Introduction
Post-stroke depression (PSD), one of the most common
complications of stroke, refers to different degrees of depression,
for >2 weeks, in patients with stroke. A study has shown that at
least 60% of patients with stroke suffer from PSD (1), the clinical manifestations of which are
similar to those of primary depression, such as low mood, loss of
interest, slow behavior, insomnia/hypersomnia and guilt without a
source (2). PSD is not conducive to
the recovery of brain function, weakens the treatment effect and
reduces the quality of life, and increases the mortality rate
(3), the pathogenesis of which is
still investigated. Studies have shown that the incidence of PSD is
higher in patients with damage to the left brain, is decreased in
5-HT and NE, or derepression of dexamethasone (DST), and in elderly
and female patients with stroke (4,5).
Therefore, it is particularly important to clarify the pathogenesis
of PSD and develop targeted therapeutic drugs and protocols, for
improving the prognosis of PSD patients.
As an important material basis for learning and
memory function, Ca2+/CaM-dependent protein kinase II
(CaMKII) is mainly present in postsynaptic densities in the
hippocampus (6) and involved in
glutamatergic excitatory transmission (7). Glutamate is an excitatory
neurotransmitter that causes Ca2+ influx. When
intracellular Ca2+ concentration is increased,
Ca2+/CaM binds to CaMKII to activate CaMKII and induce
long-term potentiation (7,8).
Connexin 43 (Cx43) is mainly present in astrocytes
and vascular endothelial cells and is involved in gap junction,
maintaining direct communication between blood-brain barrier (BBB)
and cells (10). If BBB is damaged,
inflammatory factors and harmful substances enter the brain,
inducing neuritic degeneration and brain damage which are related
to the occurrence and development of PSD (11). Studies have shown that Cx43
expression is decreased and gap junction is widened in the
hippocampus tissue and cortical areas in patients with depression
(12,13). As an indicator for judging whether
BBB is damaged, Cx43 is closely related to depression (14). A study has shown that the
pathophysiological process of depression is closely associated with
the glutamatergic system (9).
CaMKII, an important component of glutamatergic
excitatory transmission, is speculated to have a relationship with
depression. However, there are no reports on whether CaMKII is
indeed involved in PSD, whether BBB damage is related to PSD, and
whether CaMKII affects Cx43 expression with Cx43 considered as an
important indicator for judging BBB damage. Therefore, in the
present study, CaMKII and Cx43 expression levels in PSD rats and
the effect of CaMKII inhibitor on Cx43 were explored in order to
provide a new understanding of the etiology of PSD and a
theoretical basis for the treatment and development of PSD.
Materials and methods
Experimental animals
Thirty-five SPF male SD rats, 12–15 months of age
and weighing 300–350 g, were provided by Changzhou Cavens
Experimental Animal Co., Ltd., with license SCXK (Su) 2011-0003.
After 1 week of conventional adaptive feeding (rats were fed with
basal feed and were free to drink water and move; 12-h light/dark
cycle), rats were screened according to their behavior, based on
the open field test and step-through test. Ten rats were included
in the control group, 13 rats in the PSD group and 12 rats in the
KN93 group (rats were treated with KN93, an inhibitor of CaMKII, on
the basis of the PSD group), based on the principle that the three
groups of behavioral scores should be similar. The ages of rats in
the three groups were 12.63±2.26, 13.21±2.19 and 13.87±2.77 months,
respectively, and the body weights were 322.32±27.54, 330.21±31.23
and 326.53±28.32 g, respectively, without significant differences
(P>0.05). The study was approved by the Ethics Committee of
Wenzhou Seventh People's Hospital (Wenzhou, China).
Compound modeling of PSD
Compound modeling of PSD (15) is to establish a depression model
after a stroke model. A rat model of ischemic stroke was
established using the suture embolic method in the PSD and KN93
groups. Chloral hydrate (10%) was intraperitoneally injected at a
dose of 300 mg/kg for anesthesia. The muscle was incised and
separated at the center of the neck after the preoperative
preparation, and the bilateral common carotid artery was
permanently ligated with a line no. 9. Rats in the control group
underwent a sham operation and were not ligated after incision. The
rats were intraperitoneally injected with 3 units of penicillin for
3 consecutive days after operation to prevent infection and
peritonitis. Longa scoring (16) was
performed at the 6th hour after operation, and rats with a Longa
score of 2–3 points were considered as a successful modeling of
stroke. Twelve rat models of stroke were successfully established
in the PSD and KN93 groups, respectively. After the rats with
ischemic stroke were orphaned for 2 weeks, 2 weeks of unpredictable
stress was performed, including behavioral limitation, foot shock,
tail squeezing, thermal stimulation and a reversal of day and
night. Fifty micrograms of KN93 were dissolved into 10 µl of normal
saline containing 10% DMSO to obtain KN93 injection, which was
intrathecally injected into the rats in the KN93 group. Equal
amount of normal saline containing 10% DMSO was intrathecally
injected into the rats in the PSD group. After 4 days of KN93
injection, the open field test and step-through test were carried
out to observe rat behavior.
Materials and reagents
TransScript II All-in-One First-Strand cDNA
Synthesis SuperMix for PCR and TransScript II SYBR-Green Two-Step
RT-qPCR SuperMix kit (AH321-01 and AQ301-01; both from Transgen
Biotech Co., Ltd.); RT-qPCR primers [Sangon Biotech (Shanghai) Co.,
Ltd.]; Trizol kit (10296028; Thermo Fisher Scientific, Inc.); RIPA
lysis buffer, BCA kit and ECL chromogenic reagent (P0013C, P0012S,
and P0018FS; all from Beyotime Institute of Biotechnology);
monoclonal rabbit anti-rat CaMKII, monoclonal rabbit anti-rat Cx43,
and monoclonal rabbit anti-rat β-actin antibodies, as well as
polyclonal horseradish peroxidase (HRP)-labeled goat anti-rabbit
secondary antibody (ab5683, ab79010, ab179467, and ab6728; all from
Abcam); KN93 (CSN11255; CSNpharm).
Longa score
Longa scoring was performed on the 7th, 14th and
18th day, with a total score of 4 points. The higher the score, the
more severe the neurological deficit was (16). The specific scoring standards are
shown in Table I.
 | Table I.Longa score criteria. |
Table I.
Longa score criteria.
| Symptom | Score |
|---|
| No neurologic
deficit | 0 points |
| Failure to fully
extend left forepaw, a mild focal neurologic deficit | 1 point |
| Circling to the left,
a moderate focal neurologic deficit | 2 points |
| Falling to the left,
a severe focal deficit | 3 points |
| Not spontaneous
walking and a depressed level of consciousness | 4 points |
Open field test
The open field test was performed on the 7th, 14th
and 18th day. Rats were exposed to white noise at 95 dB for 1 h,
and then placed in the open field from the same corner (80 cm × 80
cm × 40 cm, a total of 25 squares). After they were adapted for 1
min, their activities in the open field within 5 min were
photographed and recorded, including the square number of
horizontal movements (four paws into the grid was considered as one
time) and the vertical standing condition (two paws in the air and
then putting them down was considered as one time). The autonomous
activity frequency was the sum of all conditions.
Step-through test
The step-through test was performed on the 7th, 14th
and 18th day. Rats were firstly placed in a dark room. Escaping to
the bright room after an electric shock, and returning to the dark
room was considered as one time. The test was stopped when rats
stayed in the bright room for 2 min or the number of electric
shocks reached 20 times. The number of electric shocks and the
durations were recorded.
RT-qPCR
On the 18th day, RT-qPCR was performed using the
TransScript II SYBR-Green Two-Step RT-qPCR SuperMix kit to detect
CaMKII and Cx43 expression levels. Rats in each group were
decapitated after anesthesia with 300 mg/kg 10% chloral hydrate.
The hippocampus tissue was taken on an ice tray, placed in normal
saline, precooled at 4°C, and stored at 0°C with the remaining
blood washed away. TRIzol was used to extract total RNA, and the
concentration and purity were detected using an UV
spectrophotometer (S117578; Shanghai Kemin Biological Technology
Co., Ltd.). A260/A280 between 1.9 and 2.0 was considered as
qualified. Twenty microliters of reverse transcription reaction
system were prepared with 1 µg of total RNA, 4 µl of TransScript II
All-in-One First-Strand cDNA Synthesis SuperMix for PCR, and 1 µl
of gDNA Remover (Transgen Biotech Co., Ltd.) and RNase-free Water
(Takara Biotechnology Co., Ltd.). The mixture was incubated at 50°C
for 15 min, and at 85°C for 5 sec. With β-actin as an internal
reference, the amplification reaction was performed, and the final
system was 20 µl: 1 µl of cDNA, each 0.4 µl of upstream and
downstream primers (10 µM), 10 µl of 2X TransScript Tip SYBR-Green
qPCR SuperMix (Transgen Biotech Co., Ltd.) and RNase-free Water
used to complement 20 µl. The two-step amplification conditions
were as follows: 94°C for 30 sec, 94°C for 5 sec and 60°C for 30
sec, for 40 cycles. The results were analyzed using the
2−ΔΔCq method (17).
Primer sequences are shown in Table
II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Upstream primers | Downstream
primers |
|---|
| β-actin |
5′-CACGGCATTGTAACCAACTG-3′ |
5′-TCTCAGCTGTGGTGGTGAGG-3′ |
| CaMKII |
5′-AAGATGTGCGACCCTGGAATG-3′ |
5′-TGTAGGCGATGCAGGCTGAC-3′ |
| Cx43 |
5′-TTGTTTCTGTCACCAGTAAC-3′ |
5′-GATGAGGAAGGAAGAGAAGC-3′ |
Western blot analysis of CaMKII and
Cx43 protein expression levels
On the 18th day, western blot analysis was performed
to detect the expression of CaMKII and Cx43. RIPA was used to lyse
and extract the total protein from the hippocampus tissue, and BCA
was used to measure its concentration. A total of 10 µl of protein
was loaded per lane. Total protein was separated with 10% SDS-PAGE
electrophoresis, transferred to the NC membrane, and blocked at
22°C for 1 h with 5% skim milk (M230-42G-5PK; Beijing Jiehui Bogao
Biotechnology Co., Ltd.). Rabbit anti-rat CaMKII (1:300) and Cx43
(1:400) primary antibodies were blocked overnight at 4°C, with
β-actin (1:4,000) as an internal reference. After the membrane was
washed 3 times with PBS, the HRP-labeled goat anti-rabbit secondary
antibody was diluted at 1:5,000, and incubated at room temperature
for 2 h. The ECL chromogenic agent was incubated at room
temperature and developed for 1 min. Two parallel experiments were
conducted simultaneously, and gel imaged using the ImageJ software
(National Institutes of Health). The gray value of each protein
band was analyzed to calculate the relative protein content of
target protein (the gray value of target protein/the gray value of
internal reference protein).
Statistical analysis
SPSS 20.0 (IBM Corp.) was used in this study.
Measurement data were expressed as mean ± SD. t-test was used for
comparisons between two groups. Analysis of variance was used for
comparisons between three or more groups, and LSD test was the post
hoc test. Paired t-test was used for comparisons between two
time-points in the same group, and Chi-square test for comparisons
between multiple time-points in the same group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of Longa scores among
groups
On the 14th and 18th day, the Longa scores in the
control group were 0.48±0.15 and 0.51±0.48, respectively; in the
PSD group were 3.24±0.84 and 3.68±0.58, respectively; and in the
KN93 group were 3.89±0.76 and 1.57±0.34, respectively. On the 14th
day, the Longa score was not different between the PSD and KN93
groups (P>0.05), but in both groups the Longa score was higher
than that in the control group (both P<0.05). On the 18th day,
the Longa score was higher in the PSD group than that in the
control and KN93 groups (both P<0.05), and higher in the KN93
group than that in the control group (P<0.05). In the control
group, the score was not significantly different between the 18th
and 14th day (P>0.05). In the PSD group, the score on the 18th
day was significantly higher than that on the 14th day, whereas in
the KN93 group, the score on the 18th day was significantly lower
than that on the 14th day (both P<0.05) (Fig. 1).
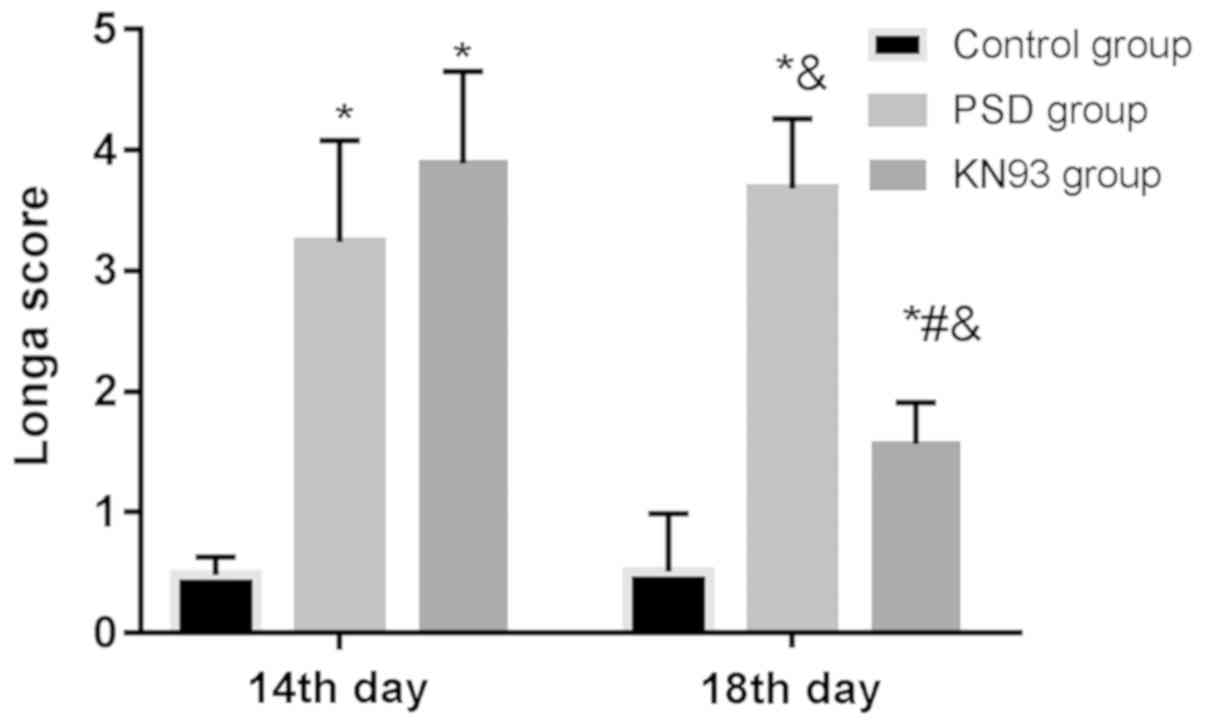 | Figure 1.Comparison of Longa scores among
groups. On the 14th day, the Longa score was not different between
the PSD and KN93 groups (P>0.05), but the scores in the two
groups were higher than that in the control group (both P<0.05).
On the 18th day, the score in the PSD group was higher than that in
the control and KN93 groups (P<0.05), and higher in the KN93
group than that in the control group (P<0.05). In the control
group, the scores on the 14th and 18th day were not significantly
different (P>0.05); in the PSD group, the score on the 18th day
was significantly higher than that on the 14th day, whereas in the
KN93 group, the score on the 18th day was significantly lower than
that on the 14th day (both P<0.05). *P<0.05, compared with
control group at the same time-point; #P<0.05,
compared with PSD group at the same time-point;
&P<0.05 compared with the 14th day in the same
group. PSD, post-stroke depression. |
Open field test
On the 7th day, there were no differences in the
results of the open field test between the control, PSD and KN93
groups (P>0.05). On the 14th day, there were no differences
between the PSD and KN93 groups (P>0.05), but the results in the
PSD and KN93 groups were lower than those in the control group
(both P<0.05). On the 18th day, the results in the PSD group
were lower than those in the control and KN93 groups (both
P<0.05), but there was no difference between the KN93 and
control groups (P>0.05). In the control group, the results were
not significantly different on the 7th, 14th or 18th day
(P>0.05). In the PSD group, the results were not different on
the 14th and 18th day (P>0.05), whereas they were lower on the
14th and 18th day than those on the 7th day (both P<0.05). In
the KN93 group, the results were lower on the 14th and 18th day
than those on the 7th day (both P<0.05), but those on the 18th
day were higher than that on the 14th day (P<0.05) (Table III).
 | Table III.Results of open field test. |
Table III.
Results of open field test.
|
| Autonomous activity
frequency (times) |
|
|
|---|
|
|
|
|
|
|---|
| Group | 7th day | 14th day | 18th day | F value | P-value |
|---|
| Control group
(n=10) | 48±15 | 42±12 | 40±13 | 1.048 | 0.363 |
| PSD group (n=12) | 43±12 | 13±8a,b | 11±9a,b | 36.500 | <0.001 |
| KN93 group
(n=12) | 46±11 | 14±9a,b | 39±11b–d | 30.120 | <0.001 |
| F value | 0.441 | 23.680 | 26.060 |
|
|
| P-value | 0.648 | <0.001 | <0.001 |
|
|
Step-through test
On the 7th day, there was no difference in the
number of electric shocks between the control, PSD and KN93 groups
(P>0.05). On the 14th day, the number in the PSD and KN93 groups
was higher than that in the control group (both P<0.05), but
there was no difference between the PSD and KN93 groups
(P>0.05). On the 18th day, the number in the PSD and KN93 groups
was higher than that in the control group (both P<0.05), and was
lower in the KN93 group than that in the PSD group (P<0.05). In
the control group, the number was not significantly different on
the 7th, 14th or 18th day (P>0.05). In the PSD group, the number
on the 14th and 18th day was significantly higher than that on the
7th day (both P<0.05), but there was no difference between the
number of shocks on the 14th and 18th day (P>0.05). In the KN93
group, the number on the 4th and 18th day was significantly higher
than that on the 7th day (both P<0.05), and was significantly
lower on the 18th day than that on the 14th day (P<0.05)
(Table IV).
 | Table IV.Results of step-through test. |
Table IV.
Results of step-through test.
| Variables | Control group
(n=10) | PSD group
(n=12) | KN93 group
(n=12) | F value | P-value |
|---|
| Electric shock
number |
| 7th
day | 1.86±0.55 | 1.92±0.61 | 1.89±0.58 | 0.029 | 0.971 |
| 14th
day | 1.76±0.42 |
5.88±1.43a,b |
6.35±1.11a,b | 55.680 | <0.001 |
| 18th
day | 1.98±0.61 |
6.98±1.56a,b |
2.21±0.76a–d | 78.530 | <0.001 |
| F
value | 0.428 | 52.560 | 103.800 |
|
|
|
P-value | 0.656 | <0.001 | <0.001 |
|
|
| Duration |
| 7th
day | 2.78±0.67 | 2.76±0.87 | 2.98±0.88 | 0.258 | 0.774 |
| 14th
day | 2.61±0.57 |
8.98±1.53a,b |
9.23±1.21a,b | 103.200 | <0.001 |
| 18th
day | 2.87±0.87 |
9.16±1.44a,b |
3.12±0.91b–d | 117.800 | <0.001 |
| F
value | 0.342 | 92.450 | 149.500 |
|
|
|
P-value | 0.714 | <0.001 | <0.001 |
|
|
On the 7th day, there was no difference in the
duration of electric shock between the control, PSD and KN93 groups
(P>0.05). On the 14th day, the duration in the PSD and KN93
groups was higher than that in the control group (both P<0.05),
but there was no difference between the PSD and KN93 groups
(P>0.05). On the 18th day, the duration in the PSD group was
higher than that in the control and KN93 groups (both P<0.05),
but there was no difference between the control and KN93 groups
(P>0.05). In the control group, the duration was not
significantly different on the 7th, 14th or 18th day (P>0.05).
In the PSD group, the duration on the 14th and 18th day was
significantly higher than that on the 7th day (both P<0.05), but
there was no difference on the 14th and 18th day (P>0.05). In
the KN93 group, the duration on the 7th day was lower than that on
the 14th and 18th day (both P<0.05), and was significantly lower
on the 18th day than that on the 14th day (P<0.05) (Table IV).
RT-qPCR detection of CaMKII and Cx43
expression
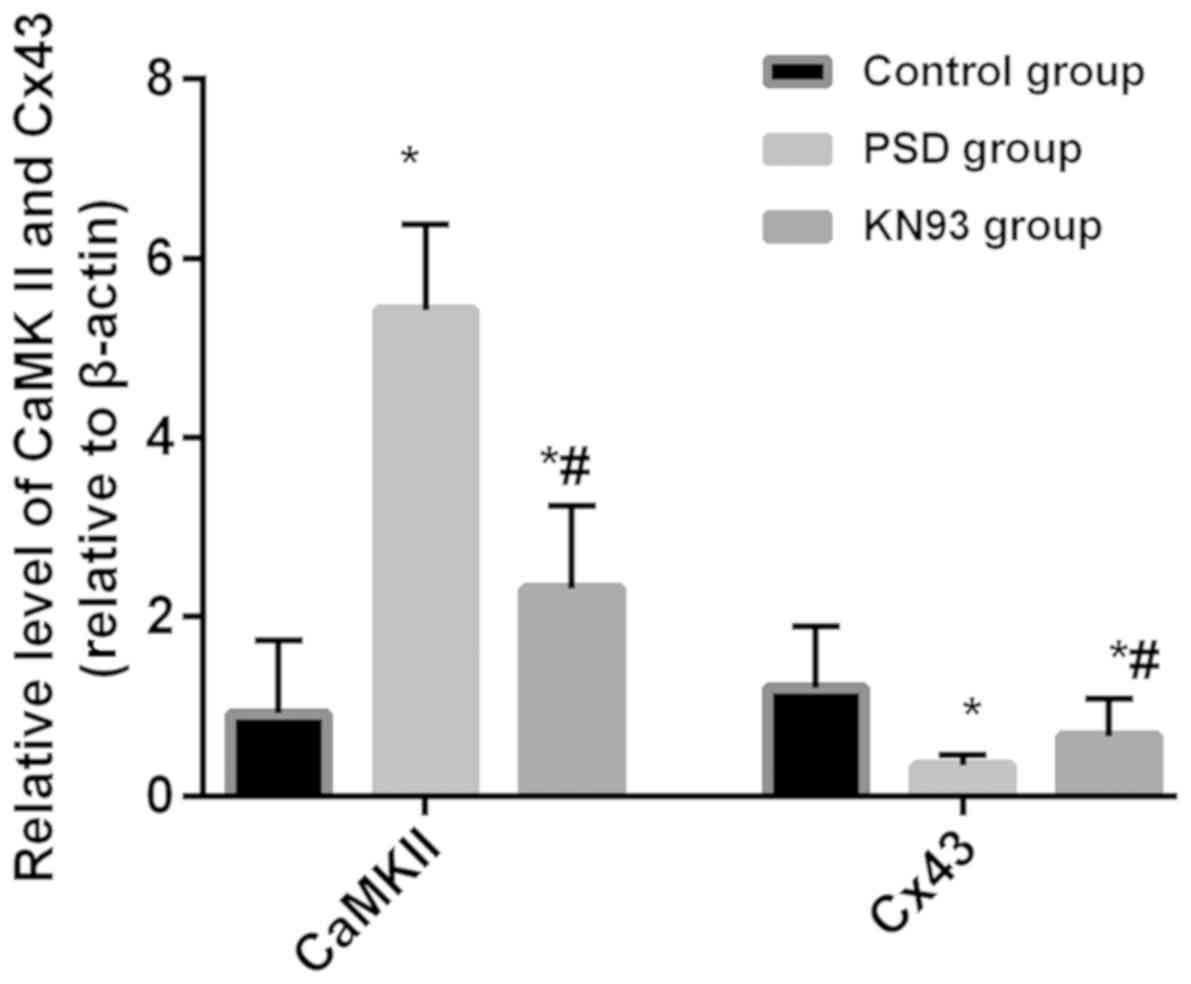
The relative expression of CaMKII mRNA in the
control, PSD and KN93 groups was 0.92±0.82, 5.42±0.96 and
2.32±0.92, respectively, and of Cx43 mRNA expression was 1.21±0.68,
0.34±0.12 and 0.67±0.42, respectively. The relative expression of
CaMKII mRNA in the PSD and KN93 groups was significantly higher
than that in the control group (both P<0.05), and was
significantly lower in the KN93 group than that in the PSD group
(P<0.05). The expression of Cx43 mRNA in the PSD and KN93 groups
was significantly lower than that in the control group (both
P<0.05), and was significantly higher in the KN93 group than
that in the PSD group (P<0.05) (Fig.
2).
Western blot analysis of CaMKII and
Cx43 expression
The relative protein expression of CaMKII in the
control, PSD and KN93 groups was 0.89±0.34, 4.32±0.87 and
2.32±0.84, respectively, and of Cx43 was 1.11±0.87, 0.45±0.21 and
0.97±0.66, respectively. The relative protein expression levels of
CaMKII in the PSD and KN93 groups was significantly higher than
that in the control group (both P<0.05), and was significantly
lower in the KN93 group than that in the PSD group (P<0.05). The
relative protein expression of Cx43 in the PSD group was lower than
that in the control and KN93 groups (both P<0.05), and there was
no difference in the expression of Cx43 between the KN93 and PSD
groups (P>0.05) (Fig. 3).
Discussion
With the aging of the population and the increase in
social competition, the incidence, disability rate and mortality
rate of PSD are increasing year by year, but the pathogenesis of it
has not yet been elucidated, which may be related to brain injury
site, neurotransmitter, endocrine, age and sex (18,19).
CaMKII is an important component of the glutamatergic nervous
system that plays an important role in the pathogenesis of PSD
(20). There is also a study showing
that the pathological process of depression is closely related to
the integrity of BBB, and Cx43 is an important protein for
maintaining BBB, and an important indicator for judging the
integrity of BBB (21). Therefore,
in the present study, the expression of CaMKII and Cx43 in the
hippocampus tissue of PSD rats and their association with PSD were
explored, in order to provide a new understanding for the etiology
of PSD and a theoretical basis for the treatment and development of
PSD.
On the 14th day, the Longa score was not different
between the PSD and KN93 groups, but was higher in the two groups
than that in the control group, indicating that the rat model of
stroke was successfully established in the PSD and KN93 groups. On
the 18th day, the score was higher in the PSD group than that in
the control and KN93 groups, and higher in the KN93 group than that
in the control group. In the control group, the score was not
significantly different between the 18th and 14th day; in the PSD
group, the score was significantly higher on the 18th day than that
on the 14th day; in the KN93 group, the score was significantly
lower on the 18th day than that on the 14th day. The results
suggest that after treated with KN93, the nerve damage of rats in
the KN93 group was repaired, and the nerve function was recovered
to a certain extent. However, stroke becomes more severe in the PSD
group, and the increase in the Longa score indicates the aggravated
neurological deficit.
Studies have shown that compared with healthy rats,
PSD rats have less spontaneous and inquiry activities, less
curiosity to new things and environment, lower reactivity to avoid
injury, and more reaction time, because of fear of the new
environment (22,23). On the 7th day, the results of the
open field test and the step-through test were not different
between the control, PSD and KN93 groups, suggesting that the
grouping is reasonable, and ensures the comparability of subsequent
test results. On the 14th day, compared with the control group,
rats in the PSD and KN93 groups had different degrees of passive
avoidance defects, significantly decreased reactivity to external
stimuli, and significantly reduced number of activity in the open
field test, indicating that different degrees of PSD occur in rats
after PSD compound modeling, showing successful compound modeling.
In a study on learning and memory levels of PSD rats, Wu et
al (24) have found that
compared with normal rats, PSD rats have weaker activity in the
open field test, and prolonged reaction time in the step-through
test. On the 18th day, compared with the PSD group, the passive
avoidance defects of rats in the KN93 group were improved, and the
number of activities was significantly increased in the open field
test, indicating that the depression in rats was alleviated after
treated with KN93. The results of RT-qPCR and western blot analysis
showed that on the 18th day, compared with the control group, the
PSD group had higher CaMKII expression but lower Cx43 expression,
indicating that compared with healthy rats, CaMKII expression is
upregulated but Cx43 expression is downregulated in PSD rats.
Kozoriz et al (25) observed
Cx43 expression in middle cerebral artery occlusion and the
relationship with neuronal injury, and found that Cx43 protects the
nerves in the model of stroke, and Cx43 expression is significantly
increased in the short term after stroke, followed by a sustained
and significant decrease. In this study, Cx43 expression was
decreased on the 18th day in the PSD group, which is consistent
with the findings of Kozoriz et al (25). CaMKII expression in the KN93 group
was lower than that in the PSD group, but higher than that in the
control group; Cx43 expression in the KN93 group was higher than
that in the PSD group, but not different from that in the control
group. The results suggest that KN93 can downregulate CaMKII
expression, upregulate Cx43 expression, and alleviate the
depression in PSD rats. Margrie et al (26) have found that KN93, an antagonist of
CaMKII, can completely block the long-term depression in young
chickens caused by low frequency stimulation, which is consistent
with the results of the present study. However, the way in which
CaMKII participates in depression was not previously explored in
depth (26). The results of the
present study show that CaMKII involved in PSD may be related to
Cx43 protein expression, which provides a new idea for
understanding the pathogenesis of depression and PSD. Therefore, it
is speculated that KN93 is an inhibitor of CaMKII, so CaMKII
expression is downregulated after KN93 treatment. In this study,
KN93 was found to upregulate Cx43 expression, suggesting that
CaMKII may negatively regulate Cx43. Cx43 is an important protein
that maintains BBB (9). The increase
in CaMKII expression in PSD rats inhibits Cx43 expression. The rat
BBB is damaged, and harmful substances enter the brain, which cause
damage to related nerves and participate in PSD. KN93 alleviates
the progress of PSD by upregulating Cx43 expression, which needs
more experiments for verifiction, and the specific regulatory
pathway needs more research.
In this study, an ischemic stroke animal model was
established using carotid artery ligation. This model has
advantages, such as simple operation, low mortality, and long
observation time after operation. The disadvantage is that it only
causes incomplete cerebral ischemia, that is, chronic
hypoperfusion. The pathological changes of cerebrovascular vessels
in this model are close to the pathological basis of clinical
stroke (15). Some of the stroke
models in this study may be further developed into vascular
dementia, but it is not excluded that these rats still have stroke.
At the same time, long-term ligation of the bilateral common
carotid arteries may lead to damage of the BBB, resulting in
decreased expression of Cx43 and may be a pathological pathway for
the reduction of clinical PSD Cx43 expression. Because carotid
artery ligation simulates a stroke model, clinical stroke may also
lead to BBB damage leading to decreased Cx43 expression, and
decreased Cx43 expression induces depression in patients, which may
be the possible pathological process of secondary depression in
stroke patients. However, this is only our speculation based on
other related research, and more sophisticated experiments are
required. The severity of stroke does have an impact on the
severity of depression. This is indeed not considered and is one of
the limitations of this study. However, there is no difference in
the open field test and step-through test on the 7th day in the
three groups. On the 14th and 18th day, the results of the open
field test and step-through test were decreased and were lower than
those on the 7th day. The effects of stroke severity on the
severity of depression were evenly distributed among the three
groups. The data of this study were comparable, and more
experimental methods will be applied in future studies.
Stroke consists of ischemic stroke and hemorrhagic
stroke. No less than 60% of patients with stroke have ischemic
stroke, and the incidence of ischemic stroke is much higher than
that of hemorrhagic stroke (27,28). In
this study, the rat model of stroke is ischemic stroke, without a
rat model of hemorrhagic stroke, which saves cost and time of the
test, but limits the universality of the results. Another
limitation of this study is that MRI was not used to evaluate
cerebral infarction, and the Longa score was the only means used,
which may increase bias in outcomes due to subjective factors.
In this study, CaMKII was found to negatively
regulate Cx43 expression and be involved in PSD. However, the
specific signal transduction pathway has not been elucidated, and
it needs to be further explored for harmful substances entering the
brain and participating in the pathogenesis of PSD after BBB
damage, due to the downregulation of Cx43 expression, as well as
the specific biological role of the downstream target genes of
Cx43.
In conclusion, CaMKII leads to PSD through
regulating Cx43 protein expression and gap junction function, and
the inhibitor of CaMKII, KN93, can improve depression.
Acknowledgements
Not applicable.
Funding
This study was supported by the Intervention Study
of Ditankaiqiao Tang for Post-stroke Depression through
CaMKII-Cx43-Glu Pathway (no. LY16H270002) and the Influence of
Ditan Decoction on the PV-Glu/SKCa-DA Neuron Pathway (no.
81774230).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and MJ performed PCR and western blot analysis.
ST drafted the manuscript. TQ assisted with open field test. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Wenzhou Seventh People's Hospital (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei C, Gao J, Chen L, Zhang F, Ma X, Zhang
N, Zhang W, Xue R, Luo L and Hao J: Factors associated with
post-stroke depression and emotional incontinence: Lesion location
and coping styles. Int J Neurosci. 126:623–629. 2016.PubMed/NCBI
|
|
2
|
Valiengo L, Casati R, Bolognini N, Lotufo
PA, Benseñor IM, Goulart AC and Brunoni AR: Transcranial direct
current stimulation for the treatment of post-stroke depression in
aphasic patients: A case series. Neurocase. 22:225–228. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quaranta D, Marra C and Gainotti G:
Post-stroke depression: Main phenomenological clusters and their
relationships with clinical measures. Behav Neurol. 25:303–310.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen L, Piao L and Piao H: Clinical
significance of SEP and plasma 5-HT in post stroke depression. J
Apo Nerv Dis. 12:1122–1125. 2016.(In Chinese).
|
|
5
|
Andersen G, Vestergaard K,
Ingemann-Nielsen M and Lauritzen L: Risk factors for post-stroke
depression. Acta Psychiatr Scand. 92:193–198. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunha MP, Budni J, Pazini FL, Oliveira Á,
Rosa JM, Lopes MW, Leal RB and Rodrigues AL: Involvement of PKA,
PKC, CAMK-II and MEK1/2 in the acute antidepressant-like effect of
creatine in mice. Pharmacol Rep. 66:653–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coultrap SJ and Bayer KU: CaMKII
regulation in information processing and storage. Trends Neurosci.
35:607–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isobe T and Okuyama T: The amino-acid
sequence of S-100 protein (PAP I-b protein) and its relation to the
calcium-binding proteins. Eur J Biochem. 89:379–388. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang X, Chu H, Tang Y and Dong Q: The role
of connexin43 in hemorrhagic transformation after thrombolysis in
vivo and in vitro. Neuroscience. 329:54–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Najjar S, Pearlman DM, Mackenzie TA,
Hernandez F Jr and Brown JR: Role of glial activation and BBB
disruption in the pathophysiology of depression. Neurol Psychiatry
Brain Res. 22:17–18. 2016. View Article : Google Scholar
|
|
11
|
Major S, Friedman A and Dreier JP:
Recurrent spreading depression (SD) causes early opening of the
blood-brain barrier (BBB). J Cereb Blood Flow Metab. 25 (Suppl
1):S2602005. View Article : Google Scholar
|
|
12
|
Li X, Rao F, Deng CY, Wei W, Liu FZ, Yang
H, Wang ZY, Kuang SJ, Chen XY, Xue YM, et al: Involvement of ERK1/2
in Cx43 depression induced by macrophage migration inhibitory
factor in atrial myocytes. Clin Exp Pharmacol Physiol. 44:771–778.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Zhang SS, Xiao S, Basheer WA, Baum
R, Epifantseva I, Hong T and Shaw RM: Cx43 isoform GJA1-20k
promotes microtubule dependent mitochondrial transport. Front
Physiol. 8:9052017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu X, Balesar R, Lu J, Farajnia S, Zhu Q,
Huang M, Bao AM and Swaab DF: Erratum to: Increased glutamic acid
decarboxylase expression in the hypothalamic suprachiasmatic
nucleus in depression. Brain Struct Funct. 222:38632017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overstreet DH: Modeling depression in
animal models. Methods Mol Biol. 829:125–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative geneexpression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
No authors listed, . Correction to:
In-hospital risk prediction for post-stroke depression: Development
and validation of the post-stroke depression prediction scale.
Stroke. 48:e1512017.PubMed/NCBI
|
|
19
|
Swartz RH, Bayley M, Lanctôt KL, Murray
BJ, Cayley ML, Lien K, Sicard MN, Thorpe KE, Dowlatshahi D, Mandzia
JL, et al: Post-stroke depression, obstructive sleep apnea, and
cognitive impairment: Rationale for, and barriers to, routine
screening. Int J Stroke. 11:509–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Ling S, Yang Y, Hu Z, Davies H and
Fang M: Systematic hypothesis for post-stroke depression caused
inflammation and neurotransmission and resultant on possible
treatments. Neuro Endocrinol Lett. 35:104–109. 2014.PubMed/NCBI
|
|
21
|
Freitas-Andrade M, She J, Bechberger J,
Naus CC and Sin WC: Acute connexin43 temporal and spatial
expression in response to ischemic stroke. J Cell Commun Signal.
12:193–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Fei P, Mu J, Wang H, Li W and
Song J: Decreased expression of neuronal Per-Arnt-Sim domain
protein 4 gene in the hippocampus of a post-stroke depression rat
model. Exp Ther Med. 7:1045–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Zhao M and Sui RB: Cerebellar
fastigial nucleus electrical stimulation alleviates depressive-like
behaviors in post-stroke depression rat model and potential
mechanisms. Cell Physiol Biochem. 41:1403–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu C, Zhang J and Chen Y: Study on the
behavioral changes of a post-stroke depression rat model. Exp Ther
Med. 10:159–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozoriz MG, Bechberger JF, Bechberger GR,
Suen MW, Moreno AP, Maass K, Willecke K and Naus CC: The connexin43
C-terminal region mediates neuroprotection during stroke. J
Neuropathol Exp Neurol. 69:196–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Margrie TW, Rostas JA and Sah P:
Presynaptic long-term depression at a central glutamatergic
synapse: A role for CaMKII. Nat Neurosci. 1:378–383. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feigin VL, Krishnamurthi RV, Parmar P,
Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran AE, Sacco
RL, Truelsen T, et al GBD 2013 Writing Group; GBD 2013 stroke panel
experts group, : Update on the global burden of ischemic and
hemorrhagic stroke in 1990–2013: The GBD 2013 Study.
Neuroepidemiology. 45:161–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacco RL, Adams R, Albers G, Alberts MJ,
Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J,
Harbaugh R, et al American Heart Association; American Stroke
Association Council on Stroke; Council on Cardiovascular Radiology
and Intervention; American Academy of Neurology, : Guidelines for
prevention of stroke in patients with ischemic stroke or transient
ischemic attack: A statement for healthcare professionals from the
American Heart Association/American Stroke Association Council on
Stroke: Co-sponsored by the Council on Cardiovascular Radiology and
Intervention: The American Academy of Neurology affirms the value
of this guideline. Stroke. 37:577–617. 2006. View Article : Google Scholar : PubMed/NCBI
|