Introduction
Liver cirrhosis is a common chronic liver disease in
China, which is characterized by diffuse fibrosis as well as
pseudo-lobular and nodular formation. According to statistics,
patients with liver cirrhosis account for ~10% of the total number
of patients in China (1). A study
has revealed that the pathogenic factors of liver cirrhosis include
hepatitis viruses, drugs and poisons, cholestasis, metabolism and
inheritance, schistosomiasis and alcohol. These pathogenic factors
stimulate liver cells for a long time, induce the activation of
hepatic stellate cells, and make the generated extracellular
matrices more than degraded ones, thus resulting in the
extracellular matrix deposition and liver tissue remodeling
(2).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt)/mechanistic target of rapamycin (mTOR) signal transduction
pathway can promote cell growth, proliferation, differentiation and
invasion, and it also plays key roles in anti-apoptosis, immune
regulation and pro-angiogenesis. In addition, a study has evidenced
that this pathway exerts crucial effects on the occurrence and
development of liver fibrosis (3).
Inflammation is a key factor triggering liver fibrosis and
simulating the progression of liver cirrhosis (4). PI3K and mTOR can upregulate
anti-inflammatory cytokines, inhibit the expression of
pro-inflammatory cytokines, the inflammatory response of liver
cells, and the proliferation and invasion of hepatic stellate
cells, and promote the degradation of the liver extracellular
matrix (5).
A study has demonstrated that astragaloside IV
(AS-IV), one of the active components of Astragalus saponins
in Astragalus membranaceus, has various pharmacological
effects such as antioxidation, anti-inflammation and anti-infection
(6). AS-IV can alleviate the
inflammatory damage induced by orthotopic liver transplantation in
rats through inhibiting the transcriptional activity of nuclear
factor κ-light-chain-enhancer of activated B cells (NF-κB)
(7). According to another study,
AS-IV pretreatment can reduce cerebral ischemia-reperfusion injury
in rats, which is mainly due to its ability to inhibit neutrophil
adhesion-associated molecules, thereby inhibiting inflammation
(8). Recent studies have manifested
that AS-IV pretreatment can protect kidney injury, and its
mechanisms include reducing inflammation and inhibiting apoptosis
(9). In the present study, a rat
model of liver cirrhosis was established to investigate whether
AS-IV pretreatment can protect rats from liver cirrhosis and the
effect of AS-IV on the PI3K/Akt/mTOR signaling pathway, providing
basic research on the prevention and treatment of liver cirrhosis
using AS-IV.
Materials and methods
Materials and reagents
AS-IV (Aladdin Reagent Co., Ltd., Shanghai, China),
alanine transaminase (ALT) and aspartate transaminase (AST)
detection kits, as well as interleukin (IL)-6, IL-1β and tumor
necrosis factor-α (TNF-α) (cat. nos. H052, H007, H002; all from
Nanjing Jiancheng Bioengineering Institute, Nanjing, China), rabbit
anti-rat phosphorylated (p)-PI3K, PI3K, p-Akt, Akt, p-mTOR, mTOR
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) polyclonal
antibodies, secondary goat anti-rabbit polyclonal antibody (cat.
nos. bs-6417R, bs-10657R, bs-3043R, bs-0115R, bs-5329R, bs-1992R,
bs-0755R, bs-0295G; all from BIOSS, Beijing, China) and Masson's
staining solution (Wuhan Sanying Biotechnology, Wuhan, China), and
hematoxylin and eosin (H&E) staining kit, bicinchoninic acid
(BCA) protein quantitation kit and tissue lysate (all from Beyotime
Institute of Biotechnology, Nantong, China).
Experimental animal grouping and
treatment
A Sprague-Dawley (SD) male rat model (4 months of
age, 210–240 g; Beijing Vital River Laboratory Animal Technology
Co., Ltd., Beijing, China) of liver cirrhosis was induced by the
intraperitoneal injection of 50% carbon tetrachloride
(CCl4) (the volume ratio of CCl4 to olive oil
was 1:1) twice a week for 8 weeks. A total of 36 SD rats were
randomly divided into three groups: the normal control group
(n=10), the model control group (n=13), and the AS-IV group (n=13).
The rats were kept in a cage with controlled temperature and light
conditions (24°C and 12/12 light cycles) and had free access to
water and food. The humidity was 60±10%. The normal control group
was intraperitoneally injected with olive oil twice a week, and
given intragastric administration of 0.5% carboxymethyl cellulose
(CMC)-Na (10 ml/kg/day); the model control group was given
intragastric administration of 0.5% CMC-Na (10 ml/kg/day) during
modeling; and the AS-IV group underwent the intragastric
administration of AS-IV (20 ml/kg/day) during modeling. The rats
were sacrificed at the end of the 8th week, and underwent solid
instead of liquid fasting overnight on the day before the end of
the experiment. The next day, the rats were anesthetized, and their
abdominal venous blood and liver were taken. After the blood was
placed at room temperature and centrifuged at 2,600 × g for 10 min,
the supernatant serum was carefully drawn and stored at −80°C for
standby application. After the liver was weighed, the left lobe
liver tissues were fixed in formalin for the histopathological
study, and the remaining liver tissues were stored at −80°C for
western blotting. The study was approved by the Ethics Committee of
the Sixth People's Hospital of Qingdao (Qingdao, China)
Determination of biochemical indexes
in serum of rats
The activities of AST and ALT in serum of rats in
each group were detected via AST and ALT kits, and the content of
TNF-α, IL-6 and IL-1β in serum of rats in each group was detected
using enzyme-linked immunosorbent assay (ELISA). All the operations
were carried out according to the manufacturer's instructions.
Detection of the liver histopathology
of rats
The fixed liver tissues in each group were selected.
These tissues underwent dehydration and transparency at first,
immersed in wax and embedded into wax blocks next, and cut into
blank slices finally. After that, H&E staining and Masson's
trichrome staining were carried out based on the histopathological
conventional methods. After the stained slices were dehydrated with
gradient alcohol and made transparent via xylene, they were sealed
with gum. Then the histopathological changes in the liver of rats
in each group were observed using a microscope (Olympus Corp.,
Tokyo, Japan).
Detection of the expression of
proteins relevant to the PI3K/Akt/mTOR signaling pathway via
western blotting
About 50 mg of rat liver tissues frozen at −80°C
were taken from each group. After the total protein was extracted
with the tissue lysate, the protein concentration was determined
via BCA assay, and then the proteins were mixed with the loading
buffer and underwent thermal denaturation. A total of 40 µg
proteins were taken from each group for separation by 15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then
the membrane was transferred through wet processes, and the
separated proteins were electrotransferred onto a polyvinylidene
difluoride (PVDF) membrane. The blocking solution bovine serum
albumin (BSA) was adopted for blocking for 2 h at room temperature,
and primary antibodies including p-PI3K, PI3K, p-Akt, Akt, p-mTOR,
mTOR and GAPDH (diluted at 1:1,000) were added for incubation in a
refrigerator at 4°C overnight, followed by membrane washing with
Tris-buffered saline with Tween-20 (TBST) for 3 times. Afterwards,
secondary antibody (diluted at 1:2,000) were added for incubation
at room temperature for 2 h. After the membrane washing with TBST
for 3 times, color development was conducted using the enhanced
chemiluminescent (ECL) developing solution (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and records were scanned via a
gel imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
densitometry of the blots was performed using ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
17.0 software (IBM Corp., Armonk, NY, USA) was applied for data
processing, and the data were expressed as mean ± standard
deviation. The t-test was implemented for the intergroup
comparison. ANOVA was used for comparison between multiple groups
and LSD test was the post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
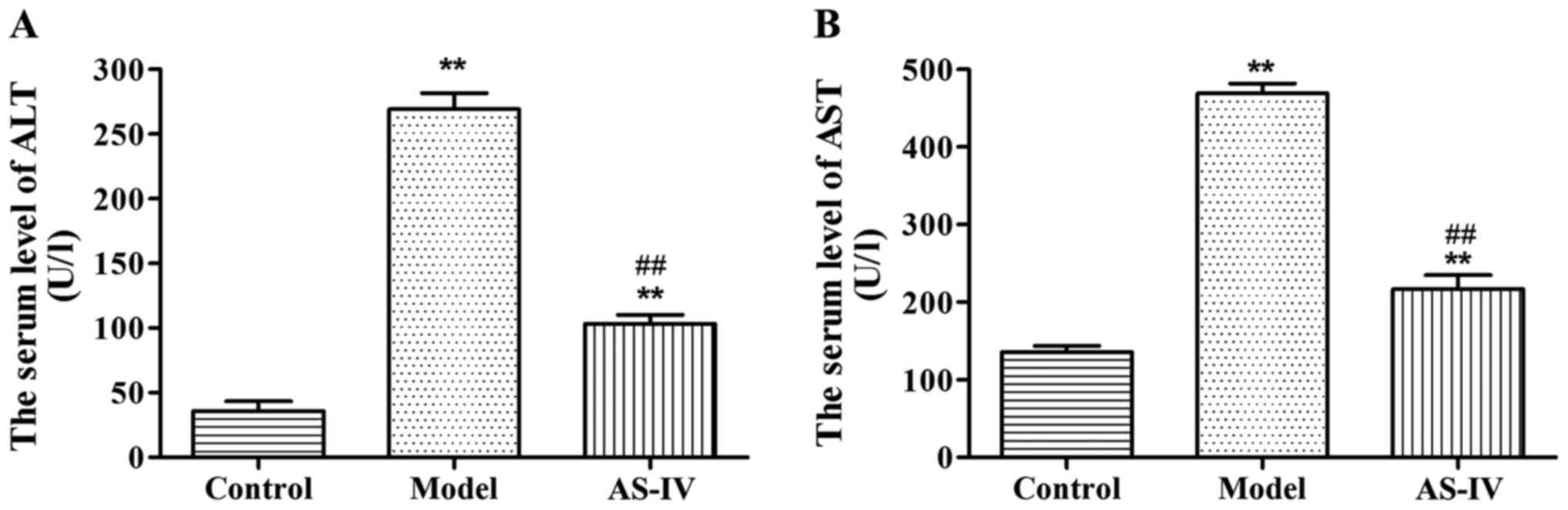
Effects of AS-IV on AST and ALT in
serum of rats
Compared with those in the normal control group, the
levels of AST and ALT in serum of rats in the model group were
significantly increased (p<0.01). Compared with those in the
model group, the levels of AST and ALT in serum of rats in the
AS-IV administration group were remarkably decreased (p<0.01),
indicating that the degree of rat liver injury in the AS-IV
administration group was lowered than that in the model group
(Fig. 1).
Effects of AS-IV on inflammatory
cytokines in serum of rats
Compared with those in the normal control group, the
content of TNF-α, IL-6 and IL-1β in serum of rats in the model
group was obviously increased (p<0.01). Compared with those in
the model group, the expression levels of TNF-α, IL-6 and IL-1β
significantly declined in serum of rats in the AS-IV group
(p<0.01), indicating that the inflammatory response of rats in
the AS-IV group was reduced compared with that of rats in the model
group (Fig. 2).
Effect of AS-IV on the liver
histopathology of rats
There were intact liver lobule structures and
central veins in the liver of rats in the normal control group.
Destructed liver structures and inflammatory cell infiltration
appeared in the liver of rats in the model group, while the above
symptoms of rat liver tissues in the AS-IV treatment group were
remarkably improved (Fig. 3).
Effect of AS-IV on the expression of
rat collagens
Compared with the blank control group, obvious
collagen hyperplasia was observed in liver tissues of the model
group, and the fiber rope formed was relatively thick; whereas
collagen deposition in liver tissues in the AS-IV treatment group
was significantly reduced compared with that of the model group,
and the fiber rope formed was relatively thin (Fig. 4).
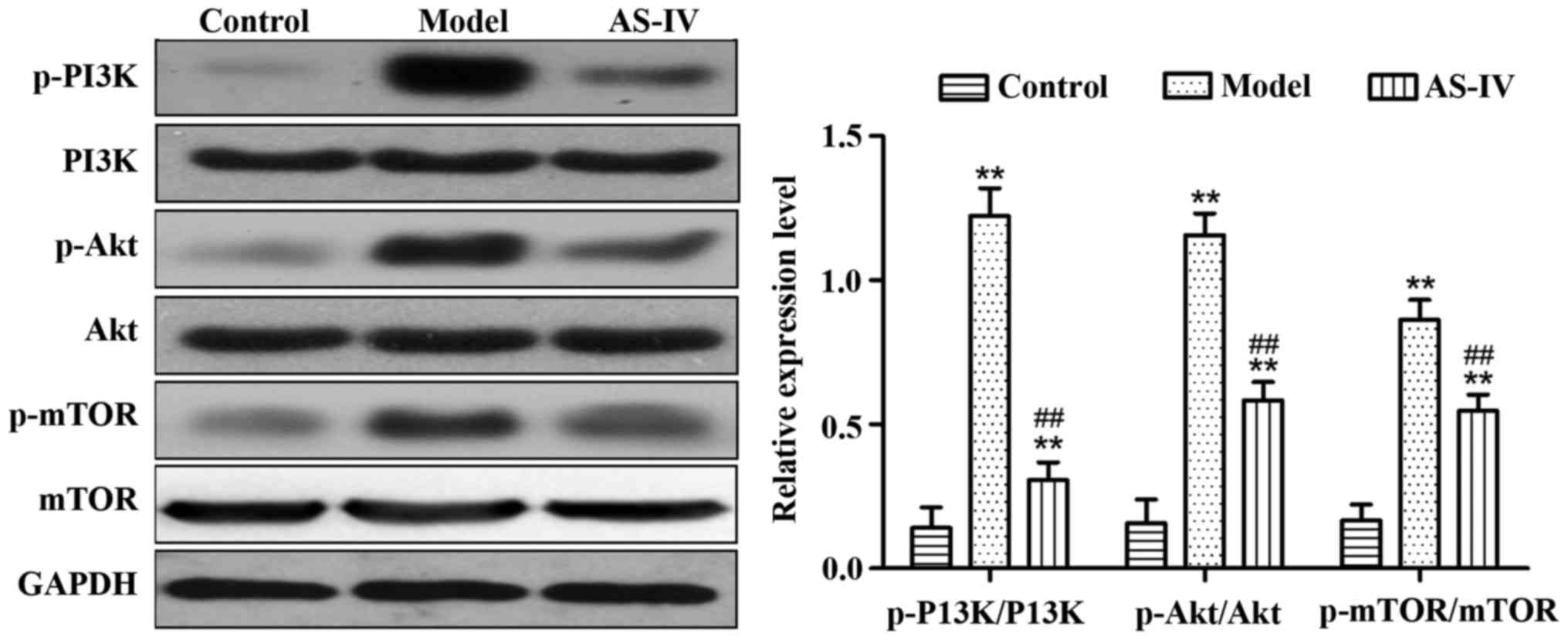
Effect of AS-IV on the expression of
proteins relevant to the PI3K/Akt/mTOR signaling pathway in liver
tissues of rats
Compared with those in the control group, the
expression levels of p-PI3K/PI3K, p-Akt/Akt, and p-mTOR/mTOR
proteins in liver tissues of the model group significantly
increased (p<0.01). Compared with the model group, AS-IV
obviously reduced the expression of p-PI3K/PI3K, p-Akt/Akt, and
p-mTOR/mTOR proteins in rat liver tissues (p<0.01) (Fig. 5).
Discussion
Liver cirrhosis results from many chronic liver
diseases developing to the late stage. The long-term stimulation of
damage factors leads to diffuse necrosis of liver cells, and
promotes the proliferation of fibrous tissues and the regeneration
of nodular hepatocytes, thus ultimately contributing to the
remodeling of liver lobule structures and blood vessels (10). A large number of basic and clinical
studies have manifested that treatments can reverse the progression
of liver fibrosis (11,12). A great controversy currently exists
in the treatment of liver cirrhosis, and some studies have revealed
that early treatment can reverse liver cirrhosis (13–16).
PI3K phosphorylates phosphoinositides via the
phosphorylation of serine 308 and threonine 473 by
phosphoinositide-dependent kinase-1 (17). PI3 on the inositol ring generates
phosphatidylinositol 4,5-bisphosphate (PIP2) and
phosphatidylinositol (3,4,5)-trisphosphate (PIP3), and then
acts as the second messenger in cells. Akt acts as a
serine/threonine protein kinase, and can bind to PIP2
and PIP3 under the action of the
phosphatidylinositol-dependent protein kinase, which promotes the
transfer of Akt from the cytoplasm to the cell membrane (18). According to a study, platelet-derived
growth factors can induce the phosphorylation of PI3K and Akt.
Activated Akt can promote the phosphorylation of downstream
substrates such as mTOR, Bad and the caspase family, having a
variety of biological effects (19).
Inhibiting the PI3K/Akt signaling pathway can obviously reduce the
proliferation of hepatic stellate cells and the expression levels
of type I collagen messenger ribonucleic acid (mRNA) and protein
(20).
mTOR/p70S6K protein plays a crucial role in the
activation process of hepatic stellate cells (21). Inhibiting the activation of
mTOR/p70S6K protein, a downstream target of the PI3K/Akt signaling
pathway, can inhibit the proliferation and activation of hepatic
stellate cells as well as the synthesis and secretion of collagens
(22). This study showed that
low-dose rapamycin can suppress the progression of liver fibrosis,
whose mechanism is to inhibit the activation of mTOR/p70S6 so as to
suppress the activation of hepatic fibroblasts (17). Another study has revealed that the
mTOR inhibitors, sirolimus and everolimus, can significantly slow
the development of liver fibrosis, and inhibit the migration of
hepatic stellate cells and the production of collagens (23).
In the present study, the rat model of liver
cirrhosis was established through the intraperitoneal injection of
CCl4, and then the protective role of AS-IV intervention
in the liver of rats and its effect on the PI3K/Ak/mTOR signaling
pathway were investigated. Compared with those in the normal
control group, the levels of AST, ALT, TNF-α, IL-6 and IL-1β in
serum of rats in the model group were obviously increased. Compared
with those in the model group, the levels of TNF-α, IL-6 and IL-1β
significantly declined in serum of rats in the AS-IV group,
indicating that the liver injury degree and inflammatory response
of rats in the AS-IV group were lower than those in the model
group. The histopathological detection revealed that there were
intact liver lobule structures and central veins in the liver of
rats in the normal control group. Destructed liver structures,
inflammatory cell infiltration, and the significant increase in
collagen expression appeared in the liver of rats in the model
group, while the above symptoms of rat liver tissues in the AS-IV
treatment group were remarkably improved, and collagen deposition
in liver tissues in the AS-IV treatment group was significantly
reduced compared with that in the model group. Based on western
blotting results, compared with those in the control group, the
expression levels of p-PI3K/PI3K, p-Akt/Akt, and p-mTOR/mTOR
proteins in rat liver tissues in the model group increased
significantly. AS-IV obviously reduced the expression ratios of
p-PI3K/PI3K, p-Akt/Akt, and p-mTOR/mTOR proteins in rat liver
tissues, thus inhibiting the PI3K/Akt/mTOR signaling pathway.
In summary, AS-IV protects CCl4-induced
liver cirrhosis, and its mechanism may play a role in inhibiting
the PI3K/Akt/mTOR signaling pathway. This study provides a clear
research basis for the treatment of liver cirrhosis with AS-IV in
clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RW and HL wrote the manuscript and conducted the
experimental animal grouping and treatment. RC analyzed the
biochemical indexes. YS and TL contributed to western blotting. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Sixth People's Hospital of Qingdao (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liaw YF, Lin DY, Chen TJ and Chu CM:
Natural course after the development of cirrhosis in patients with
chronic type B hepatitis: A prospective study. Liver. 9:235–241.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou
HY, Chu CM and Liaw YF: Long-term outcome after spontaneous HBeAg
seroconversion in patients with chronic hepatitis B. Hepatology.
35:1522–1527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y and Liu YP: Research advances in
PI3K/Akt signaling pathway and tumor multidrug resistance. Zhonghua
Linchuang Yishi Zazhi. 5:446–449. 2011.
|
|
4
|
Pettinelli P, Obregón AM and Videla LA:
Molecular mechanisms of steatosis in nonalcoholic fatty liver
disease. Nutr Hosp. 26:441–450. 2011.PubMed/NCBI
|
|
5
|
Weichhart T and Säemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67 Suppl 3:iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SG, Xu Y, Chen JD, Yang CH and Chen
XH: Astragaloside IV stimulates angiogenesis and increases nitric
oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules.
18:12809–12819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng MX, Chen ZZ, Cai YL, Liu CA and Tu
B: Astragaloside IV protects against ischemia reperfusion in a
murine model of orthotopic liver transplantation. Transplant Proc.
43:1456–1461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei
XY, Gao L and Gao GD: Astragaloside IV protects against focal
cerebral ischemia/reperfusion injury correlating to suppression of
neutrophils adhesion-related molecules. Neurochem Int. 60:458–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin Y, Li G, Liu H and Ai D: AS-IV
protects against kidney IRI through inhibition of NF-κB activity
and PUMA upregulation. Int J Clin Exp Med. 8:18293–18301.
2015.PubMed/NCBI
|
|
10
|
Pinzani M, Rosselli M and Zuckermann M:
Liver cirrhosis. Best Pract Res Clin Gastroenterol. 25:281–290.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang TT, Liaw YF, Wu SS, Schiff E, Han
KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al: Long-term
entecavir therapy results in the reversal of fibrosis/cirrhosis and
continued histological improvement in patients with chronic
hepatitis B. Hepatology. 52:886–893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serpaggi J, Carnot F, Nalpas B, Canioni D,
Guéchot J, Lebray P, Vallet-Pichard A, Fontaine H, Bedossa P and
Pol S: Direct and indirect evidence for the reversibility of
cirrhosis. Hum Pathol. 37:1519–1526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bortolotti F and Guido M: Reversal of
liver cirrhosis: A desirable clinical outcome and its pathogenic
background. J Pediatr Gastroenterol Nutr. 44:401–406. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Desmet VJ: Comments on cirrhosis reversal.
Dig Liver Dis. 37:909–916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reif S, Lang A, Lindquist JN, Yata Y,
Gabele E, Scanga A, Brenner DA and Rippe RA: The role of focal
adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in
hepatic stellate cell proliferation and type I collagen expression.
J Biol Chem. 278:8083–8090. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Chu ES, Chen HY, Man K, Go MY,
Huang XR, Lan HY, Sung JJ and Yu J: microRNA-29b prevents liver
fibrosis by attenuating hepatic stellate cell activation and
inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget.
6:7325–7338. 2015.PubMed/NCBI
|
|
19
|
Hua H, Zhu Y and Song YH: Ruscogenin
suppressed the hepatocellular carcinoma metastasis via
PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 101:115–122.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gäbele E, Reif S, Tsukada S, Bataller R,
Yata Y, Morris T, Schrum LW, Brenner DA and Rippe RA: The role of
p70S6K in hepatic stellate cell collagen gene expression and cell
proliferation. J Biol Chem. 280:13374–13382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coffey JC, Wang JH, Smith MJ, Laing A,
Bouchier-Hayes D, Cotter TG and Redmond HP: Phosphoinositide
3-kinase accelerates postoperative tumor growth by inhibiting
apoptosis and enhancing resistance to chemotherapy-induced
apoptosis. Novel role for an old enemy. J Biol Chem.
280:20968–20977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai CX, Buddha H, Castelino-Prabhu S,
Zhang Z, Britton RS, Bacon BR and Neuschwander-Tetri BA: Activation
of insulin- PI3K/Akt-p70S6K pathway in hepatic stellate cells
contributes to fibrosis in nonalcoholic steatohepatitis. Dig Dis
Sci. 62:968–978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patsenker E, Schneider V, Ledermann M,
Saegesser H, Dorn C, Hellerbrand C and Stickel F: Potent
antifibrotic activity of mTOR inhibitors sirolimus and everolimus
but not of cyclosporine A and tacrolimus in experimental liver
fibrosis. J Hepatol. 55:388–398. 2011. View Article : Google Scholar : PubMed/NCBI
|