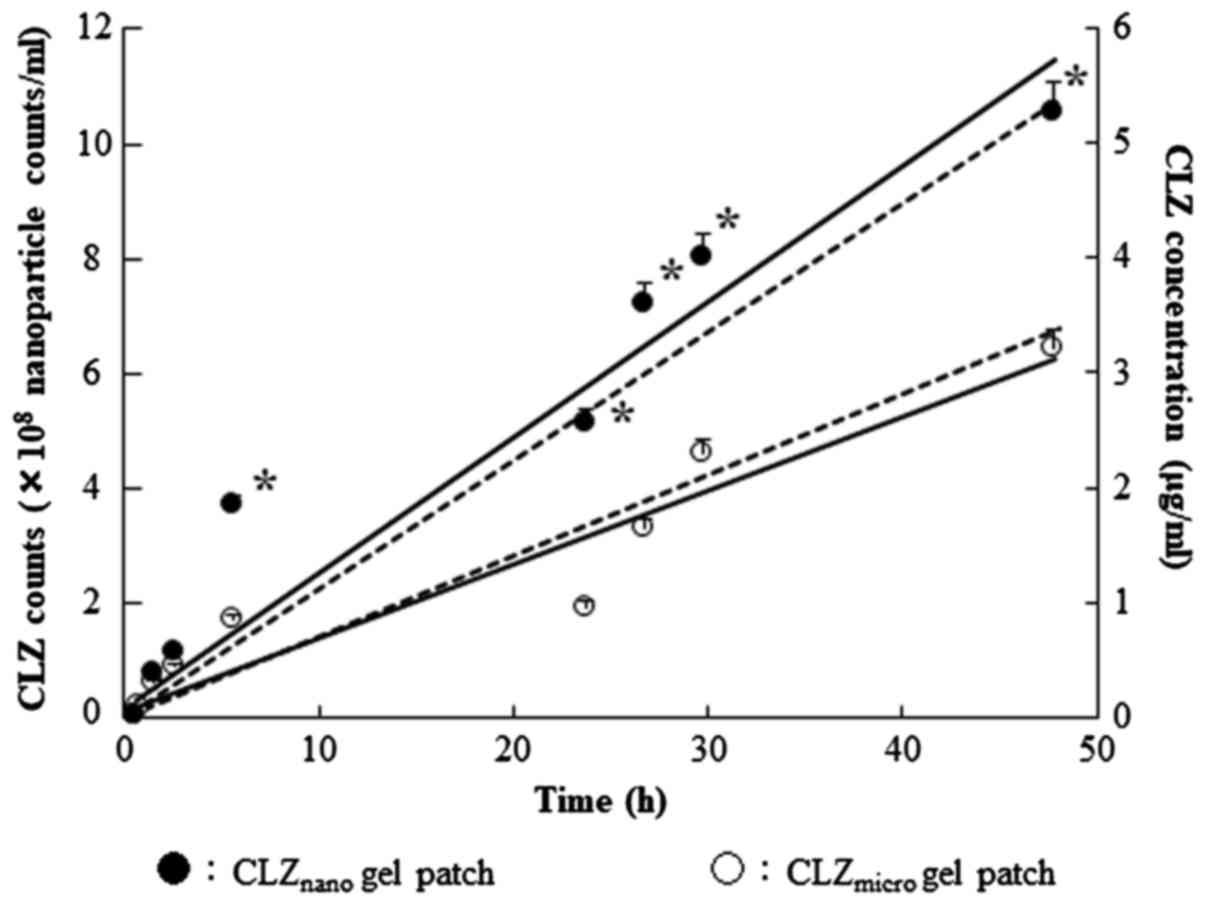
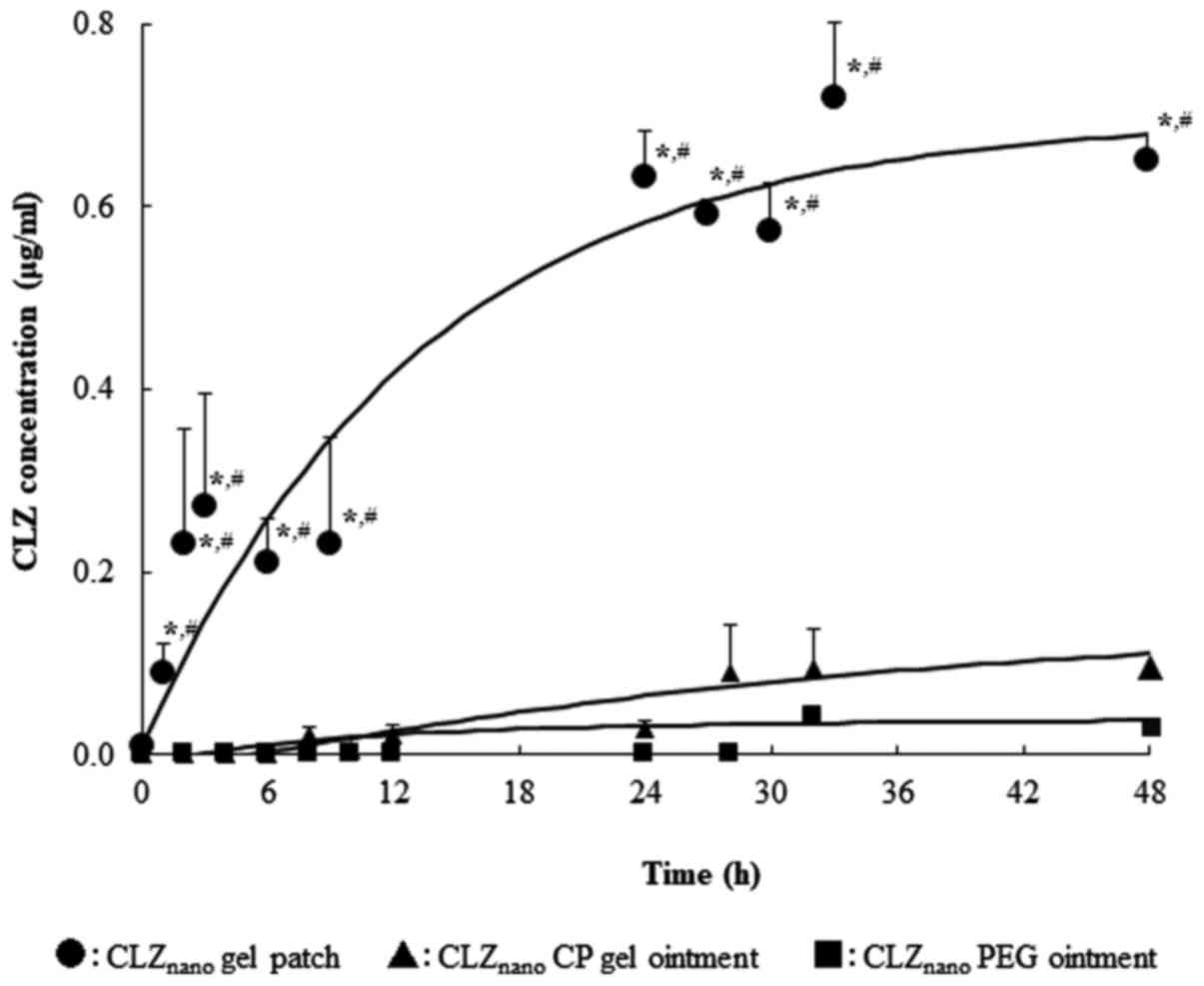
|
1
|
Kimura Y, Tani T, Kanbe T and Watanabe K:
Effect of cilostazol on platelet aggregation and experimental
thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.PubMed/NCBI
|
|
2
|
Kanbayashi J, Liu Y, Sun B, Shakur Y,
Yoshitake M and Czerwiec F: Cilostazol as a unique antithrombotic
agent. Curr Pharm Des. 9:2289–2302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bramer SL and Forbes WP: Relative
bioavailability and effects of a high fat meal on single dose
cilostazol pharmacokinetics. Clin Pharmacokinet. 2(37 Suppl):
S13–S23. 1999. View Article : Google Scholar
|
|
4
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
Effect of particle size reduction on dissolution and oral
absorption of a poorly water-soluble drug, cilostazol, in beagle
dogs. J Control Release. 111:56–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jinno J, Kamada N, Miyake M, Yamada K,
Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K and Kimura T:
In vitro-in vivo correlation for wet-milled tablet of poorly
water-soluble cilostazol. J Control Release. 130:29–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasenack N and Müller BW: Micron-size drug
particles: Common and novel micronization techniques. Pharm Dev
Technol. 9:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotoh F, Tohgi H, Hirai S, Terashi A,
Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, et
al: Cilostazol stroke prevention study: A placebo-controlled
double-blind trial for secondary prevention of cerebral infarction.
J Stroke Cerebrovasc Dis. 9:147–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugibayashi K: Development &
Applications of Transdermal Drug Delivery Systems. CMC publishing
Company; Tokyo: pp. 167–176. 2011
|
|
9
|
Honeywell-Nguyen PL and Bouwstra JA:
Vesicles as a tool for transdermal and dermal delivery. Drug Discov
Today Technol. 2:67–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walters KA and Roberts MS: The structure
and function of the skin. Marcel Dekker; New York, NY: pp. 1–40.
2002
|
|
11
|
Cevc G and Vierl U: Nanotechnology and the
transdermal route: A state of the art review and critical
appraisal. J Control Release. 141:277–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scheuplein RJ: Mechanism of percutaneous
absorption. I. Routes of penetration and influence of solubility. J
Invest Dermatol. 45:334–346. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe T: The present conditions and the
prospects of a skin applications drug. Drug Delivery System.
22:450–457. 2007. View Article : Google Scholar
|
|
14
|
Tsujimoto H, Hara K, Yokoyama T, Yamamoto
H, Takeuchi H, Kawashima Y, Akagi K, Miwa N and Haung CC:
Percutaneous absorption study of biodegradable PLGA nano-spheres
via human skin biopsies. J Soc Powder Technol. 41:867–875. 2004.
View Article : Google Scholar
|
|
15
|
Bal SM, Ding Z, van Riet E, Jiskoot W and
Bouwstra JA: Advances in transcutaneous vaccine delivery: Do all
ways lead to Rome? J Control Release. 148:266–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshioka C, Ito Y and Nagai N: An oral
formulation of cilostazol nanoparticles enhances intestinal drug
absorption in rats. Exp Ther Med. 15:454–460. 2017.PubMed/NCBI
|
|
17
|
Franz TJ: Percutaneous absorption on the
relevance of in vitro data. J Invest Dermatol. 64:190–195. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N, Iwamae A, Tanimoto S, Yoshioka C
and Ito Y: Pharmacokinetics and antiinflammatory effect of a novel
gel system containing ketoprofen solid nanoparticles. Biol Pharm
Bull. 38:1918–1924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DeLouise LA: Applications of
nanotechnology in dermatology. J Invest Dermatol. 132:964–975.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao X, Cui Y, Levenson RM, Chung LW and
Nie S: In vivo cancer targeting and imaging with semiconductor
quantum dots. Nat Biotechnol. 22:969–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moghimi SM, Hunter AC and Murray JC:
Nanomedicine: Current status and future prospects. FASEB J.
19:311–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Jamal WT, Al-Jamal KT, Tian B,
Cakebread A, Halket JM and Kostarelos K: Tumor targeting of
functionalized quantum dot-liposome hybrids by intravenous
administration. Mol Pharm. 6:520–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boisselier E and Astruc D: Gold
nanoparticles in nanomedicine: Preparations, imaging, diagnostics,
therapies and toxicity. Chem Soc Rev. 38:1759–1782. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Debbage P: Targeted drugs and
nanomedicine: Present and future. Curr Pharm Des. 15:153–172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang HC, Barua S, Sharma G, Dey SK and
Rege K: Inorganic nanoparticles for cancer imaging and therapy. J
Control Release. 155:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, Peng X, Wang Y, Wang Y, Shin DM,
El-Sayed MA and Nie S: A reexamination of active and passive tumor
targeting by using rod-shaped gold nanocrystals and covalently
conjugated peptide ligands. ACS Nano. 4:5887–5896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ilbasmiş-Tamer S, Yilmaz S, Banoğlu E and
Değim IT: Carbon nanotubes to deliver drug molecules. J Biomed
Nanotechnol. 6:20–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang LW, Yu WW, Colvin VL and
Monteiro-Riviere NA: Biological interactions of quantum dot
nanoparticles in skin and in human epidermal keratinocytes. Toxicol
Appl Pharmacol. 228:200–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garala K, Faldu N, Basu B, Bhalodia R,
Mehta K and Joshi B: Chemical penetration enhancement. J Pharm Res.
2:1804–1808. 2009.
|
|
30
|
Nishihata T, Kamada A, Sakai K, Takahashi
K, Matsumoto K, Shinozaki K, Tabata Y, Keigami M, Miyagi T and
Tatsumi N: Percutaneous absorption of diclofenac in rats and
humans: Aqueous gel formulation. Int J Pharm. 46:1–7. 1988.
View Article : Google Scholar
|
|
31
|
Ahmed TA, Ibrahim HM, Ibrahim F, Samy AM,
Fetoh E and Nutan MT: In vitro release, rheological, and stability
studies of mefenamic acid coprecipitates in topical formulations.
Pharm Dev Technol. 16:497–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goddard ED and Gruber JV: Principles of
polymer science and technology in cosmetics and personal care.
Marcel Dekker, Inc.; New York-Basel: 1999, View Article : Google Scholar
|