 25:965–974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim I, Kim JY, Hwang YJ, Hwang KA, Om AS,
Kim JH and Cho KJ: The beneficial effects of aged black garlic
extract on obesity and hyperlipidemia in rats fed a high-fat diet.
J Med Plants Res. 5:3159–3168. 2011.
|
|
29
|
Gambino R, Musso G and Cassader M: Redox
balance in the pathogenesis of nonalcoholic fatty liver disease:
Mechanisms and therapeutic opportunities. Antioxid Redox Signal.
15:1325–1365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cervinková Z, Lotková H, Kriváková P,
Rousar T, Kucera O, Tichý L, Cervinka M and Drahota Z: Evaluation
of mitochondrial function in isolated rat hepatocytes and
mitochondria during oxidative stress. Altern Lab Anim. 35:353–361.
2007.PubMed/NCBI
|
|
31
|
Jaeschke H, Gores GJ, Cederbaum AI, Hinson
JA, Pessayre D and Lemasters JJ: Mechanisms of hepatotoxicity.
Toxicol Sci. 65:166–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Berk M, McIntyre TM, Gores GJ and
Feldstein AE: The lysosomal-mitochondrial axis in free fatty
acid-induced hepatic lipotoxicity. Hepatology. 47:1495–1503. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kučera O, Roušar T, Staňková P, Haňáčková
L, Lotková H, Podhola M and Cervinková Z: Susceptibility of rat
non-alcoholic fatty liver to the acute toxic effect of
acetaminophen. J Gastroenterol Hepatol. 27:323–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|