Introduction
It is widely known that hyperlipidemia, particularly
hypercholesterolemia, is a notable risk factor for the development
of cardiovascular disease (CVD), which is the main cause of
mortality worldwide (1), and was
recently identified as a causative factor for rotator cuff tear
(2). At present, statins, such as
lovastatin and atorvastatin, are the most commonly used
lipid-lowering drugs, as they efficiently reduce plasma lipids;
however, they also present a number of undesirable side effects,
such as hepatotoxicity (3),
rhabdomyolysis (4) and skeletal
muscle injury (5), which have
limited their usage. Therefore, it is necessary to identify and
develop effective and natural agents that may be valuable in
regulating lipid metabolism. In recent years, Traditional Chinese
Medicine has attracted greater attention in metabolic syndrome
treatments, and has become a common therapy for controlling
symptoms in patients with hyperlipidemia (6).
Mulberry leaf (ML), the leaf of Morus alba
L., is a Traditional Chinese Medicine that has been used in
clinical settings in China for decades. Previous reports have
demonstrated that ML extract and powder exhibit anti-type 2
diabetes (7–9), antioxidant (10,11),
anti-inflammatory (12),
anti-obesity (13,14) and anti-atherosclerosis (15) properties. A number of previous
studies have demonstrated that ML powder was effective in reducing
serum triglyceride (TG) and low-density lipoprotein
(LDL)-cholesterol (C) in patients with mild hyperlipidemia
(16,17) and in hyperlipidemia rats (18). In addition, ML may significantly
decrease plasma total cholesterol (TC) and increase high-density
lipoprotein (HDL)-C (19,20). Valacchi et al (21) have previously reported that a
combination of ML and mulberry fruit had a beneficial effect on the
regulation of cholesterol transport via modulating the expression
of scavenger receptor class B type I (SR-BI) and ATP-binding
cassette transporter A1 (ABCA1) in obese mice. As HDL-C is a key
factor in reverse cholesterol transport (RCT) (22), which is a mechanism of cholesterol
clearance in vivo (23), and
SR-BI and ABCA1 are RCT-related proteins, it was hypothesized that
ML may regulate the process of RCT to reduce serum cholesterol
levels in patients with hyperlipidemia. The aim of the present
study was to confirm the effects of ML on serum cholesterol
reduction and RCT-related protein expression to identify the
potential target.
Materials and methods
Reagents
ML powder was produced by Shaoxing Royal Tea Village
Co., Ltd., (Shaoxing, China) via a process of hot air drying and
ball milling technology, and the content of total flavonoids in the
powder was 53% which was measured by ultraviolet-visible
spectroscopy. ML powder was suspended in pure water according to
the dosage with the final concentration as 0.09, 0.06 and 0.03 g/ml
prior to intragastric administration. Biochemical assay kits for TC
(15110401), TG (15090101), HDL-C (15091401), LDL-C (15100703),
aspartate aminotransferase (AST; 15092901), alanine
aminotransferase (ALT; 15092401), were purchased from Medicalsystem
Biotechnology Co., Ltd (Ningbo, China). ELISA kits for the
determination of TG (cat. no. 15090101), TC (cat. no. 20151127) and
total bile acid (TBA; 20151128) in the liver and feces were
obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). High-fat diet (HFD) consisted of standard fodder (82%),
lard (10%), cholesterol (1%), cholate (1%) and yolk powder (5%) and
was produced by Trophic Animal Feed High-tech Co., Ltd. (Nantong,
China).
Animals and treatments
A total of 48 male Sprague-Dawley (SD) rats (age,
6–8 weeks; weight, 180–200 g) were purchased from the Animal Supply
Center of Zhejiang Academy of Medical Science [Hangzhou, China;
certificate no. SCXK (Zhe) 2014–0001]. Rats were housed in an
environmentally controlled breeding room (temperature, 25±1°C;
humidity, 55±5%; 12-h light/dark cycle) for one-week
acclimatization prior to experiments. All rats were fed rodent
laboratory chow with tap water ad libitum, and were fasted for 12 h
prior to experiments with ad libitum access to water. All
procedures were performed in strict accordance with the P.R. China
Legislation on the Use and Care of Laboratory Animals (24) and with the Animal Management Rules of
the Health Ministry of China (25).
The present study was approved by the Ethics Committee of Zhejiang
Chinese Medical University (Hangzhou, China).
SD rats were divided into six groups (n=8/group)
including the normal control (normal), model control (model),
atorvastatin (positive control) and three ML-treated (0.9, 0.6, 0.3
g/kg) groups. Animals were given ad libitum access to HFD
for 5 weeks, except for the normal group, which were administered
the control diet. At the same time, normal and model groups were
orally administered distilled water, whereas the atorvastatin and
ML groups were administered with 6.0 mg/kg atorvastatin (China
Meheco Group Co., Ltd., Beijing, China), or 0.9, 0.6 or 0.3 g/kg ML
on a daily basis. At the end of experiment all rats were
anesthetized and sacrificed. Livers were harvested for
histopathology, immunohistochemical staining and western blot
analysis.
Biochemical analysis and liver TC, TG
and TBA assay
Following sacrifice, blood was collected from rat
abdominal aortas and centrifuged at 206 × g for 15 min at 4°C to
separate the serum. Serum TC, TG, HDL-C, LDL-C, ALT and AST levels
were measured using a fully automatic blood biochemistry analyzer
(TBA-40FR; Toshiba Medical Systems Corporation, Otawara, Japan)
with commercial biochemical kits according to the manufacturer's
protocol. Liver homogenate was prepared by grinding 0.5 g liver
tissue in 4.5 ml absolute ethyl alcohol followed by centrifugation
at 825 × g for 10 min and the supernatant was separated. Levels of
TC, TG and TBA in the liver were measured using ELISA kits and a
PowerWave 340 microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA), according to the manufacturer's protocol.
Fecal TC and TBA levels
determination
Prior to sacrifice, fecal samples were collected
from each rat and dried in an oven at ≤80°C. Subsequently, 0.3 g
fecal powder was extracted with 3.4 ml absolute ethyl alcohol using
an ultrasonic apparatus (KQ-500DE; Kunshan Ultrasonic Instruments
Co., Ltd., Kunshan, China) followed by centrifugation at 825 × g
for 10 min at room temperature, and the supernatant was separated
as the sample for determination. TC and TBA levels were measured
using ELISA kits and the PowerWave 340 microplate reader according
to the manufacturer's protocol.
Oil red O/hematoxylin staining
The Oil red O/hematoxylin staining procedure was
performed at room temperature as follows: 5-µm frozen liver
sections were fixed in 4% paraformaldehyde solution for 10 min, the
sections were washed with PBS and stained with Oil red O
(WSIG20100803; Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) for 15 min, followed by washing with PBS and staining with
Harris's hematoxylin (20151216; Nanjing Jiancheng Bioengineering
Institute) staining for 3 min. Lipid droplets were observed under a
light microscope (magnification, −200; B5-223IEP; Motic China Group
Co., Ltd., Xiamen, China).
Liver histopathological analysis
Liver tissues were fixed in 10% neutral-buffered
formalin for 1 week at 25–27°C, dehydrated in a 70–100% gradient of
ethyl alcohol, washed in xylene, embedded in paraffin and cut into
5-µm sections. The liver sections were deparaffinized in xylene,
rehydrated in a reverse-gradient series of ethyl alcohol, and
stained with hematoxylin for 5 min and eosin for 5 min (H&E;
Merck KGaA, Darmstadt, Germany) at room temperature. Pathological
changes were observed under a light microscope (magnification,
−200), and analyzed with Motic Images Advanced 3.2 software (Motic
China Group Co., Ltd.).
Immunohistochemistry analysis
The location of SR-BI and low-density lipoprotein
LDL-receptor (LDL-R) in liver tissue was evaluated via
immunohistochemistry. In brief, liver specimens fixed in 10%
formalin at room temperature for 1 week, were embedded in paraffin
wax, cut into 5-µm-thick sections, deparaffinized in xylene and
rehydrated in a reverse-gradient series of ethyl alcohol. Following
treatment with 3% hydrogen peroxide for 15 min at room temperature
to block endogenous peroxidase activity, the sections were
incubated with primary antibodies against SR-BI (1:100; ab52629;
Abcam, Cambridge, UK) and LDL-R (1:100; sc-11824; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 12 h at 4°C then washed
with PBS. Biotinylated goat anti-polyvalent secondary antibodies
[1:200; Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC kit;
ab64264; Abcam] were added and incubated for 20 min at room
temperature. The specimens were subsequently incubated with
streptavidin peroxidase for 10 min at room temperature and DAB
(1:50) was applied to visualize the labeling. The positive area was
identified with brown staining under the B5-223IEP light microscope
(magnification, −400).
Western blot analysis
Liver tissues (~100 mg) were homogenized with liquid
nitrogen, lysed with radioimmunoprecipitation buffer (P0013B;
Beyotime Institute of Biotechnology, Haimen, China) and
protease/phosphatase inhibitors for 30 min on ice, and centrifuged
at 18,246 × g for 15 min at 4°C. Total proteins were quantified
using the BCA method with a protein quantitation kit (P0012;
Beyotime Institute of Biotechnology). Protein samples (50 µg/lane)
were separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% skimmed milk
in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 120
min at room temperature. Following overnight incubation at 4°C with
the following primary antibodies: Peroxisome proliferator-activated
receptor-α (PPARα; 1:1,000; 15540-1-AP, ProteinTech Group, Inc.,
Chicago, IL, USA), SR-BI (1:1,000; ab52629, Abcam), LDL-R (1:1,000;
sc-11824, Santa Cruz Biotechnology, Inc.), ATP-binding cassette
transporter (ABC)G5 (1:1,000; bs-5013R; BIOSS, Beijing, China),
ABCG8 (1:1,000; bs-10149R; BIOSS), farnesoid-X receptor (FXR;
1:1,000; NR1H4, YN2161; ImmunoWay Biotechnology Company, Plano, TX,
USA) cholesterol 7α-hydroxylase 1 (CYP7A1; 1:1,000; sc-14426, Santa
Cruz Biotechnology, Inc.), and GAPDH (1:1,000; B661204-0001, Sangon
Biotech Co., Ltd., Shanghai, China); the membranes were washed
three times with TBST (10 min each time) and subsequently incubated
with HRP-conjugated rabbit anti-mouse immunoglobulin G secondary
antibodies (cat. no. 58802; 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) for 1 h at room temperature. Visualization
was performed with an enhanced chemiluminescence detection reagent
(GE Healthcare, Chicago, IL, USA), and GAPDH was used as an
internal control. Expression levels were quantified using ImageJ
1.46r image analysis software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance with Fisher's
least-significant difference post hoc analysis for multiple
comparisons was applied to compare differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
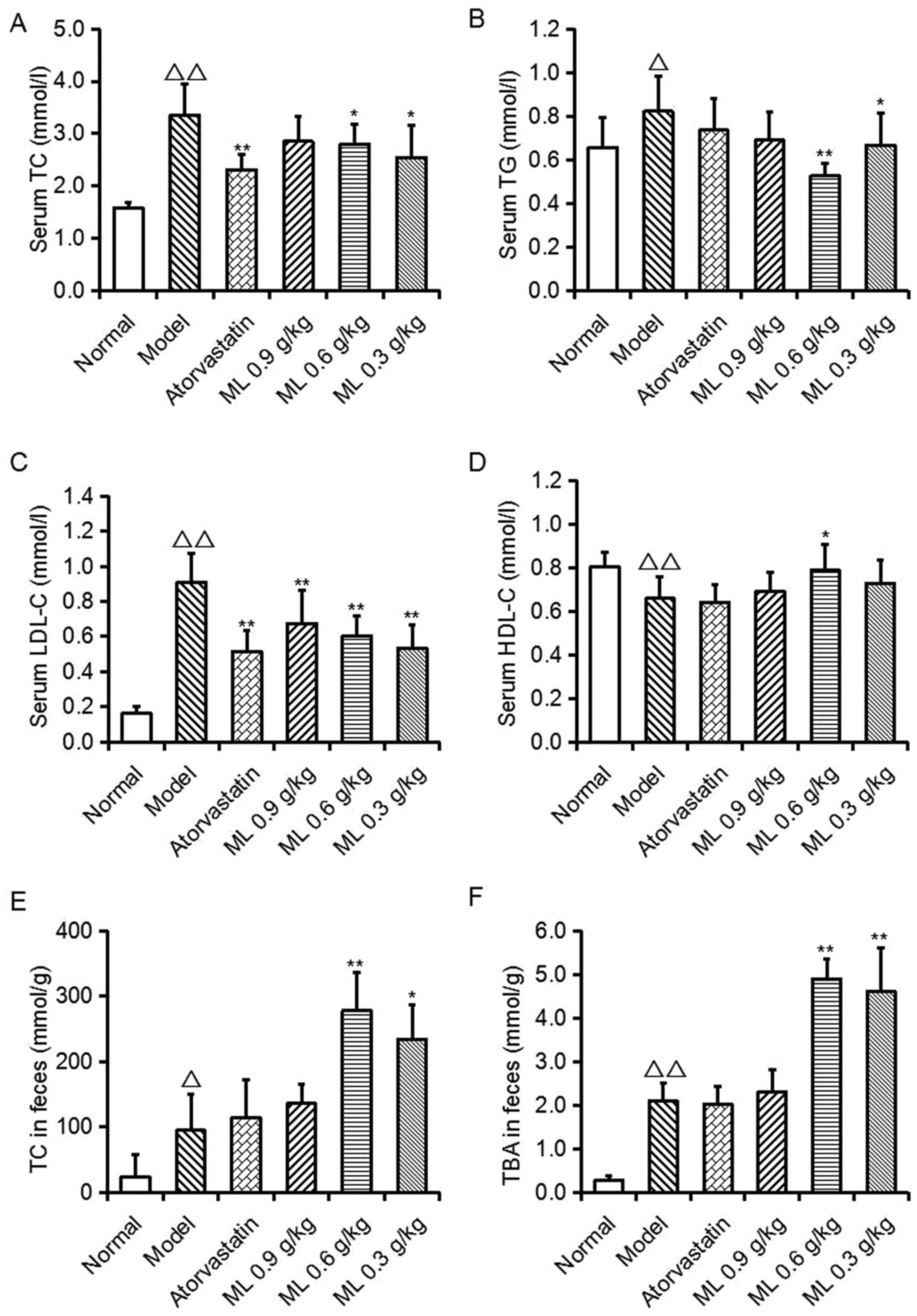
Effects of ML on serum lipid levels
and fecal TC and TBA levels
As presented in Fig.
1, serum TC (P<0.01), TG (P<0.05) and LDL-C (P<0.01)
levels, and fecal TC (P<0.05) and TBA (P<0.01) levels in the
model group were significantly increased, compared with the normal
group, whereas serum HDL-C levels were significantly decreased
(P<0.01). Compared with the model group, atorvastatin
significantly lowered serum TC and LDL-C levels (P<0.01),
however no other significant differences were observed. ML
treatment reduced TC (0.6 and 0.3 g/kg; P<0.05), TG (0.6 g/kg,
P<0.01; 0.3 g/kg, P<0.05) and LDL-C (all, P<0.01)
concentrations, and 0.6 g/kg ML significantly increased HDL-C
levels (P<0.05). Conversely, in ML treatment groups, fecal TC
(0.6 g/kg, P<0.01; 0.3 g/kg, P<0.05) and TBA (0.6 and 0.3
g/kg, P<0.01) levels were significantly increased, compared with
the model group. These findings suggest that the serum cholesterol
reduction of ML may be associated with the excretion of TC and
TBA.
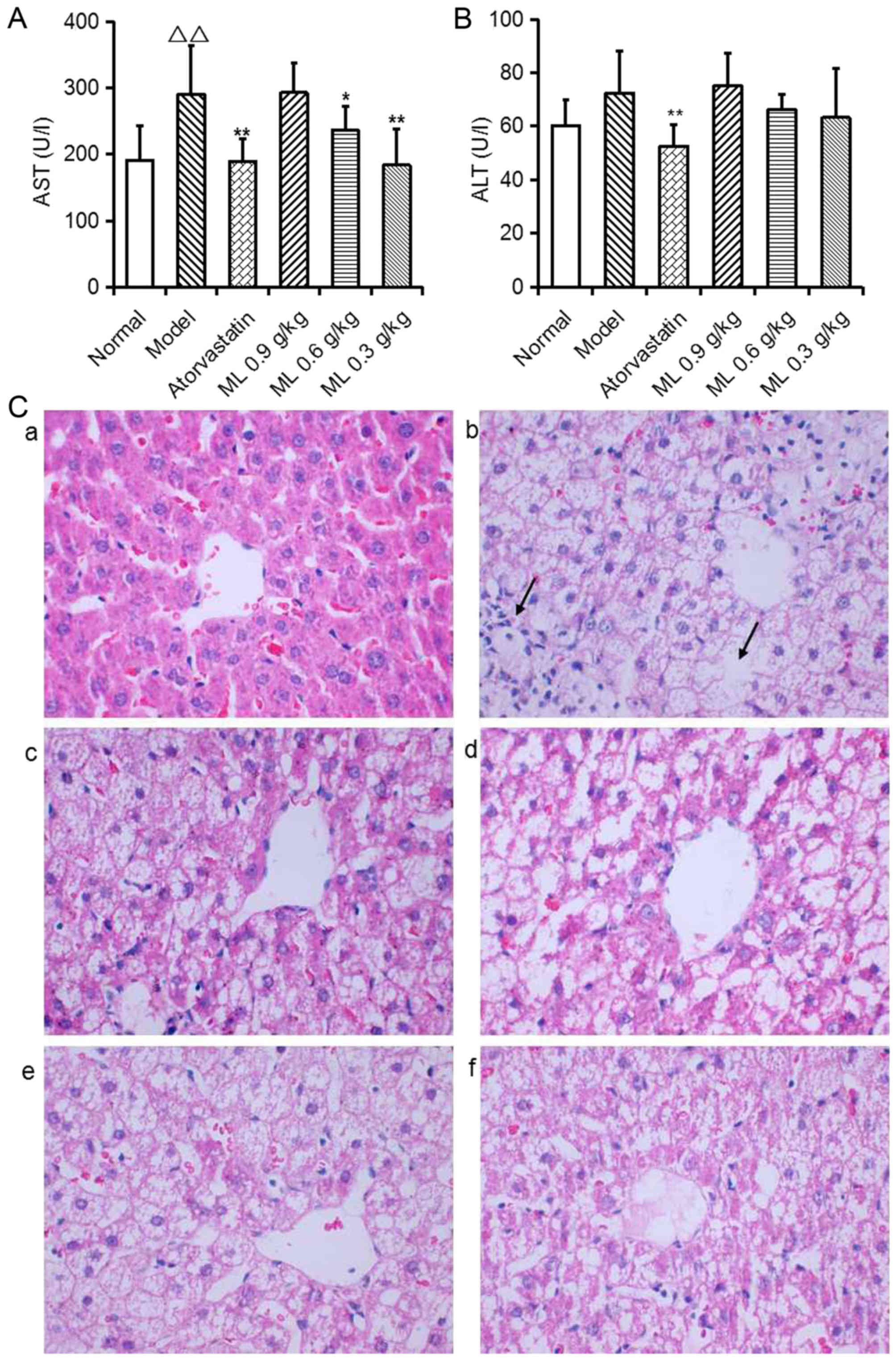
Effects of ML on liver pathological
changes and hepatic lipidosis
Serum AST activity was significantly increased in
model rats compared with the normal group (P<0.01; Fig. 2A), and ALT activity exhibited a
similar, marked trend (Fig. 2B).
Representative images of liver histology demonstrated that normal
rats presented normal liver histology, as hepatocytes were observed
with a common radial array encircling the central veins and no
hepatocyte lipid degeneration was observed (Fig. 2C-a). In model rats, the lobular
structures of hepatocytes were disrupted and inflammatory cell
infiltration was observed (Fig.
2C-b). Atorvastatin significantly reduced serum AST and ALT
activity compared with the model group (Fig. 2A and B), and notably improved
hepatocyte lipid degeneration (Fig.
2C-c). ML reduced serum AST activity in a dose-dependent manner
(0.6 g/kg, P<0.05; 0.3 g/kg, P<0.01; Fig. 2A), and induced a marked decrease in
ALT activity (Fig. 2B), compared
with the model group. ML also markedly reduced neutrophil
infiltration (Fig. 2C-d-f).
Liver TC (Fig. 3A)
and TBA (Fig. 3B) levels in the
model group were significantly increased (P<0.01 and
<0.05, respectively) compared with normal rats, and liver
TG exhibited a similar, marked increase (Fig. 3A). Lipid droplets were visible in the
hepatic plates, and oil red O staining revealed a pale pink
staining in the normal rat hepatic tissue (Fig. 3C-a), while the model rats exhibited
severe hepatic steatosis (Fig.
3C-b). ML significantly decreased liver TC (0.9 and 0.6 g/kg,
P<0.01; 0.3 g/kg, P<0.05; Fig.
3A) and TBA (0.9 g/kg, P<0.05; Fig. 3B) levels, and the color and density
of oil red O staining of the liver was weakened, indicating that
Atorastatin and ML markedly alleviated hepatocyte lipid
degeneration (Fig. 3C-c-f).
Effects of ML on expressions of
cholesterol absorption-related proteins in liver
According to the effects of ML on serum and liver
lipid level and fecal TC and TBA level, two of the ML treated
groups (0.6 and 0.3 g/kg) were chosen to observe the expression of
cholesterol absorption and excretion-related proteins in the
liver.
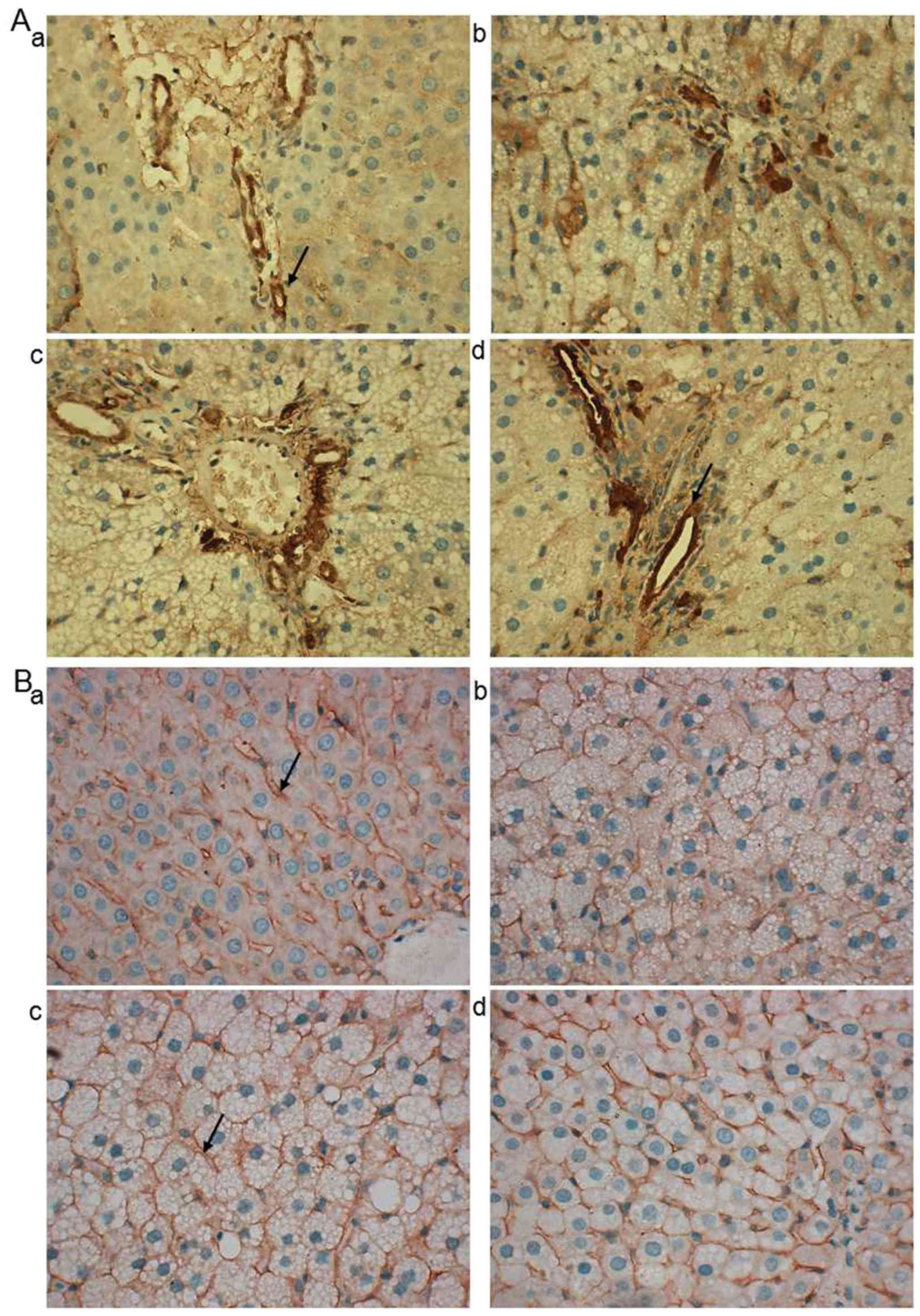
LDL-R and SR-BI are associated with the
transportation of cholesterol from peripheral blood to the liver
(26,27). As demonstrated in representative
images of immunohistochemistry, LDL-R protein was predominantly
located at the hepatic portal vein area (Fig. 4A). In the normal, Atorastatin and ML
groups, LDL-R was primarily located at the hepatocytes close to the
vein (Fig. 4A-a, c and d) while in
the model group it was observed in a wider range (Fig. 4A-b). SR-BI was predominantly located
at the hepatocyte membrane and no notable differences were observed
between the groups (Fig. 4B-a-d).
Western blotting revealed that the SR-BI protein expression in
model rats was significantly decreased compared with normal rats
(P<0.01), whereas LDL-R expression was significantly increased
(P<0.01; Fig. 5A and B).
Atorastatin and ML significantly reduced SR-BI (0.6 g/kg,
P<0.01) and LDL-R (both, P<0.01) levels compared with the
model group (Fig. 5A and B).
 | Figure 5.Expressions of cholesterol
absorption, conversion and bile acid excretion-associated proteins
in the livers of HFD-fed rats. (A) Western blotting images of SR-BI
and LDL-R, ABCG5, ABCG8, PPARα, FXR and CYP7A1 protein expression.
(B) Relative expression of SR-BI and LDL-R protein. (C) Relative
expression of ABCG5 and ABCG8 protein. (D) Ratio of ABCG5/ABDG8
expression. (E) Relative expression of PPARα, FXR and CYP7A1
protein. Data are presented as the mean ± standard error of the
mean (n=8). ∆P<0.05,
∆∆P<0.01, vs. normal; *P<0.05,
**P<0.01 vs. model. SR-BI, scavenger receptor class B
type I; LDL-R, low-density lipoprotein receptor; ABCG5, ATP-binding
cassette transporter G5; ABCG8, ATP-binding cassette transporter
G8; PPARα, peroxisome proliferator-activated receptor-α; FXR,
farnesoid-X receptor; CYP7A1, cholesterol 7α-hydroxylase 1; HFD,
high-fat diet; ML, mulberry leaf. |
Effects of ML on expressions of
cholesterol excretion related proteins in liver
ABCG5, ABCG8, FXR, PPARα and CYP7A1 are associated
with the conversion of cholesterol into TBA in the liver, and the
excretion of TBA in feces. Compared with the normal group, a
decrease was observed in ABCG5 and ABCG8 (P<0.01) protein
expression in the model group (Fig. 5A
and C), and the ratio of ABCG5/ABCG8 in the model group was
significantly increased, compared with that of the normal group
(P<0.01; Fig. 5D). This indicated
that the balance of ABCG5 and ABCG8 was changed. ABCG5 and ABCG8
protein expressions were significantly decreased in Atorastatin and
ML-treated rats compared with the model group (both 0.6 g/kg,
P<0.05; 0.3 g/kg, P<0.01), however the ratio of ABCG5/ABCG8
was significantly lower compared with that of the model rats (both,
P<0.01), indicating that ML was able to maintain the balance of
ABCG5 and ABCG8 expression in the liver.
Compared with normal rats, expression of PPARα
protein was increased significantly in the model group (P<0.05),
whereas FXR and CYP7A1 were significantly decreased (both,
P<0.01; Fig. 5E). ML at 0.6 and
0.3 g/kg significantly decreased the expression of PPARα and FXR
protein in liver, compared with model rats (all, P<0.01),
whereas 0.3 g/kg ML significantly increased the protein expression
of CYP7A1 (P<0.01; Fig. 5A and
E).
Discussion
Hypercholesterolemia accompanied by high serum LDL-C
and low HDL-C is a main factor for the development of
atherosclerotic disease (28,29).
Excess diet-derived cholesterol is the primary cause for
hypercholesterolemia (30). In the
present study, rats fed with HFD for 5 weeks exhibited
significantly increased serum TC and LDL-C levels. ML treatment
significantly reduced the accumulation of TC in plasma and the
liver, and alleviated hepatocyte lipid deposition in high-fat
diet-fed rats. H&E and oil red O staining images presented
lipid accumulation in the livers of rats fed an HFD, and a marked
increase of liver TG levels was observed in the HFD group compared
with the normal group. There are two potential reasons for these
findings: i) Intra-group variance induced a non-significant
difference between HFD group and normal group; and ii) HFD, which
increased the serum TC level, induced TC accumulation in the
liver.
RCT is a process that encompasses the transport of
excess cholesterol from peripheral tissues to the liver for biliary
and fecal excretion (31). It is an
effective method for cholesterol homeostasis in vivo
(23) and a protective mechanism to
contract atherosclerotic injury (32). Various cholesterol transporters,
including SR-BI, ABCG5, ABCG8 and CYP7A1 are associated with RCT,
and increase cholesterol transportation and conversion (33). Therefore, medicines which regulate
expression of these proteins are potential candidates for
hypercholesterolemia treatment.
LDL-R has a role in regulating plasma cholesterol
level and cholesterol homeostasis by limiting hepatic uptake of
circulating cholesterol (29).
However, contrary to recent findings (32), the present study demonstrated that
the expression of LDL-R in liver cells was significantly increased
in rats with hyperlipidemia, whereas it was decreased in ML-treated
rats. The LDL-R pathway is complex (34,35), and
with the previous identification of the LDL-R-proprotein convertase
subtilisin/kexin type 9 (PCSK9)-LDL axis in the LDL-R pathway for
cholesterol homeostasis, Kosenko et al (36) demonstrated that plasma LDL particles
reduce PCSK9-mediated LDL-R degradation in a dose-dependent manner
by binding to PCSK9. This finding suggested that decreased plasma
LDL may induce a lower expression of LDLR in hepatocytes. However,
whether there is an association among serum LDL, hepatic LDL-R,
PCSK9 and ML required further study.
SR-BI has a crucial role in cholesterol homeostasis
and hepatic SR-BI mediates the final step in RCT via the uptake of
HDL-C for routing to the bile (27,37). The
suppression of hepatic SR-BI expression impairs HDL-mediated RCT
and induces hypercholesterolemia (38,39). In
the present study, it was demonstrated that SR-BI protein
expression in the liver exhibited a decrease in model rats and ML
treatment did not block this decline, which suggested that the
SR-BI signal pathway may not be the target of ML in mediating the
transportation of cholesterol.
ABCG5 and ABCG8 are expressed in the liver and
intestine. They typically form a heterodimer in the endoplasmic
reticulum, which pumps neutral sterols back into the gut lumen to
prevent the accumulation of other sterols (40,41) and
partially contributes to the trans-intestinal cholesterol efflux
pathway (42). ABCG5/ABCG8 promotes
SR-BI-transported cholesterol excretion in the bile, and eventually
in feces, to prohibit the development of hypercholesterolemia, and
inactivation of either protein induces sitosterolemia (43). In the current study, TC and TBA
levels in the liver and feces were significantly increased in model
rats compared with the normal group; in ML-treated rats, liver TC
and TBA levels were significantly decreased while fecal TC and TBA
levels were significantly increased compared with the model rats,
indicating that in addition to the compensatory excretion of
cholesterol in rats with HFD intake, ML promoted the clearance of
cholesterol by TBA from feces in rats with hypercholesterolemia.
Western blotting results demonstrated that ML could maintain the
balance of ABCG5 and ABCG8 protein expression in the liver.
Together, these findings suggested that maintaining the
stabilization and activity of ABCG5/ABCG8 protein may be a
potential mechanism for ML to lower serum cholesterol.
The conversion of cholesterol into BA via intestinal
and biliary lumen for fecal excretion is the final step to remove
cholesterol from the body. CYP7A1 is a rate-limiting enzyme in
cholesterol conversion to BA. Promoting CYP7A1 expression
accelerates the cholesterol conversion into BA and the excretion in
feces (44,45). PPARα serves a role in the clearance
of circulating cholesterol via upregulating CYP7A1 expression in
hepatocytes (45). Farnesoid-X
receptor (FXR) is an intracellular BA receptor, which can be
activated by BA, and has a pivotal role for BA and lipid
homeostasis; overexpression of FXR decreases BA pools and reduces
fecal BA excretion (46) due to the
suppression of hepatic CYP7A1 (47).
In the present study, 5-week HFD intake increased PPARα protein
expression, and decreased FXR and CYP7A1 expression in liver,
whereas increased levels of TC and TBA in feces were observed. ML
treatment markedly inhibited PPARα and FXR protein expression,
upregulated CYP7A1 expression, increased fecal TBA levels, and
simultaneously reduced cholesterol in the liver and plasma.
Together, these findings suggest that ML may promote the conversion
of cholesterol into BA and excretion to accelerate the clearance of
circulating cholesterol. The present study focused primarily on
extrinsic cholesterol excretion from hepatocytes, and the effect of
ML on cholesterol synthesis will be considered in future studies.
Meanwhile, changes in liver and fecal TBA levels provide rationale
that regulating enterohepatic circulation of BA may be a potential
mechanism of ML on reduced serum TC levels.
As detailed above, ML exhibited a beneficial effect
on anti hyperlipidemia, but no obvious dose-effect association was
observed on serum TG and HDL-C levels, and fecal TC and TBA levels.
Previous studies have demonstrated that ML contains
1-dexoynokifimycin (48),
polyphenols and flavonoids (49),
which are active components on blood lipid regulation,
anti-atherosclerosis and anti-oxidation (50). In these studies, the test sample was
administered an extract of ML in which the active components were
enriched to induce a more notable effect. ML is edible and may be
used to make tea, which may be a convenient method to prevent
hyperlipidemia in the future. In the present study, ML powder was
produced via a process of hot air drying and ball milling
technology as the test sample. As this sample contained all the
components of ML, it was not possible to identify an exact
dose-effect relationship, but the present results have identified
an effective dosage for intake. However, if ML is to be considered
as a candidate medicine for hypercholesterolemia treatment, it is
essential that the chemical material basis and the dose-effect
relationship are elucidated.
The findings of the present study suggest a positive
role of ML on cholesterol clearance by promoting cholesterol and
TBA execration via FXR- and CYP7A1-mediated pathways, and that RCT
regulation may be a potential mechanism of ML on anti
hypercholesterolemia.
Acknowledgements
The authors would like to thank Dr Gao Jianli of
Zhejiang Chinese Medical University (Hangzhou, China) for their
assistance and revision of the manuscript.
Funding
The present study was supported by Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY15H280007), National Natural Science Foundation of China (grant
no. 81503328), and The Zhejiang Provincial Key Laboratory Project
(grant no. 2012E10002).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH designed and organized the study. YW and LL
performed the animal experiments. CY performed the western blot
analysis. ZL performed the data analysis and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhejiang Chinese Medical University (Hangzhou,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Navar-Boggan AM, Peterson ED, D'Agostino
RB Sr, Neely B, Sniderman AD and Pencina MJ: Hyperlipidemia in
early adulthood increases long-term risk of coronary heart disease.
Circulation. 131:451–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia GH, Liu JN, Wong A, Cordasco F,
Dines DM, Dines JS, Gulotta LV and Warren R: Hyperlipidemia
increases the risk of retear after arthroscopic rotator cuff
repair. J Shoulder Elbow Surg. 26:2086–2090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bjornsson E, Jacobsen EI and Kalaitzakis
E: Hepatotoxicity associated with statins: Reports of idiosyncratic
liver injury post-marketing. J Hepatol. 56:374–380. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Graham DJ, Staffa JA, Shatin D, Andrade
SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ and
Platt R: Incidence of hospitalized rhabdomyolysis in patients
treated with lipid-lowering drugs. JAMA. 292:2585–2590. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakamoto K, Honda T, Yokoya S, Waguri S
and Kimura J: Rab-small GTPases are involved in fluvastatin and
pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J.
21:4087–4094. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu SM, Shih WT, Yang YH, Chen PC and Chu
YH: Use of traditional Chinese medicine in patients with
hyperlipidemia: A population-based study in Taiwan. J
Ethnopharmacol. 168:129–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai S, Sun W, Fan Y, Guo X, Xu G, Xu T,
Hou Y, Zhao B, Feng X and Liu T: Effect of mulberry leaf (Folium
Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling
pathway in type 2 diabetes mellitus rats. Pharm Biol. 54:2685–2691.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salemi Z, Barzin Tond S, Fallah S, Shojaii
A and Seifi M: The effect of Morus alba leaves extract and powder
on resistin levels and liver transaminase enzymes activities in
diabetes. Cell Mol Biol (Noisy-le-Grand). 62:112–118.
2016.PubMed/NCBI
|
|
9
|
Zhang Y, Ren C, Lu G, Cui W, Mu Z, Gao H
and Wang Y: Purification, characterization and anti-diabetic
activity of a polysaccharide from mulberry leaf. Regul Toxicol
Pharmacol. 70:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan MA, Rahman AA, Islam S, Khandokhar P,
Parvin S, Islam MB, Hossain M, Rashid M, Sadik G, Nasrin S, et al:
A comparative study on the antioxidant activity of methanolic
extracts from different parts of Morus alba L. (Moraceae). BMC Res
Notes. 6:242013. View Article : Google Scholar
|
|
11
|
Lee YJ, Hsu JD, Lin WL, Kao SH and Wang
CJ: Upregulation of caveolin-1 by mulberry leaf extract and its
major components, chlorogenic acid derivatives, attenuates
alcoholic steatohepatitis via inhibition of oxidative stress. Food
Funct. 8:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong JW, Lee HH, Lee KW, Kim KY, Kim SG,
Hong SH, Kim GY, Park C, Kim HK, Choi YW and Choi YH: Mori folium
inhibits interleukin-1β-induced expression of matrix
metalloproteinases and inflammatory mediators by suppressing the
activation of NF-κB and p38 MAPK in SW1353 human chondrocytes. Int
J Mol Med. 37:452–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ann JY, Eo H and Lim Y: Mulberry leaves
(Morus alba L.) ameliorate obesity-induced hepatic lipogenesis,
fibrosis, and oxidative stress in high-fat diet-fed mice. Genes
Nutr. 10:462015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto M, Arai H, Tamura Y, Murayama T,
Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, et al:
Mulberry leaf ameliorates the expression profile of adipocytokines
by inhibiting oxidative stress in white adipose tissue in db/db
mice. Atherosclerosis. 204:388–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enkhmaa B, Shiwaku K, Katsube T, Kitajima
K, Anuurad E, Yamasaki M and Yamane Y: Mulberry (Morus alba L.)
leaves and their major flavonol quercetin 3-(6-malonylglucoside)
attenuate atherosclerotic lesion development in LDL
receptor-deficient mice. J Nutr. 135:729–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aramwit P, Petcharat K and Supasyndh O:
Efficacy of mulberry leaf tablets in patients with mild
dyslipidemia. Phytother Res. 25:365–369. 2011.PubMed/NCBI
|
|
17
|
Aramwit P, Supasyndh O, Siritienthong T
and Bang N: Mulberry leaf reduces oxidation and C-reactive protein
level in patients with mild dyslipidemia. Biomed Res Int.
2013:7879812013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi Y, Miyazawa M, Kamei A, Abe K
and Kojima T: Ameliorative effects of mulberry (Morus alba L.)
leaves on hyperlipidemia in rats fed a high-fat diet: Induction of
fatty acid oxidation, inhibition of lipogenesis, and suppression of
oxidative stress. Biosci Biotechnol Biochem. 74:2385–2395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lou Z, Zhang G, Su J, Xia B, Yu J and Yan
M: Effect of green tea and mulberry leaf powder on serum lipid
profile in rats with hyperlipidemia. Zhongchengyao. 38:1594–1597.
2016.(In Chinese).
|
|
20
|
Trimarco V, Izzo R, Stabile E, Rozza F,
Santoro M, Manzi MV, Serino F, Schiattarella GG, Esposito G and
Trimarco B: Effects of a new combination of nutraceuticals with
Morus alba on lipid profile, insulin sensitivity and endotelial
function in dyslipidemic subjects. A cross-over, randomized,
double-blind trial. High Blood Press Cardiovasc Prev. 22:149–154.
2015. View Article : Google Scholar
|
|
21
|
Valacchi G, Belmonte G, Miracco C, Eo H
and Lim Y: Effect of combined mulberry leaf and fruit extract on
liver and skin cholesterol transporters in high fat diet-induced
obese mice. Nutr Res Pract. 8:20–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riwanto M and Landmesser U: High density
lipoproteins and endothelial functions: Mechanistic insights and
alterations in cardiovascular disease. J Lipid Res. 54:3227–3243.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Velde AE, Vrins CL, van den Oever
K, Seemann I, Oude Elferink RP, van Eck M, Kuipers F and Groen AK:
Regulation of direct transintestinal cholesterol excretion in mice.
Am J Physiol Gastrointest Liver Physiol. 295:G203–G208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
The State Science and Technology
Commission of China: Regulations on the management of laboratory
animals. Shiyong Qiguan Yizhi Zazhi. 4:66–67. 2016.(In
Chinese).
|
|
25
|
Ministry of Science and Technology of the
People's Republic of China: 2018, Methods for managing experimental
animal licenses (trial). [online] Available at:. http://www.most.gov.cn/fggw/zfwj/zfwj2001/zf01yw/zf01kjjh/200312/t20031209_31332.htmJuly
30–2015
|
|
26
|
Brown MS and Goldstein JL: A
receptor-mediated pathway for cholesterol homeostasis. Science.
232:34–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huby T, Doucet C, Dachet C, Ouzilleau B,
Ueda Y, Afzal V, Rubin E, Chapman MJ and Lesnik P: Knockdown
expression and hepatic deficiency reveal an atheroprotective role
for SR-BI in liver and peripheral tissues. J Clin Invest.
116:2767–2776. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Braamskamp MJ, Hutten BA and Wiegman A:
Early initiation of statin treatment in children with familial
hypercholesterolaemia. Curr Opin Lipidol. 26:236–239. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yakushiji E, Ayaori M, Nishida T, Shiotani
K, Takiguchi S, Nakaya K, Uto-Kondo H, Ogura M, Sasaki M, Yogo M,
et al: Probucol-oxidized products, spiroquinone and diphenoquinone,
promote reverse cholesterol transport in mice. Arterioscler Thromb
Vasc Biol. 36:591–597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang DQ: Regulation of intestinal
cholesterol absorption. Annu Rev Physiol. 69:221–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orsoni A, Villard EF, Bruckert E,
Robillard P, Carrie A, Bonnefont-Rousselot D, Chapman MJ,
Dallinga-Thie GM, Le Goff W and Guerin M: Impact of LDL apheresis
on atheroprotective reverse cholesterol transport pathway in
familial hypercholesterolemia. J Lipid Res. 53:767–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Si Y, Zhai L, Guo S, Zhao J, Sang
H, Pang X, Zhang X, Chen A and Qin S: Celastrus orbiculatus thunb.
Reduces lipid accumulation by promoting reverse cholesterol
transport in hyperlipidemic mice. Lipids. 51:677–692. 2016.
|
|
33
|
Zhu RG, Sun YD, Hou YT, Fan JG, Chen G and
Li TP: Pectin penta-oligogalacturonide reduces cholesterol
accumulation by promoting bile acid biosynthesis and excretion in
high-cholesterol-fed mice. Chem Biol Interact. 272:153–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maxwell KN, Fisher EA and Breslow JL:
Overexpression of PCSK9 accelerates the degradation of the LDLR in
a post-endoplasmic reticulum compartment. Proc Natl Acad Sci USA.
102:2069–2074. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zelcer N, Hong C, Boyadjian R and Tontonoz
P: LXR regulates cholesterol uptake through Idol-dependent
ubiquitination of the LDL receptor. Science. 325:100–104. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kosenko T, Golder M, Leblond G, Weng W and
Lagace TA: Low density lipoprotein binds to proprotein convertase
subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits
PCSK9-mediated low density lipoprotein receptor degradation. J Biol
Chem. 288:8279–8288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji Y, Wang N, Ramakrishnan R, Sehayek E,
Huszar D, Breslow JL and Tall AR: Hepatic scavenger receptor BI
promotes rapid clearance of high density lipoprotein free
cholesterol and its transport into bile. J Biol Chem.
274:33398–33402. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Braun A, Zhang S, Miettinen HE, Ebrahim S,
Holm TM, Vasile E, Post MJ, Yoerger DM, Picard MH, Krieger JL, et
al: Probucol prevents early coronary heart disease and death in the
high-density lipoprotein receptor SR-BI/apolipoprotein E double
knockout mouse. Proc Natl Acad Sci USA. 100:7283–7288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chulsky S, Paland N, Lazarovich A and
Fuhrman B: Urokinase-type plasminogen activator (uPA) decreases
hepatic SR-BI expression and impairs HDL-mediated reverse
cholesterol transport. Atherosclerosis. 233:11–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berge KE, Tian H, Graf GA, Yu L, Grishin
NV, Schultz J, Kwiterovich P, Shan B, Barnes R and Hobbs HH:
Accumulation of dietary cholesterol in sitosterolemia caused by
mutations in adjacent ABC transporters. Science. 290:1771–1775.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Mitsche MA, Lutjohann D, Cohen JC,
Xie XS and Hobbs HH: Relative roles of ABCG5/ABCG8 in liver and
intestine. J Lipid Res. 56:319–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van der Veen JN, van Dijk TH, Vrins CL,
van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK and
Kuipers F: Activation of the liver X receptor stimulates
trans-intestinal excretion of plasma cholesterol. J Biol Chem.
284:19211–19219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dikkers A, Freak de Boer J, Annema W,
Groen AK and Tietge UJ: Scavenger receptor BI and ABCG5/G8
differentially impact biliary sterol secretion and reverse
cholesterol transport in mice. Hepatology. 58:293–303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao Y, Bei W, Hu Y, Cao L, Huang L, Wang
L, Luo D, Chen Y, Yao X, He W, et al: Hypocholesterolemia of
Rhizoma Coptidis alkaloids is related to the bile acid by
up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine.
19:686–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li T, Matozel M, Boehme S, Kong B, Nilsson
LM, Guo G, Ellis E and Chiang JY: Overexpression of cholesterol
7alpha-hydroxylase promotes hepatic bile acid synthesis and
secretion and maintains cholesterol homeostasis. Hepatology.
53:996–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sinal CJ, Tohkin M, Miyata M, Ward JM,
Lambert G and Gonzalez FJ: Targeted disruption of the nuclear
receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell.
102:731–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai SY, He H, Nguyen T, Mennone A and
Boyer JL: Retinoic acid represses CYP7A1 expression in human
hepatocytes and HepG2 cells by FXR/RXR-dependent and independent
mechanisms. J Lipid Res. 51:2265–2274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kojima Y, Kimura T, Nakagawa K, Asai A,
Hasumi K, Oikawa S and Miyazawa T: Effects of mulberry leaf extract
rich in 1-deoxynojirimycin on blood lipid profiles in humans. J
Clin Biochem Nutr. 47:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chan KC, Yang MY, Lin MC, Lee YJ, Chang WC
and Wang CJ: Mulberry leaf extract inhibits the development of
atherosclerosis in cholesterol-fed rabbits and in cultured aortic
vascular smooth muscle cells. J Agric Food Chem. 61:2780–2788.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He X, Fang J, Ruan Y, Wang X, Sun Y, Wu N,
Zhao Z, Chang Y, Ning N, Guo H and Huang L: Structures,
bioactivities and future prospective of polysaccharides from Morus
alba (white mulberry): A review. Food Chem. 245:899–910. 2018.
View Article : Google Scholar : PubMed/NCBI
|