Introduction
Thyroid cancer is one of the most common endocrine
tumors and its incidence is increasing globally (1). The first symptom of thyroid cancer is
usually the development of thyroid nodules. More than 90% of these
nodules are benign; however, malignant thyroid nodules account for
>90% of all endocrine malignancies (2). The aggressiveness of thyroid cancer
increases with the reduction of cell adherence (3) Anaplastic thyroid cancer (ATC) is the
most aggressive type of thyroid cancer and there are currently no
effective methods of treating patients with ATC. However,
understanding of the molecular pathogenesis of this cancer is
increasing and a number of clinical trials have been performed to
try and identify novel methods of treating this type of thyroid
cancer (4).
Vitamin D is a fat-soluble prohormone that exerts
important roles in calcium metabolism and homeostasis, and its
active metabolite is 1,25-dihydroxyvitamin D (1,25-D3) (5). The primary effect of 1,25-D3 is to
regulate bone and calcium homeostasis. However, this active form of
vitamin D3 also exhibits non-classical effects, including
regulation of the cell cycle and various anti-tumor effects, and is
therefore attracting increased attention (6–8). It has
been demonstrated that 1,25-D3 is implicated in various types of
cancer (9–11), including thyroid cancer, where its
concentration was reduced (12,13).
Active vitamin D may serve as a ligand of the vitamin D receptor
(VDR). Vitamin D signaling affects the expression of its target
genes via the vitamin D response element (VDRE). A previous study
has demonstrated the interaction of vitamin D signaling and nuclear
receptor ligands in certain types of cancer (14). However, the results of clinical
trials have indicated that the anti-tumor efficacy of 1,25-D3 is
disappointing (15–17). Furthermore, the administration of
large amounts of 1,25-D3 may induce hypercalcemia, which limits the
scope of its use (18).
It remains controversial whether vitamin D exhibits
an anti-cancer effect (19–22); therefore, it is important to
investigate the factors that may affect the anti-cancer effect of
vitamin D in cancer cells. The metabolism of vitamin D is highly
complex and a series of enzymes are involved in its synthesis,
activation and inactivation (23).
The enzyme 25-hydroxyvitamin-D3-24-hydroxylase (CYP24A1), is
responsible for neutralizing active 1,25-D3 and is an important
component of the mitochondrial enzyme cytochrome P450 (24). This 1,25-D3-inactivating enzyme is
critical in determining the antitumor activity of 1,25-D3. It has
been demonstrated that high expression of CYP24A1 facilitates
carcinogenesis in colorectal cancer (25). Thus, the aim of the current study was
to identify whether CYP24A1 affects the anti-tumor effect of
1,25-D3 in thyroid cancer.
The protein kinase B (Akt) signaling pathway
enhances cell survival and tumorigenesis (26–28).
Abnormal activation of Akt may be associated with the progression
of thyroid tumors (29,30). In addition, β-catenin, another
important signaling molecule in cells, has attracted extensive
attention due to its critical role in differentiation and
regulation of patterning (31). The
activity of β-catenin in tumors is enhanced by various mutations
(32–34). Furthermore, the alteration of
β-catenin activity has been identified in the majority of
aggressive thyroid tumors (35).
Based on these results, the current study sought to explore the
effect of 1,25-D3 on the proliferation and metastasis of thyroid
cancer cells following the inhibition of CYP24A1 and to determine
its molecular mechanisms of action.
Materials and methods
Cell culture
The anaplastic thyroid cancer cell line KAT-18 was
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were kept in RPMI 1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA) with 10% fetal bovine serum (FBS
Gibico; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
incubator containing 5% CO2.
Cell transfection
To knockdown CYP24A1 expression, cells were seeded
in 24-well plates at a density of 2×105/well. When cells
reached 50–60% confluence, they were cultured in serum-free medium
overnight. Cells were then transfected with 100 nM human
CYP24A1siRNA (cat. no. sc-44652; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or 100 nM scramble control siRNA (cat. no.
sc-37007; Santa Cruz Biotechnology, Inc.) using X-tremeGENE™ siRNA
transfection reagent (Roche Diagnostics, Basel, Switzerland)
following the manufacturer's protocol. Following 36 h incubation,
cells were treated with or without 100 nM 1,25-D3 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 4 h; this concentration of
1,25-D3 was selected according to the results of previous studies
(36,37). Cells in all relevant groups received
the same concentration of 1,25-D3 (100 nM). All subsequent
experiments were independently performed ≥3 times.
Cell grouping
Cells were divided into different groups as follows:
A control group (untreated cells); cells treated with 100 nM
1,25-D3 for 4 h (VD3); cells transfected with scramble control
siRNA and treated with 100 nM 1,25-D3 (siscr+VD3); cells
transfected with CYP24A1 siRNA and treated with 100 nM 1,25-D3
(siCYP+VD3); cells transfected with scramble control siRNA (siscr)
and cells transfected with CYP24A1siRNA (siCYP).
Cell proliferation assay
Cells were seeded at 1×105 cell/well in
24-well plates. A Cell Counting Kit 8 (CCK-8) assay was used to
measure the proliferation of cells in each group. The CCK-8
solution from the Cell Counting Kit (Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China) was added to each well (10
µl/well). Cells were then maintained at 37°C for 4 h. The
absorbance of the reaction regent was determined at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell migration assay
Cells (2 ml) at a density of 1×105/ml
were placed on the upper cell culture chambers containing Transwell
inserts (Corning Incorporated, Corning, NY, USA) and maintained in
medium containing 0.2% FBS. The lower chamber was supplemented with
the medium containing 15% FBS. The chambers were incubated for 24 h
and then cells retained on the upper chamber were removed. Cells
that migrated to the lower chamber were fixed with 4%
paraformaldehyde at room temperature for 20 min. Cells were then
stained with 0.1% crystal violet at room temperature for 20 min and
observed using an optical microscope at a magnification of
×200.
Scratch wound assay
Following transfection, cells were cultured in
complete medium for a further 12 h. A sterile pipette tip was used
to scratch the wells and cells were then incubated in serum-free
solution following washing with PBS. The distance of the scratch
was measured at 0 and 24 h following incubation using an inverted
microscope at a magnification of ×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
regent (Invitrogen; Thermo Fisher Scientific, Inc.). The
purification and concentration of RNA was measured at 260/280 nm
using an ultra-micro spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.). The integrity of RNA was detected
using 1% agarose gel electrophoresis. A total of 1 µg RNA was
reverse transcribed using M-MLV reverse transcriptase (Promega
Corporation, Madison, WI, USA), ribonuclease inhibitor and
oligo(dT18) (Takara Bio, Inc., Otsu, Japan), 5X buffer (Takara Bio,
Inc.) and dNTP (Takara Bio, Inc.) in 20 µl volumes. The temperature
protocol used for reverse transcription was: 25°C for 10 min; 42°C
for 50 min; and 70°C for 15 min. A SYBR® Premix Taq™ II
kit (Takara Bio, Inc.) was used to amplify cDNA on a Mx3000
platform (Agilent Technologies, Inc., Santa Clara, CA, USA)
following the manufacturer's protocol. The thermocycling conditions
were as follows: 95°C for 4 min; 35 cycles of 95°C for 30 sex; 60°C
for 30 sec; and 72°C for 10 min. The relative expression level was
calculated using the 2−ΔΔCq method (38). The primers used were as follows:
E-cadherin, forward, 5′-TCACATCCTACACTGCCCAG-3′ and reverse,
5′-AGTGTCCCTGTTCCAGTAGC-3′; N-cadherin forward,
5′-ATATTTCCATCCTGCGCGTG-3′ and reverse, 5′-GTTTGGCCTGGCGTTCTTTA-3′;
vimentin, forward, 5′-GAGAGGAAGCCGAAAACACC-3′ and reverse,
5′-TTCCTGAATCTGAGCCTGCA-3′; matrix metalloproteinase (MMP) 2,
forward, 5′-ACCACAGCCAACTACGATGA-3′ and reverse,
5′-GCTCCTGAATGCCCTTGATG-3′; MMP9, forward,
5′-GCGTCTTCCCCTTCACTTTC-3′ and reverse, 5′-ATAGGGTACATGAGCGCCTC-3′;
metalloproteinase inhibitor (TIMP) 1, forward,
5′-ACTACCTGCAGTTTTGTGGC-3′ and reverse, 5′-CTGGAAGCCCTTTTCAGAGC-3′;
CYP24A1 forward, 5′-CCGTAATCCCCAAGTGCAAC-3′ and reverse,
5′-CCCAGAACTGTTGCCTTGTC-3′; VDR, forward,
5′-ACTCCACCACCCAAAAGTCA-3′ and reverse, 5′-GCATTCCCCAAACTCAAGCA-3′;
β-actin, forward, 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse,
5′-GCTGTCACCTTCACCGTTCC-3′.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer
containing protease inhibitors (Roche Diagnostics, Basel,
Switzerland) was used to extract the total protein. Protein
concentration was determined using Bio-Rad protein assay kits
(Bio-rad Laboratories, Inc.). Proteins (20 µg/lane) were resolved
using 8–10% SDS-PAGE and transferred onto a PVDF membrane (EMD
Millipore, Billerica, MA, USA). Subsequently, membranes were
blocked using a blocking buffer (20 mM, Tris-base, 150 mM NaCl)
containing 0.1% Tween-20 and non-fat milk for 2 h at room
temperature. Following washing with PBS, the membrane was incubated
with primary antibodies against CYP24A1 (cat. no. sc-365700; 1:700;
Santa Cruz Biotechnology, Inc.), VDR (cat. no. sc-13133; 1:500;
Santa Cruz Biotechnology, Inc.), Akt1/2 (cat. no. sc81434; 1:1,000;
Santa Cruz Biotechnology, Inc.), phosphorylated (p)-Akt (cat. no.
ab81283; 1:5,000; Abcam, Cambridge, UK), β-catenin (cat. no.
ab32572; 1:5,000; Abcam), N-cadherin (cat. no. C2542; 1:100;
Sigma-Aldrich; Merck KGaA), E-cadherin (cat. no. SAB4503751;
1:1,000, Sigma-Aldrich; Merck KGaA), vimentin (cat. no. V5255;
1:500; Sigma-Aldrich; Merck KGaA), MMP-2 (cat. no. 40994; 1:1,000;
Cell signaling technology, Inc., Danvers, MA, USA), MMP-9 (cat. no.
13667; 1:1,000; Cell signaling technology, Inc.), TIMP1 (cat. no.
8946; 1:1,000; Cell signaling technology, Inc.) and β-actin (cat.
no. 3700; 1:1,000; Cell signaling technology, Inc.) overnight at
4°C. Subsequently, the membrane was incubated with horseradish
peroxidase conjugated secondary antibodies (cat. no. ab6728;
1:2,000; Abcam) for 1 h at room temperature. Immunoblots were
visualized using enhanced chemiluminescence (Beyotime Institute of
Biotechnology, Haimen, China). ImageJ software version 1.46
(National Institutes of Health, Bethesda, MD, USA) was used to
quantify protein expression.
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance followed by a Turkey's post-hoc test
was performed to compare differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA,
USA) was used to perform data analysis.
Results
The effect of CYP24A1 knockdown and
1,25-D3 treatment on cell proliferation
The association between enhanced CYP24A1 activity
and tumorigenesis has been previously reported (8). Following transfection with CYP24A1
siRNA, CYP24A1 expression was significantly downregulated (Fig. 1A-C), meaning that the combined effect
of CYP24A1 and 1,25-D3 on thyroid cancer cells could be explored
effectively. The results of the CCK-8 assay indicated that the
proliferation of thyroid cancer cells was significantly inhibited
in the VD3 group and the siCYP group, compared with the control
(Fig. 1D). Furthermore, cell
proliferation was significantly inhibited in the siCYP+VD3 group
compared with the VD3 and siCYP groups (Fig. 1D). These results suggest that CYP24A1
knockdown and 1,25-D3 treatment synergistically suppress cell
proliferation.
 | Figure 1.(A) Quantitative analysis of CYP24A1
mRNA expression following transfection with siRNA. **P<0.01 vs.
control. (B) Determination of CYP24A1 protein expression following
transfection with siRNA. *P<0.05 vs. control. (C) Western blot
analysis of CYP24A1 expression following transfection with siRNA.
β-actin was used as a sample loading control. (D) Determination of
cell proliferation using a Cell Counting Kit-8. *P<0.05,
**P<0.01 and ***P<0.001 vs. control; #P<0.05
vs. VD3 group. Control, untreated cells; VD3, cells treated with
1,25-D3 for 4 h; siscr+VD3, cells transfected with scramble control
siRNA and treated with 1,25-D3; siCYP+VD3, cells transfected with
CYP24A1 siRNA and treated with 1,25-D3; siscr, cells transfected
with scramble control siRNA; siCYP, cells transfected with
CYP24A1siRNA; siRNA, small interfering RNA; CYP24D1,
25-hydroxyvitamin-D3-24-hydroxylase; 1,25-D3, 1,25-dihydroxyvitamin
D. |
The effect of CYP24A! knockdown and
1,25-D3 treatment on the expression of CYP24A1 and VDR
Impaired 1,25-D3-VDR signaling serves an important
role in the progression of thyroid cancer (39). It has also been demonstrated that the
expression of CYP24A1 is increased by 1,25-D3 (8,40). Thus,
levels of CYP24A1 and VDR in the presence of 1,25-D3 were measured.
The results revealed that 1,25-D3 treatment increased CYP24A1 mRNA
(Fig. 2A) and protein (Fig. 2B and C) expression whereas CYP24A1
expression was significantly decreased following treatment with
siCYP. By contrast, VDR expression did not change significantly
following treatment with 1,25-D3 or transfection with siCYP
(Fig. 2).
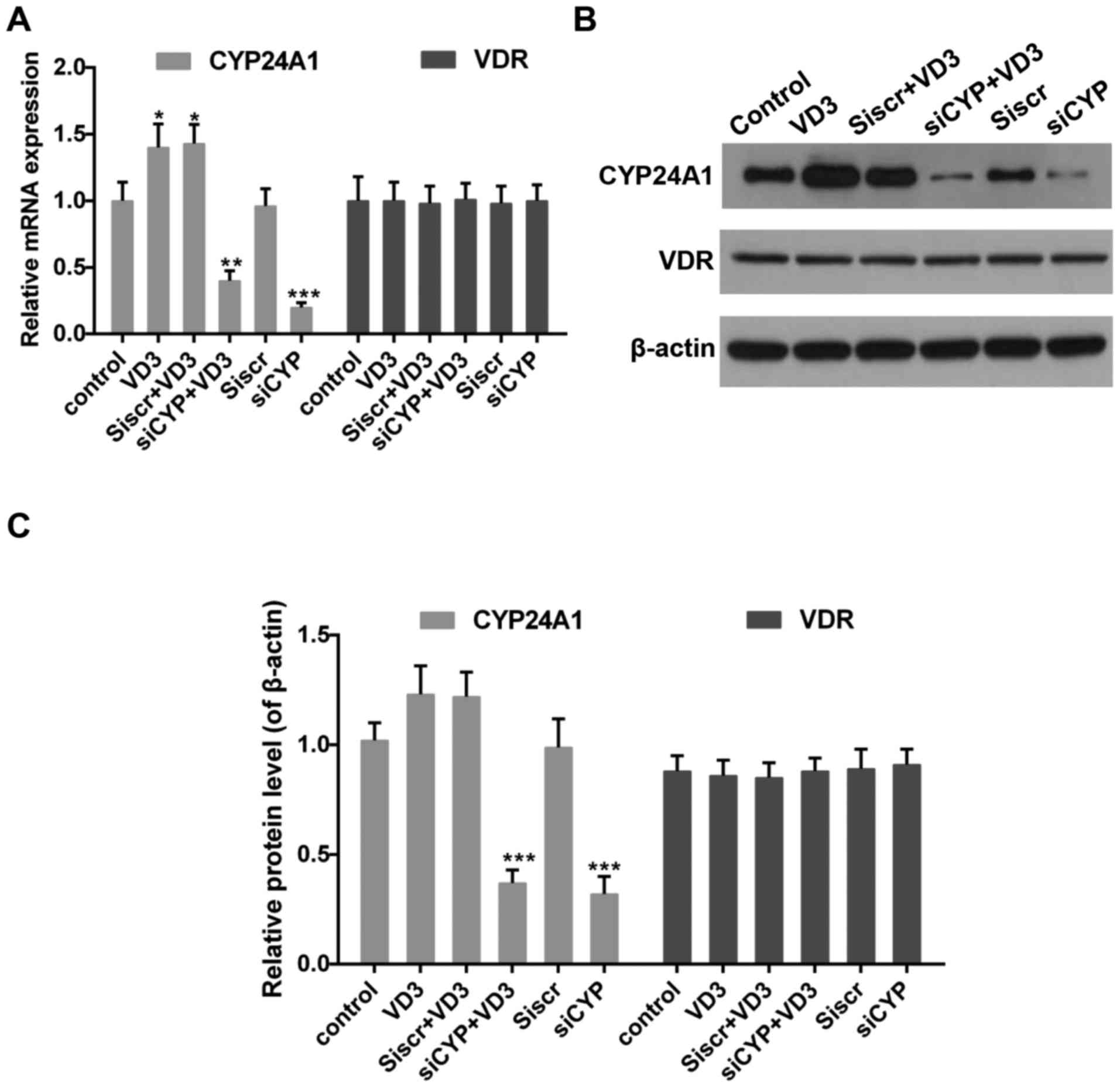 | Figure 2.(A) Quantitative analysis of CYP24A1
and VDR mRNA expression. *P<0.05, **P<0.01 and ***P<0.001
vs. control. (B) Western blotting and (C) quantification of CYP24A1
and VDR protein expression. β-actin was used as the sample loading
control. ***P<0.001 vs. control. Control, untreated cells; VD3,
cells treated with 1,25-D3 for 4 h; siscr+VD3, cells transfected
with scramble control siRNA and treated with 1,25-D3; siCYP+VD3,
cells transfected with CYP24A1 siRNA and treated with 1,25-D3;
siscr, cells transfected with scramble control siRNA; siCYP, cells
transfected with CYP24A1siRNA; siRNA, small interfering RNA;
CYP24D1, 25-hydroxyvitamin-D3-24-hydroxylase; VDR, vitamin D
receptor; 1,25-D3, 1,25-dihydroxyvitamin D. |
The effect of CYP24A1 knockdown and
1,25-D3 treatment on cell migration
The migration of thyroid cancer cells was measured
using Transwell and scratch assays. The number of migrating cells
significantly decreased following treatment with 1,25-D3 or
transfection with CYP24A1siRNA, compared with untreated cells
(Fig. 3). Furthermore, the number of
migrating cells was significantly lower in the group of cells that
underwent transfection with CYP24A1siRNA and treatment with
1,25-D3, compared with the group that underwent treatment with
1,25-D3 alone (Fig. 3B).
 | Figure 3.(A) Measurement of cell migration
using a Transwell assay. Magnification, ×200. (B) Relative numbers
of migrating cells. **P<0.01 and ***P<0.001 vs. control;
##P<0.01 vs. VD3 group. Control, untreated cells;
VD3, cells treated with 1,25-D3 for 4 h; siscr+VD3, cells
transfected with scramble control siRNA and treated with 1,25-D3;
siCYP+VD3, cells transfected with CYP24A1 siRNA and treated with
1,25-D3; siscr, cells transfected with scramble control siRNA;
siCYP, cells transfected with CYP24A1siRNA; siRNA, small
interfering RNA; CYP24D1, 25-hydroxyvitamin-D3-24-hydroxylase;
1,25-D3, 1,25-dihydroxyvitamin D. |
The results of the scratch wound assay demonstrated
that the relative migration distance was 0.5±0.06 mm in the control
group, 0.78±0.11 mm in the VD3 group, 0.74±0.09 mm in the siscr+VD3
group, 0.98±0.13 mm in the siCYP+VD3 group, 0.47±0.06 mm in the
siscr group and 0.57±0.08 mm in the siCYP group (Fig. 4). Significant differences were
observed in the relative wound width between the VD3, Siscr+VD3,
siCYP+VD3 groups compared with the control group. Furthermore, the
migration distance was significantly higher in the siCYP+VD3
compared with the VD3 group.
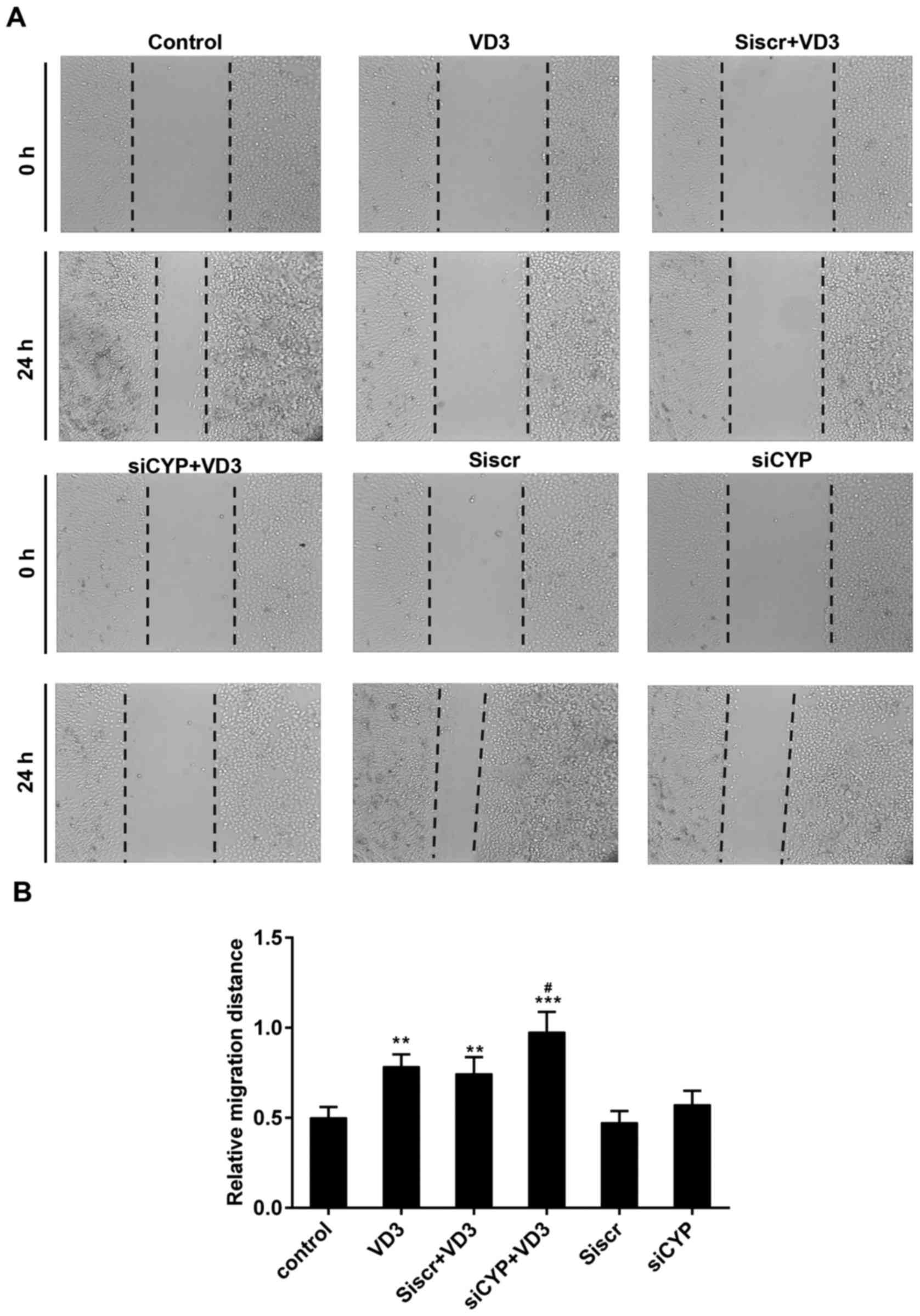 | Figure 4.(A) Assessment of tumor cell
migration using a scratch assay. Magnification, ×100. (B) Relative
wound width of tumor cells in each group. **P<0.01 and
***P<0.001 vs. control; #P<0.05 vs. VD3 group.
Control, untreated cells; VD3, cells treated with 1,25-D3 for 4 h;
siscr+VD3, cells transfected with scramble control siRNA and
treated with 1,25-D3; siCYP+VD3, cells transfected with CYP24A1
siRNA and treated with 1,25-D3; siscr, cells transfected with
scramble control siRNA; siCYP, cells transfected with CYP24A1
siRNA; siRNA, small interfering RNA; CYP24D1,
25-hydroxyvitamin-D3-24-hydroxylase; 1,25-D3, 1,25-dihydroxyvitamin
D. |
The effect of CYP24A1 knockdown and
1,25-D3 treatment on the expression of epithelial-mesenchymal
transition (EMT)-associated genes
The EMT is considered to be responsible for tumor
cell migration and invasion, and production of the extracellular
cell matrix (ECM) during tumor progression (41,42).
E-cadherin is highly expressed in epithelial cells whereas
N-cadherin and vimentin are abundant in mesenchymal cells (43). The results of the western blotting
revealed that the expression of E-cadherin was significantly
increased in cells treated with 1,25-D3 or CYP24A1siRNA (Fig. 5A and B). Furthermore, treatment with
1,25-D3 and CYP24A1siRNA synergistically enhanced the mRNA
expression of this epithelial related gene (Fig. 5C). By contrast, the expression of
N-cadherin and Vimentin significantly decreased following treatment
with 1,25-D3 or CYP24A1, with the greatest decrease in the group
that underwent treatment with 1,25-D3 and CYP24A1.
 | Figure 5.(A and B) Western blot analysis
measuring E-cadherin, N-cadherin and vimentin expression. β-actin
was used as sample loading control. (C) The expression of
E-cadherin, N-cadherin and vimentin mRNA. *P<0.05, **P<0.01
and ***P<0.001 vs. control; #P<0.05 and
##P<0.01 vs. VD3 group. Control, untreated cells;
VD3, cells treated with 1,25-D3 for 4 h; siscr+VD3, cells
transfected with scramble control siRNA and treated with 1,25-D3;
siCYP+VD3, cells transfected with CYP24A1 siRNA and treated with
1,25-D3; siscr, cells transfected with scramble control siRNA;
siCYP, cells transfected with CYP24A1siRNA; siRNA, small
interfering RNA; CYP24D1, 25-hydroxyvitamin-D3-24-hydroxylase;
1,25-D3, 1,25-dihydroxyvitamin D. |
The effect of CYP24A1 knockdown and
1,25-D3 treatment on the expression of MMPs
MMPs serve a critical role in the migration and
metastasis of tumor cells, which is essential for inducing
degradation of the ECM (44). The
expression of MMP-2 mRNA and protein was significantly decreased in
the siCYP+VD3 group compared with VD3 and control groups (Fig. 6). By contrast, the expression of
MMP-9 mRNA and protein was unaffected in all treatment groups;
there was a slight decrease in MMP-9 expression in the siCYP+VD3
group, although this was not significant. The mRNA and protein
expression of TIMP1, which inhibits the expression of MMPs
(45), was increased following
treatment with 1,25-D3 or CYP24A1siRNA. This increase was
significantly greater in the siCYP+VD3 group compared with the VD3
group.
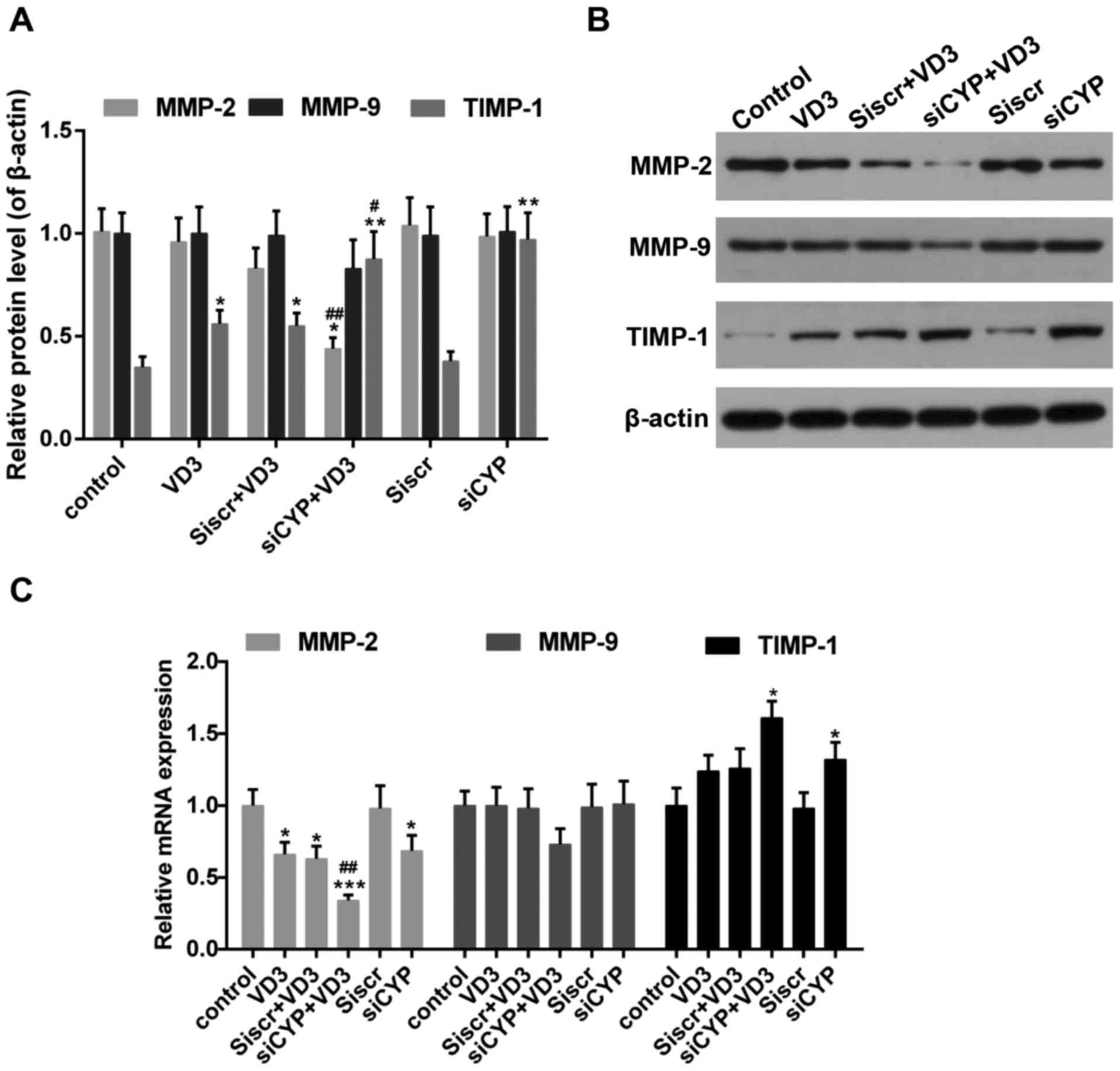 | Figure 6.(A and B) Western blot analysis
measuring MMP-2, MMP-9 and TIMP1 expression. β-actin was used as
sample loading control. (C) The expression of MMP-2, MMP-9 and
TIMP1 mRNA. *P<0.05, **P<0.01 and ***P<0.001 vs. control;
#P<0.05 and ##P<0.01 vs. VD3 group.
Control, untreated cells; VD3, cells treated with 10 nM 1,25-D3 for
4 h; siscr+VD3, cells transfected with scramble control siRNA and
treated with 1,25-D3; siCYP+VD3, cells transfected with CYP24A1
siRNA and treated with 1,25-D3; siscr, cells transfected with
scramble control siRNA; siCYP, cells transfected with CYP24A1siRNA;
siRNA, small interfering RNA; CYP24D1,
25-hydroxyvitamin-D3-24-hydroxylase; 1,25-D3, 1,25-dihydroxyvitamin
D; MMP, matrix metalloproteinase; TIMP, metalloproteinase
inhibitor. |
The effect of CYP24A1 knockdown and
1,25-D3 treatment on the activity of AKT and β-catenin
AKT activation and β-catenin inhibition are
correlated with the pathogenesis of thyroid cancer (31,46). As
presented in Fig. 7A and B, the
phosphorylation of AKT was significantly decreased in cells
following treatment with 1,25-D3 or CYP24A1siRNA. Furthermore, the
expression of p-AKT was significantly decreased in the siCYP+VD3
group compared with the VD3 group. β-catenin activity was also
significantly decreased in the siCYP+VD3 groups compared with the
control and VD3 groups.
 | Figure 7.(A and B) Western blot analysis of
p-Akt and β-catenin expression. β-actin was used as sample loading
control. **P<0.01 and ***P<0.001 vs. control;
##P<0.01 vs. VD3 group. Control, untreated cells;
VD3, cells treated with 10 nM 1,25-D3 for 4 h; siscr+VD3, cells
transfected with scramble control siRNA and treated with 1,25-D3;
siCYP+VD3, cells transfected with CYP24A1 siRNA and treated with
1,25-D3; siscr, cells transfected with scramble control siRNA;
siCYP, cells transfected with CYP24A1 siRNA; siRNA, small
interfering RNA; CYP24D1, 25-hydroxyvitamin-D3-24-hydroxylase;
1,25-D3, 1,25-dihydroxyvitamin D; p-phosphorylated; Akt, protein
kinase B. |
Discussion
To determine the influence of the enzyme CYP24A1 on
the anti-tumor effect of vitamin D3, its expression was decreased
using specific siRNA. The proliferation of thyroid cancer cells was
assessed using a CCK-8 assay and it was demonstrated that thyroid
cell proliferation was significantly inhibited following treatment
with 1,25-D3 or CYP24A1 knockdown. Furthermore, CYP24A1 knockdown
significantly enhanced the anti-proliferative effects of 1,25-D3 72
h after treatment, compared with cells that were treated with
1,25-D3 alone. It has been reported that the susceptibility of
cancer cells to vitamin D3 gradually decreases during tumor
progression (15); nevertheless, its
mechanisms of action remain unclear. In the present study, the
effect of 1,25-D3 on the expression of CYP24A1 and VDR, which are
important factors that affect the anti-tumor effect of vitamin D3,
was assessed. It was demonstrated that 1,25-D3 treatment increased
CYP24A1 expression but had no influence on VDR expression. The
elevated expression of CYP24A1 following 1,25-D3 treatment was in
line with a previous study (47).
This may be a self-defense mechanism of cancer cells and may partly
explain why the anti-tumor effect of 1,25-D3 is weakened following
treatment.
Tumor metastasis is usually accompanied by tumor
cell invasion and migration to other tissues, and the initiation of
the EMT, which may lead to the loss of the epithelial phenotype and
gain of fibroblast-like mesenchymal morphology (48). The results of a Transwell assay
assessing cell migration indicated that 1,25-D3 treatment and
CYP24A1 knockdown significantly inhibited the migration of thyroid
cancer cells. Cell migration was significantly decreased in the
group that underwent CYP24A1 knockdown and treatment with 1,25-D3
compared with the groups that underwent 1,25-D3 treatment
alone.
In addition, the results of a scratch wound assay
indicated that the migratory ability of thyroid cancer cells was
decreased following treatment with 1,25-D3, as well as following
CYP24A1 knockdown. Likewise, CYP24A1 knockdown enhanced the
inhibitory effect of 1,25-D3 on cell migration. The decrease in the
expression of the epithelial-related protein E-cadherin and the
increase in the expression of the mesenchymal-related proteins
N-cadherin and vimentin were reversed by 1,25-D3 treatment and also
by CYP24A1 knockdown. This effect was more pronounced in the group
that underwent 1,25-D3 treatment and CYP24A1 knockdown compared
with the group that underwent 1,25-D3 treatment alone, suggesting
that a decrease in CYP24A1 expression inhibits the EMT.
MMPs are key enzymes that affect degradation of the
ECM, an important step in the progression of the EMT (49). The results demonstrated that the
expression of MMP-2 expression decreased and TIMP1 expression
increased in the three treatment groups (VD3, siCYP and siCYP+VD3);
MMP-9 expression was similar among all groups. Taken together,
these results suggest that CYP24A1 knockdown facilitates the
anti-tumor effect of 1,25-D3 by inhibiting the migration of thyroid
cancer cells and the EMT.
A deep understanding of the underlying mechanisms is
required to facilitate the development of precise targets during
the intervention and treatment of thyroid cancer. Previous studies
have identified the role of Akt and β-catenin in multiple
biological events (31,46,50). In
the present study, it was noted that the treatment with 1,25-D3
suppressed Akt and β-catenin activity and that this effect was most
prominent when 1,25-D3 treatment was initiated following CYP24A1
knockdown. These results are in line with those of a previous
study, which also claimed that the deactivation of β-catenin could
induce the expression of E-cadherin (51). However, in the current study, the
association between β-catenin and EMT markers remained obscure;
further studies are required to validate this. Furthermore, it has
been reported that activation of the phosphoinositide 3-kinase/Akt
pathway can phosphorylate β-catenin at a specific site, leading to
its accumulation and stabilization in the nucleus (52). However, it remains unclear whether
Akt affects the activity of β-catenin. It has been reported that
β-catenin may be at the convergence of multiple signaling pathways
in thyroid cancer (31). Therefore,
a signaling pathway that was not assessed in the current study may
participate in the regulation of thyroid cancer. It is important to
undertake in-depth studies to determine the definite mechanisms of
action of 1,25-D3 and CYP24A1. If the synergetic effect of 1,25-D3
and CYP24A1 is confirmed in vivo, it would be a big step
forward in developing a novel therapeutic strategy to treat thyroid
cancer.
In conclusion, the results of the present study
demonstrated that the downregulation of CYP24A1 facilitated the
anti-tumor effect of vitamin D3. This anti-tumor effect primarily
occurred via the suppression of tumor cell proliferation and
migration. Furthermore, the expression of the EMT-related genes
E-cadherin, N-cadherin and Vimentin, and MMP-2 and TIMP1 were
regulated following treatment with 1,25-D3 and CYRP24A1 knockdown.
This effect may be induced via the deactivation of Akt and
β-catenin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NH performed the experiments and data analysis and
contributed significantly to the preparation of the manuscript. HZ
contributed to the experimental design.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973-2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherma SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dobrinja C: Papillary thyroid cancer
gender disparity. 2014.
|
|
4
|
Kapiteijn E, Schneider TC, Morreau H,
Gelderblom H, Nortier JW and Smit JW: New treatment modalities in
advanced thyroid cancer. Ann Oncol. 23:10–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boot AC: Vitamin-D deficiency. Ned
Tijdschr Geneeskd. 150:1315–1316. 2006.(In Dutch). PubMed/NCBI
|
|
6
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: Potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clinckspoor I, Verlinden L, Mathieu C,
Bouillon R, Verstuyf A and Decallonne B: Vitamin D in thyroid
tumorigenesis and development. Prog Histochem Cytochem. 48:65–98.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balla B, Tobias B, Kósa JP, Podani J,
Horváth P, Nagy Z, Horanyi J, Jaray B, Szekely E, Krenács L, et al:
Vitamin D-neutralizing CYP24A1 expression, oncogenic mutation
states and histological findings of human papillary thyroid cancer.
J Endocrinol Invest. 38:313–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hershberger PA, Modzelewski RA, Shurin ZR,
Rueger RM, Trump DL and Johnson CS: 1,25-Dihydroxycholecalciferol
(1,25-D3) inhibits the growth of squamous cell carcinoma and
down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res.
59:2644–2649. 1999.PubMed/NCBI
|
|
10
|
Bises G, Kállay E, Weiland T, Wrba F,
Wenzl E, Bonner E, Kriwanek S, Obrist P and Cross HS:
25-hydroxyvitamin D3-1alpha-hydroxylase expression in normal and
malignant human colon. J Histochem Cytochem. 52:985–989. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trump DL, Potter DM, Muindi J, Brufsky A
and Johnson CS: Phase II trial of high-dose, intermittent
calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in
androgen-independent prostate cancer. Cancer. 106:2136–2142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett RG, Wakeley SE, Hamel FG, High RR,
Korch C and Goldner WS: Gene expression of vitamin D metabolic
enzymes at baseline and in response to vitamin D treatment in
thyroid cancer cell lines. Oncology. 83:264–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stepien T, Krupinski R, Sopinski J, Kuzdak
K, Komorowski J, Lawnicka H and Stepien H: Decreased 1–25
dihydroxyvitamin D3 concentration in peripheral blood serum of
patients with thyroid cancer. Arch Med Res. 41:190–194. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peehl DM and Feldman D: Interaction of
nuclear receptor ligands with the Vitamin D signaling pathway in
prostate cancer. J Steroid Biochem Mol Biol. 92:307–315. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Trump DL and Johnson CS: Vitamin D
in combination cancer treatment. J Cancer. 1:101–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans TR, Colston KW, Lofts FJ, Cunningham
D, Anthoney DA, Gogas H, de Bono JS, Hamberg KJ, Skov T and Mansi
JL: A phase II trial of the vitamin D analogue Seocalcitol (EB1089)
in patients with inoperable pancreatic cancer. Br J Cancer.
86:680–685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalhoff K, Dancey J, Astrup L, Skovsgaard
T, Hamberg KJ, Lofts FJ, Rosmorduc O, Erlinger S, Hansen JB,
Steward WP, et al: A phase II study of the vitamin D analogue
Seocalcitol in patients with inoperable hepatocellular carcinoma.
Br J Cancer. 89:252–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikhail N: Clinical significance of
vitamin D deficiency in primary hyperparathyroidism, and safety of
vitamin D therapy. South Med J. 104:29–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho YL, Christensen C, Saunders DE,
Lawrence WD, Deppe G, Malviya VK and Malone JM: Combined effects of
1,25-dihydroxyvitamin D3 and platinum drugs on the growth of MCF-7
cells. Cancer Res. 51:2848–2853. 1991.PubMed/NCBI
|
|
20
|
Liu G, Hu X and Chakrabarty S: Vitamin D
mediates its action in human colon carcinoma cells in a
calcium-sensing receptor-dependent manner: Downregulates malignant
cell behavior and the expression of thymidylate synthase and
survivin and promotes cellular sensitivity to 5-FU. Int J Cancer.
126:631–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka H, Abe E, Miyaura C, Kuribayashi T,
Konno K, Nishii Y and Suda T: 1 alpha,25-Dihydroxycholecalciferol
and a human myeloid leukaemia cell line (HL-60). Biochem J.
204:713–719. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lechner D, Kállay E and Cross HS:
1alpha,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces
CYP24A1 in colon cells. Mol Cell Endocrinol. 263:55–64. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones G, Prosser DE and Kaufmann M:
Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res.
55:13–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barry EL, Rees JR, Peacock JL, Mott LA,
Amos CI, Bostick RM, Figueiredo JC, Ahnen DJ, Bresalier RS, Burke
CA and Baron JA: Genetic variants in CYP2R1, CYP24A1, and VDR
modify the efficacy of vitamin D3 supplementation for increasing
serum 25-hydroxyvitamin D levels in a randomized controlled trial.
J Clin Endocrinol Metab. 99:E2133–E2137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bareis P, Kállay E, Bischof MG, Bises G,
Hofer H, Pötzi C, Manhardt T, Bland R and Cross HS: Clonal
differences in expression of 25-hydroxyvitamin
D(3)-1alpha-hydroxylase, of 25-hydroxyvitamin D(3)-24-hydroxylase,
and of the vitamin D receptor in human colon carcinoma cells:
Effects of epidermal growth factor and 1alpha,25-dihydroxyvitamin
D(3). Exp Cell Res. 276:320–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leevers SJ, Vanhaesebroeck B and
Waterfield MD: Signalling through phosphoinositide 3-kinases: The
lipids take centre stage. Curr Opin Cell Biol. 11:219–225. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandal M, Kim S, Younes MN, Jasser SA,
El-Naggar AK, Mills GB and Myers JN: The Akt inhibitor KP372-1
suppresses Akt activity and cell proliferation and induces
apoptosis in thyroid cancer cells. Br J Cancer. 92:1899–1905. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou P, Liu D, Shan Y, Hu S, Studeman K,
Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, et al:
Genetic alterations and their relationship in the
phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin
Cancer Res. 13:1161–1170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abbosh PH and Nephew KP: Multiple
signaling pathways converge on beta-catenin in thyroid cancer.
Thyroid. 15:551–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rubinfeld B, Albert I, Porfiri E,
Munemitsu S and Polakis P: Loss of beta-catenin regulation by the
APC tumor suppressor protein correlates with loss of structure due
to common somatic mutations of the gene. Cancer Res. 57:4624–4630.
1997.PubMed/NCBI
|
|
33
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a beta-catenin-Tcf complex in APC-/-
colon carcinoma. Science. 275:1784–1787. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia-Rostan G, Camp RL, Herrero A,
Carcangiu ML, Rimm DL and Tallini G: Beta-catenin dysregulation in
thyroid neoplasms: Down-regulation, aberrant nuclear expression,
and CTNNB1 exon 3 mutations are markers for aggressive tumor
phenotypes and poor prognosis. Am J Pathol. 158:987–996. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kramer C, Seltmann H, Seifert M, Tilgen W,
Zouboulis CC and Reichrath J: Characterization of the vitamin D
endocrine system in human sebocytes in vitro. J Steroid Biochem Mol
Biol. 113:9–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokar EJ and Webber MM: Cholecalciferol
(vitamin D3) inhibits growth and invasion by up-regulating nuclear
receptors and 25-hydroxylase (CYP27A1) in human prostate cancer
cells. Clin Exp Metastasis. 22:275–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ordoñez-Morán P and Muñoz A: Nuclear
receptors: Genomic and non-genomic effects converge. Cell Cycle.
8:1675–1680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kósa JP, Horváth P, Wölfling J, Kovács D,
Balla B, Mátyus P, Horváth E, Speer G, Takács I, Nagy Z, et al:
CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3
on colorectal cancer cells. World J Gastroenterol. 19:2621–2628.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu F, Gu LN, Shan BE, Geng CZ and Sang
MX: Biomarkers for EMT and MET in breast cancer: An update. Oncol
Lett. 12:4869–4876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawamata H, Kawai K, Kameyama S, Johnson
MD, Stetler-Stevenson WG and Oyasu R: Over-expression of tissue
inhibitor of matrix metalloproteinases (TIMP1 and TIMP2) suppresses
extravasation of pulmonary metastasis of a rat bladder carcinoma.
Int J Cancer. 63:680–687. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baryawno N, Sveinbjörnsson B, Eksborg S,
Chen CS, Kogner P and Johnsen JI: Small-molecule inhibitors of
phosphatidylinositol 3-kinase/Akt signaling inhibit
Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma
growth. Cancer Res. 70:266–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tashiro K, Abe T, Oue N, Yasui W and Ryoji
M: Characterization of vitamin D-mediated induction of the CYP 24
transcription. Mol Cell Endocrinol. 226:27–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu Y, Sun X, Feng J, Deng LL, Liu Y, Li
B, Zhu M, Lu C and Zhou L: MT2-MMP induces proteolysis and leads to
EMT in carcinomas. Oncotarget. 7:48193–48205. 2016.PubMed/NCBI
|
|
50
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sastre-Perona A, Riesco-Eizaguirre G,
Zaballos MA and Santisteban P: β-catenin signaling is required for
RAS-driven thyroid cancer through PI3K activation. Oncotarget.
7:49435–49449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee G, Goretsky T, Managlia E, Dirisina R,
Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, et al:
Phosphoinositide 3-kinase signaling mediates beta-catenin
activation in intestinal epithelial stem and progenitor cells in
colitis. Gastroenterology. 139:869–881.e1-e9. 2010. View Article : Google Scholar : PubMed/NCBI
|





















