Introduction
The liver, kidney and pancreas are the sites where
creatine is naturally synthesized from amino acids, including
methionine, arginine and glycine (1). Creatine is a product of the arginine
biosynthesis pathway in vivo, is stored in skeletal muscles
and metabolizes into creatinine (2).
Creatine is as a dietary supplement that can
increase phosphocreatine in the muscles and enhances performance
during high-intensity, short extent activities or during sessions
of high-intensity exercise with short rest periods, including
sprinting, jumping and strength training (3).
ATP stores in the body are sufficient to provide
maximal energy for one-two sec (4),
meaning ATP is subsequently required, which is not available
through the blood. The metabolism of phosphocreatine in muscles
rapidly produces ATP for an additional 5 to 8 sec of maximal effort
(4). Therefore, ATP and
phosphocreatine provide energy for <10 sec of maximal activity.
Lower energy output for sustained periods of time can be conserved
by aerobic oxidation of glycogen within the mitochondria (4). Therefore, aerobic metabolism regulates
the production of ATP during continuous exercise, highlighting the
importance of mitochondria for overall metabolic homeostasis
(4).
Mitochondria contains a circular DNA genome that is
called mitochondrial DNA (mtDNA), and it is well-known that
mitochondrial biogenesis increases in muscle cells upon exercise or
in response to an electrically stimulated contraction (5). Mitochondrial biogenesis is a complex
process that initiates the replication of mtDNA and the expression
of mitochondrial proteins that are encoded by nuclear and
mitochondrial genomes. Therefore, mitochondrial biogenesis serves a
pivotal role in optimizing cellular mitochondrial function
(6). The transcriptional coactivator
γ coactivator-1alpha (PGC-1α) regulates genes that are associated
with energy metabolism. PGC-1α interacts with the nuclear
peroxisome proliferator-activated receptor (PPAR) that permits the
interaction of PGC-1α with a number of transcription factors. PPAR
and PGC-1α are considered master regulators of mitochondrial
biogenesis as they activate nuclear respiratory factor 1 (NRF-1)
which in turn, activates mtDNA transcription factor A (TFAM) that
controls mtDNA transcription and replication (7). PGC-1α is a good sensor for the response
of the cells to free radicals (8),
and this indicates that free radicals generated in exercise may be
signals of increased mitochondrial biogenesis (9).
Pyruvate and lactate are critical fuel substrates
for muscles during exercise (10).
Pyruvate is generated by glycolysis and can serve as a substrate
for the mitochondrial TCA cycle to catabolize glucose producing
maximal ATP, or is used to produce lactate via the less efficient
ATP generation pathway (10).
Additionally, the oxidation of lactase is a substantial source of
pyruvate, where exercise increases lactate oxidation in skeletal
muscle (10). Lactate dehydrogenase
(LDH) is a key enzyme that catalyzes the conversion of pyruvate and
lactate, and regulates cellular pyruvate and lactate homeostasis
(10).
The aim of the current study was to investigate the
effects of creatine supplementation alone or combined with exercise
on the expression of genes controlling the pathway of mitochondrial
biogenesis, including PGC-1α, NRF1, TFAM and mtDNA copy number, in
skeletal and cardiac muscles.
Materials and methods
Experimental animals
In the current study, a total of 40 male Wister rats
(weight, 120–150 g; age, 3 months) were used. Rats were obtained
from the animal house of the Medical Research Institute, Alexandria
University (Alexandria, Egypt). Rats were maintained in
air-conditioned rooms (temperature, 23±1°C; humidity, 50–55%), with
a 12 h light-dark cycle, 5 rats were placed in each cage and were
fed with a standard diet (Table I)
and tap water ad libitum. The animal procedures were approved by
the Institutional Animal Care and Use Committee at the Medical
Research Institute at Alexandria University. All procedures comply
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (11) (NIH
Publications no. 8023; revised 1985) and regulations of Egypt's
Guide for the Care and Use of Laboratory Animals (12). The current study adheres to the
ARRIVE Guidelines for reporting in vivo experiments
(13). All efforts were made to
curtail the suffering of rats during the experimental period.
 | Table I.Experimental diet composition fed to
rats. |
Table I.
Experimental diet composition fed to
rats.
| Ingredients | Standard diet
(g/kg) |
|---|
| Protein | 220 |
| Fat | 43 |
| Carbohydrates | 631 |
| Cellulose | 54 |
| Vitamin mix | 10 |
| Mineral mix | 40 |
| Total energy
(kcal/g diet) | 3.8 |
Exercise protocol
The exercise protocol was swimming for 1 h with a
metal ring, which was customized for each individual rat to be 3%
of the rat's body weight and enclosed to the torso to avoid the
innate ability of rats to float on the water surface. This exercise
protocol has been indicated to characterize moderate intensity
exercise (14).
Experimental design
The animals were separated into four groups that
were monitored for 5 weeks. Two unexercised groups consisting of
the control sedentary group (CS; n=10) which exhibited only
spontaneous movement in cages, and the sedentary creatine-treated
group (SC; n=10), which included rats treated daily with oral
creatine (0.5 g/kg per day) (15)
and exhibited only spontaneous movement in cages. Two exercised
groups performed swimming exercise training 5 days/week for five
weeks and included the exercise training group (ET; n=10) and the
exercise training and creatine treated (0.5 g/kg per day) group
(ETC; n=10).
A period of 24 h after the last treatment and
exercise training the rats were weighed and sacrificed by cervical
dislocation under deep anaesthesia using ketamine/xylazine at a
dose of 100/10 mg/kg. Blood samples were collected centrifuged at
1,000 × g for 20 min at 4°C to obtain the serum; and the cardiac
and soleus muscle were removed and divided into three sections. The
first section was used for DNA extraction for the assessment of
mtDNA copy number, the second section was used for RNA extraction
to analyze gene expression and the third section was used for
nuclear extraction for the assay of PGC-1α.
Serum parameters
Serum lactate was determined using colorimetric
L-lactate Assay kit (cat. no. ab65331; Abcam) and pyruvate were
assayed using colorimetric Pyruvate Assay Kit (cat. no. ab65342;
Abcam) according to the manufacturer's protocols. Serum urea (cat.
no. UR3825), creatinine (cat. no. CR510), aspartate transaminase
(AST; cat. no. AS7938) and alanine transaminase (ALT; cat. no.
AL7930) activity were assayed using a Randox kit (Randox
Laboratories Ltd.) according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cardiac and soleus muscle tissues were used for
total RNA extraction using the RNeasy mini kit (Qiagen GmbH) and
DNA extraction using DNeasy Blood and Tissue kit (Qiagen GmbH)
according to manufacturer's protocols. Reverse transcription of
muscular RNA was performed using a miScript II RT kit (Qiagen GmbH)
according to the manufacturer protocol. The miScript II RT kit was
used to perform a one-step, single-tube reverse transcription
reaction. miScript HiFlex buffer was used to promote the conversion
of all RNA species into cDNA.
The cDNA was used to quantify the gene expression of
NRF1 and Tfam using Rotor-Gene Q qPCR (Qiagen, Inc.), which was
performed using QuantiTect SYBR Green PCR Master Mix (Qiagen GmbH).
qPCR amplification conditions started with an initial denaturation
for 10 min at 55°C, followed by amplification by 40 cycles of PCR
as follows: Denaturation at 95°C for 5 sec, annealing at 55°C for
15 sec and extension at 60°C for 15 sec. The housekeeping gene
GAPDH was used as a reference gene for normalization. Primers used
for rat genes were as follows: NRF1 (16) forward, 5′-TTACTCTGCTGTGGCTGATGG-3′
and reverse, 5′-CCTCTGATGCTTGCGTCGTCT-3′; TFAM (17) forward, 5′-GCTTCCAGGAGGCTAAGGAT-3′ and
reverse, 5′-CCCAATCCCAATGACAACTC-3′; GAPDH forward,
5′-GGGTGTGAACCACGAGAAATA-3′ and reverse,
5′-AGTTGTCATGGATGACCTTGG-3′. The values of threshold cycle (Ct)
were determined using Rotor-Gene Q-Pure Detection version 2.1.0
(build 9; Qiagen, Inc.). For each gene, the relative change in mRNA
in samples was determined using the 2−ΔΔCq method
(18) and normalized to the
housekeeping gene (GAPDH).
Nuclear extraction and PGC-1α
assessment
Immediately after the collection of blood, muscles
were excised, washed with ice-cold saline and preserved at −80°C
until subsequent assay. The nuclear extract of muscle tissues was
obtained using Nuclear Extraction Kit (cat. no. ab113474; Abcam)
according to manufacturer's protocol, which were then used for the
determination of muscular PGC-1α contents. Total protein
concentration was measured using Lowry method (19).
Mitochondrial DNA copy number
Total DNA was extracted from muscle tissues using
RNeasy kit (Qiagen GmbH). Using the extracted total DNA, the
mitochondrial DNA (mtDNA) content was assessed relative to the
nuclear DNA specific gene (PGC1α) using RT-qPCR (20). The primers used were as follows:
mtDNA forward, 5′-ACACCAAAAGGACGAACCTG-3′ and reverse,
5′-ATGGGGAAGAAGCCCTAGAA-3 and PGC1α forward,
5′-ATGAATGCAGCGGTCTTAGC-3′ and reverse, 5′-AACAATGGCAGGGTTTGTTC-3′.
Reactions were carried out using SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.), 0.5 µM forward and
reverse primer, and 50 ng genomic DNA were used with the following
conditions: 95°C for 10 min followed by 40 cycles of 95°C for 15
sec, 60°C for 30 sec and 72°C for 30 sec. The relative mtDNA copy
number was calculated using the 2−ΔΔCq method (18) as described previously (21).
Statistical analysis
Values are expressed as mean ± standard deviation
(n=10) and were analyzed using the GraphPad Prism v5.0 (GraphPad
Software, Inc.). Multiple comparisons were performed using one-way
ANOVA, followed by a Tukey post-hoc test. The correlation
coefficients (r) between different assayed parameters were
evaluated using Pearson's correlation coefficient. P<0.05 was
considered to indicate a statistically significance difference.
Results
Effect of creatine supplementation on
rats' weight
Rat weight was evaluated at the end of treatment
period to compare between groups that were supplemented with
creatine and rats not supplemented, and to record the difference
between exercised and sedentary rats. As presented in Table II, although sedentary rats
supplemented with creatine (SC) were indicated to exhibit an
increase in weight, no significant difference (P>0.05) was
observed in exercised rats supplemented with creatine (ETC) or any
other group (Table II).
 | Table II.Effect of creatine supplementation on
rats' weight. |
Table II.
Effect of creatine supplementation on
rats' weight.
| Experimental
groups | Weight (g) |
|---|
| Sedentary rats
(CS) | 151.8±10.84 |
| Exercised rats
(ET) |
148.8±13.96a |
| Sedentary rats
supplemented with creatine (SC) |
157.3±12.87a,b |
| Exercised rats
supplemented with creatine (ETC) |
146.3±9.85a–c |
Effect of creatine supplementation on
blood lactate/pyruvate level
Supplementation of creatine upon 1 h of daily
exercise (ETC rats) resulted in a significant decrease in blood
lactate level compared with sedentary rats receiving creatine daily
(SC), as presented in Fig. 1A
(2.95±0.150 ETC vs. 3.64±0.298 SC). However, this decrease was not
significantly different from rats that did not receive creatine,
whether sedentary or exercised. However, blood pyruvate level in
ETC rats exhibited a significant increase compared with sedentary
rats, those receiving and not receiving creatine (Fig. 1B; 0.16±0.0129 ETC vs. 0.13±0.022 SC
and vs. 0.13±0.018 CS). The lactate/pyruvate ratio was in
accordance with the previously mentioned data demonstrating a
significant decrease in ETC rats compared with sedentary rats
receiving or not receiving creatine (Fig. 1C).
Effect of creatine supplementation on
gene expression in soleus muscle
Training rats for 1 h per day exhibited a clear
effect on the soleus muscle, where exercised rats were demonstrated
to exhibit a significant increase in PGC-1α and mtDNA copy number
compared with sedentary rats (Fig. 2A
and D; 156.8±10.99 ET vs. 131.3±6.87 CS; 3.40±0.216 ET vs.
2.90±0.183 CS). Treatment with creatine in trained rats was
indicated to increase gene expression of all measured parameters
compared with all groups. When comparing ETC rats with SC rats,
PGC-1α expression was increased by ~20% (Fig. 2A). NRF-1 and TFAM expression
indicated a ~50 and ~53% increase, respectively (Fig. 2B and C), while mtDNA exhibited a
significant increase of 21% (Fig.
2C).
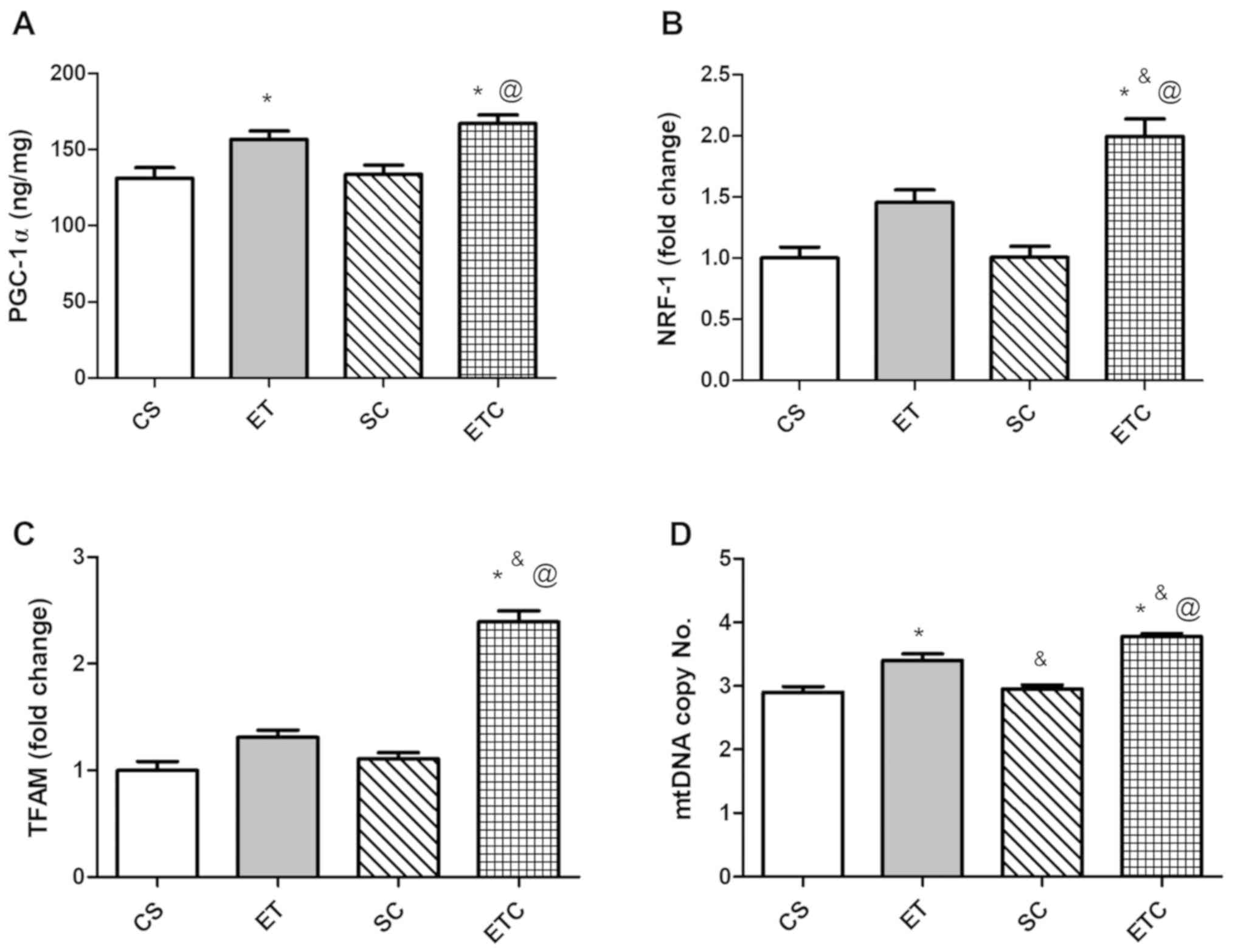 | Figure 2.Effect of creatine supplementation on
gene expression in soleus muscle. (A) PGC-1α expression.
@P<0.01 SC vs. ETC, *P<0.01 CS vs. ETC and
*P<0.05 CS vs. ET. (B) NRF-1 expression. @P<0.001
SC vs. ETC, *P<0.001 CS vs. ETC and &P<0.05 ET
vs. ETC. (C) TFAM expression. @P<0.001 SC vs. ETC,
*P<0.001 CS vs. ETC and &P<0.001 ET vs. ETC.
(D) mtDNA copy number. @P<0.001 SC vs. ETC,
*P<0.001 CS vs. ETC, *P<0.01 CS vs. ET,
&P<0.05 ET vs. ETC and &P<0.01
ET vs. SC. Values are presented as mean ± standard deviation
(n=10). PGC-1α, peroxisome proliferator-activated receptor γ
coactivator 1-α; NRF-1, nod factor receptor 1; TFAM, mitochondrial
transcription factor A; mtDNA, mitochondrial DNA; CS, sedentary
rats; SC, sedentary rats supplemented with creatine; ET, exercised
rats; ETC, exercised rats supplemented with creatine. |
Effect of creatine supplementation on
gene expression in cardiac muscle
The clear effect of creatine on cardiac muscle was
indicated in the soleus muscle. NRF-1 and TFAM genes (Fig. 3B and C) in exercised rats receiving
(ETC) or not receiving (ET) creatine demonstrated a significant
increase in expression compared with sedentary rats (CS).
Supplementation of creatine in trained rats (ETC) was observed to
increase PGC-1α expression by ~14.5% (Fig. 3A) and mtDNA by ~15% (Fig. 3D) compared with sedentary rats
recieving creatine (SC).
Effect of creatine supplementation on
liver and kidney functions
The normal concentrations of AST and ALT were
determined upon the serum analysis of sedentary (CS) and exercised
(ET) rats and are presented as means (112±16.89 U/l and 29.75±7.50
U/l; 108.5±11.90 U/l and 32±7.44 U/l, respectively). No significant
elevations of AST (P=0.9224, CS vs. SC; P=0.8720, ET vs. ETC;
Fig. 4A) and ALT (P=0.8847, CS vs.
SC; P=0.8894, ET vs. ETC; Fig. 4B)
serum levels were observed in creatine supplemented groups compared
with the healthy controls, indicating that normal transaminases
levels were neither affected by exercise nor creatine
supplementation. Additionally, creatine supplementation did not
induce significant effects on serum urea levels compared with
healthy controls (P=0.5705, CS vs. SC; P=0.6115 ET vs. ETC;
Fig. 4C). Similar observations were
made on serum creatinine levels, which showed no significant
differences between exercised and sedentary rats in a manner that
was independent of whether creatine was supplemented (Fig. 4D; 0.43±0.06 ETC, 0.38±0.10 SC,
0.36±0.09 ET and 0.4±0.08 CS), suggesting normal kidney
function.
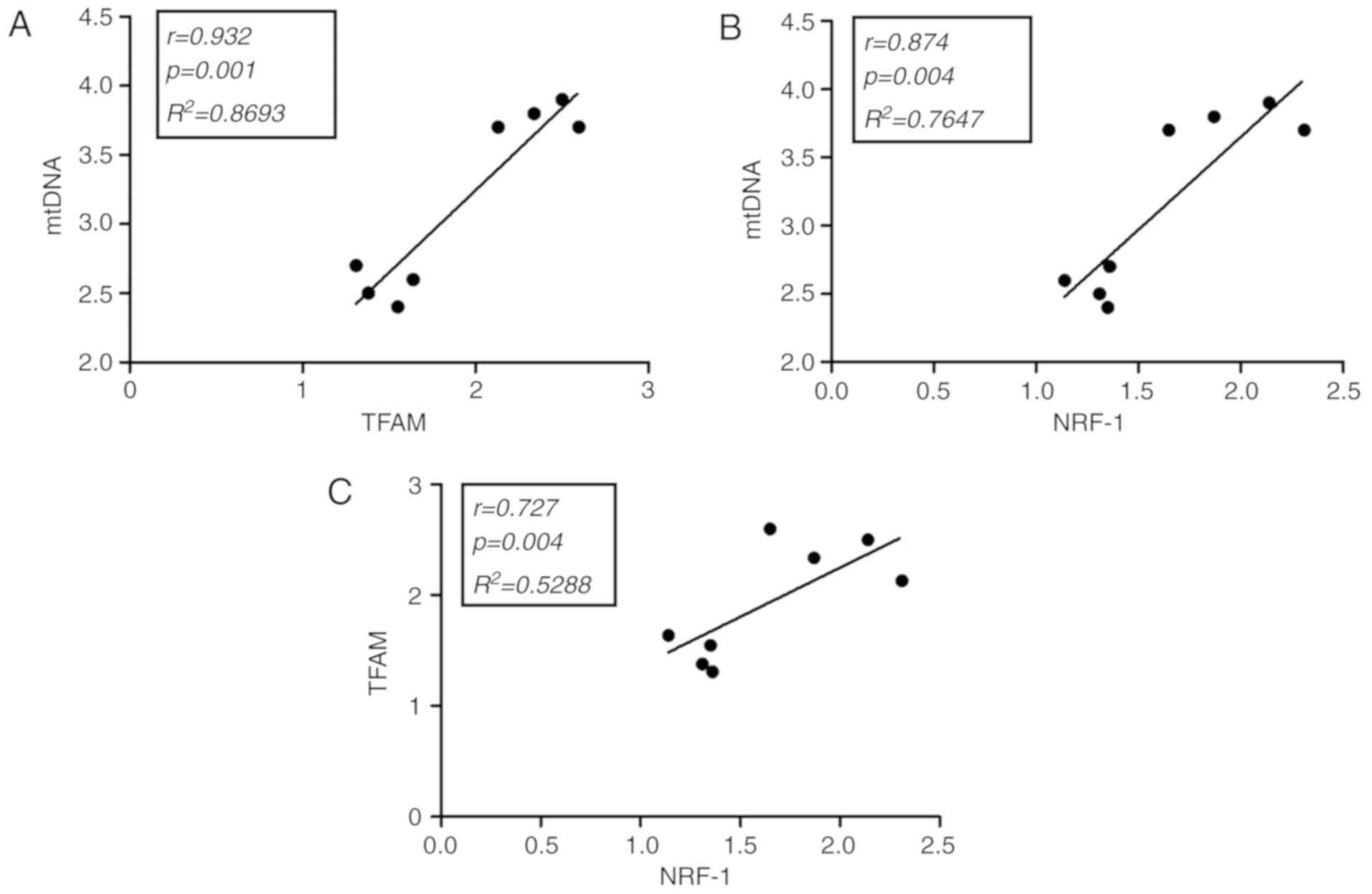
Correlation studies in soleus and
cardiac muscles
Statistical analysis of the soleus and cardiac
muscles in exercised rats supplemented with creatine indicated that
mtDNA was positively correlated with TFAM (r=0.932; P=0.001;
Fig. 5A) and NFR-1 (r=0.874;
P=0.004; Fig. 5B). Additionally,
TFAM was positively correlated with NFR-1 (r=0.727; P=0.004;
Fig. 5C). Other correlations in ETC
rats were not observed to be statistically significant (data not
shown). All results exhibited a high degree of reproducibility.
Discussion
Creatine supplementation is considered to be the
most effective nutritional supplement and ergogenic aid to enhance
anaerobic exercise performance in a number of sports (22). Additionally, research has focused on
investigating the widespread application of this supplement within
different target groups, where it can be beneficial for treating
cancer, rheumatoid arthritis, type 2 diabetes and neurodegenerative
disorders (23). To gain additional
insight into the mechanistic pathway of creatine supplementation,
the current study focused on the effect of Cr supplementation alone
or combined with exercise on the expression of genes controlling
the pathway of mitochondrial biogenesis in skeletal and cardiac
muscles, and its effect on liver and kidney functions.
The results of the present study revealed that
exercise utilizes endogenous creatine and increases the
mitochondrial biogenesis which can be identified using increased
expression of PGC-1α and mtDNA levels in soleus muscle, compared
with non-exercised groups. Exogenous Cr supplementation increased
biogenesis during exercise causing an increase of all key
regulatory parameters of mitochondrial biogenesis that can be
measured in soleus muscle (PGC-1α, NFR1, TFAM and mtDNA) and
therefore, more ATP was available for muscle consumption.
These results are in accordance with the popular
theory that creatine (Cr) is phosphorylated upon cellular uptake in
the muscle, to further exist as free Cr (40%) and phophorylcreatine
(PCr; 60%). Free Cr and phophorylcreatine serve a crucial role as
‘energy buffers’ at sites of high energy turnover, including in
skeletal muscle and the heart, to operate if the required ATP
quantity exceeds the normal rate produced by mitochondria,
retaining the ATP homeostasis at these sites. When phosphocreatine
level decreases due to the re-phosphorylation of ADP, the
production of phosphofructokinase is accelerated to increase the
speed of glycolysis (24), which is
the major source of pyruvate.
During exercise, pyruvate and lactate are essential
fuel substrates for skeletal muscles, which produce ATP, and have
been indicated to be correlated proportionally to exercise-induced
PGC-1α signaling as stated by Liang et al (10). This relationship has been reflected
in the current study, where increased PGC-1α expression in soleus
muscle of exercised rats supplemented with Cr exhibited a
significant increase in serum pyruvate level, but not the lactate.
These results demonstrated that pyruvate serves directly as a
substrate for the mitochondrial TCA cycle to catabolize glucose
producing ATP, with no need for interconversion into lactate.
These findings are supported by correlation data,
which indicated that mtDNA, NRF-1 and TFAM exhibit a statistically
significant positive correlation in cardiac and soleus muscles of
exercised rats supplemented with creatine, an effect that has not,
to the best of our knowledge, yet been indicated in sedentary rats
supplemented with creatine. The lack of immunohistochemical
analysis to confirm the increased mitochondrial number is a
limitation of the study and will be assessed in future work.
The PGC1-α gene is highly inducible in response to
physiologic conditions that require an increased mitochondrial
energy production (5). As
demonstrated by Finck et al (25), PGC-1α expression is stimulated by
exercise in skeletal muscle and by fasting in the cardiac muscle
(25). Studies have also indicated
that PGC-1-α is expressed preferentially in muscles rich in type I
fibers, including in the soleus muscle (5,26).
As indicated by Head et al (27) and Murphy et al (28), Cr supplementation increased the
sensitivity of contractile proteins to intracellular Ca2+, which
enhances muscle performance and reduces muscle fatigue. This is
attributed to the osmotic effect of Cr when entering the muscle,
and its ability to re-synthesize ATP. This effect of creatine was
further supported by an in vivo study in mice that
demonstrated how Cr supplementation decreased skeletal muscle
necrosis and improved mitochondrial respiration (29). Additional studies have demonstrated
that increased signals of mitochondria biogenesis, including
PGC-1α, NRF1 and TFAM expression, enhance mitochondrial protein
synthesis, improve its respiration and improve its functional
performance in a number of muscles types (30–32). As
oxidants are regarded to negatively impact muscle fatigue and
growth upon aerobic exercise, multiple studies have examined the
effect of Cr supplementation on oxidative stress in muscles and
proved a direct antioxidant activity of Cr affecting cell viability
and DNA damage positively (24,33).
A similar effect has been previously observed in the
cardiac muscle, with a decreased effect (26). Creatine supplementation revealed a
tendency to increase mitochondrial biogenesis compared with
sedentary rats, as indicated by the transcription levels of NRF-1
and TFAM and supported by the correlation data. There was no clear
difference to exercised rats that were not supplemented with
creatine, indicating that this was an exercise effect and not an
effect of creatine supplementation. Transcriptional coactivator
PGC-1α is the major regulator of mitochondrial biogenesis (5), and this is indicated by the increased
expression of NFR-1, TFAM and mtDNA in the skeletal muscles of
exercised rats supplemented with Cr, where an increase in the
amount of PGC-1α and mtDNA can be observed. However, their levels
fail to exhibit the same prominent increase as indicated in the
soleus muscle, which was assessed in the current study. This may be
due to the fact that PGC-1α expression is activated in the heart
upon fasting, as identified by Lehman et al (34). Fasting is a physiologic stimulus that
markedly increases the reliance of the heart on mitochondrial fat
oxidation for ATP production (35).
This phenomenon has also been demonstrated by a
study by Arany et al (26),
where hearts of mice lacking PGC-1α exhibited reduced mitochondrial
enzymatic activity, decreased ATP level and a decreased ability to
increase the work output upon electrical or chemical stimulation
(26). PGC-1α is known to coactivate
PPARα and ERRα, and nuclear receptors (NRs) that regulate genes
associated with cardiac fatty acid oxidation and mitochondrial
respiratory function (36).
In the current study, sedentary rats that were
supplemented with creatine (SC) demonstrated an increase in rat
weight, since Cr is an osmotically active substance, and any
increase in muscle Cr content can result in increased muscle water
retention and weight gain (37).
However, no significant differences in body weight were observed
between exercised rats supplemented with creatine (ETC) or any
other group. According to Becque et al (38), this increase in body mass due to Cr
supplementation was the consequence of increased fat-free mass.
While Deldicque et al (39)
and Safdar et al (40) have
reported that the administration of Cr may encourage overexpression
of genes and proteins associated with abnormal enlargement of body
parts or organs. These alterations would affect the translational
process, triggering an increase in lean mass chronically. Chrusch
et al demonstrated a significant increase in body mass, and
this increase was identified to be lean body weight rather than
water retention or fat (41).
Another study also indicated significant increases in fat-free mass
following only two resistance training sessions per week.
The results of the current study demonstrated that
creatine supplementation for 5 weeks did not disrupt liver and
kidney functions when compared with sedentary and exercised rats.
An increase in creatinine level is a normal metabolic pathway that
does not disrupt the normal functions of the kidney. This outcome
is supported by previous studies that indicated no effect of
short-, medium- and long-term Cr supplementation on kidney function
(42), nominating creatine as a
supplement with a high safety profile. These results strengthen the
possibility of using Cr supplementation for individuals that are
susceptible to impaired kidney function, including the elderly and
patients with type 2 diabetes (42).
In conclusion, the results of the current study add
to increasing number of studies investigating creatine mechanistic
pathways in the mitochondria. It can be concluded that, activity
coupled with short-term Cr supplementation increased all factors of
mitochondrial biogenesis and improved skeletal and heart muscle
functions, and this effect is unrelated to kidney or liver adverse
effects. Further studies are required to explore the possibility of
Cr supplementation in ameliorating mitochondrial diseases,
including epilepsy, skeletal and cardiac myopathies, hepatopathies
and nephropathies. In the current study, Cr supplementation with
exercise enhanced PGC-1α expression, however, whether this effect
can alter the muscle fiber types was not determined. The effect of
creatine on different muscle fibers should be assessed in future
studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MAK designed the experiments and contributed to the
writing and revising of the paper. SAM and MAG extracted RNA and
DNA, assayed the molecular parameters, analyzed the data, wrote and
revised the manuscript. NAS and YES performed experimental design,
rat training, wrote and revised the manuscript. All authors read
and approved the final manuscript.
Ethical approval and consent to
participate
The animal procedures were approved by the
Institutional Animal Care and Use Committee at the Medical Research
Institute, Alexandria University. All procedures comply with the
National Institutes of Health guide for the care and use of
Laboratory Animals (NIH Publications no. 8023, revised 1978),
regulations of Egypt's guide for the care and use of laboratory
animals (12) and the ARRIVE
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CS
|
control sedentary group
|
|
ET
|
exercise training group
|
|
ETC
|
exercise training and creatine treated
group
|
|
mtDNA
|
mitochondrial DNA
|
|
NRF-1
|
nuclear respiratory factor 1
|
|
PGC-1α
|
γ coactivator-1α
|
|
SC
|
sedentary creatine-treated group
|
|
TFAM
|
transcription factor A
|
References
|
1
|
Bemben MG, Witten M, Carter J, Eliot K,
Knehans A and Bemben D: The effects of supplementation with
creatine and protein on muscle strength following a traditional
resistance training program in middle-aged and older men. J Nutr
Health Aging. 14:155–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brosnan JT, da Silva RP and Brosnan ME:
The metabolic burden of creatine synthesis. Amino Acids.
40:1325–1331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karimian J and Esfahani PS: Supplement
consumption in body builder athletes. J Res Med Sci. 16:1347–1353.
2011.PubMed/NCBI
|
|
4
|
Holloway GP: Nutrition and training
influences on the regulation of mitochondrial adenosine diphosphate
sensitivity and bioenergetics. Sports Med. 47 (Suppl 1):S13–S21.
2017. View Article : Google Scholar
|
|
5
|
Scarpulla RC: Metabolic control of
mitochondrial biogenesis through the PGC-1 family regulatory
network. Biochim Biophys Acta. 1813:1269–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin F and Cadenas E: Mitochondria: The
cellular hub of the dynamic coordinated network. Antioxid Redox
Signal. 22:961–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang Y, Xia W, Yang J, Zhu Y, Chang H,
Liu J, Huo W, Xu B, Chen X, Li Y and Xu S: BPA-induced DNA
hypermethylation of the master mitochondrial gene PGC-1α
contributes to cardiomyopathy in male rats. Toxicology. 329:21–31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olsen RK, Cornelius N and Gregersen N:
Redox signalling and mitochondrial stress responses; lessons from
inborn errors of metabolism. J Inherit Metab Dis. 38:703–719. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez-Cabrera MC, Domenech E, Romagnoli M,
Arduini A, Borras C, Pallardo FV, Sastre J and Viña J: Oral
administration of vitamin C decreases muscle mitochondrial
biogenesis and hampers training-induced adaptations in endurance
performance. Am J Clin Nutr. 87:142–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang X, Liu L, Fu T, Zhou Q, Zhou D, Xiao
L, Liu J, Kong Y, Xie H, Yi F, et al: Exercise inducible lactate
dehydrogenase B regulates mitochondrial function in skeletal
muscle. J Biol Chem. 291:25306–25318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Care IoLARCo, Animals UoL and Resources
NIoHDoR: Guide for the care and use of laboratory animals: National
Academies. 1985.
|
|
12
|
Fahmy SR and Gaafar K: Establishing the
first institutional animal care and use committee in Egypt. Philos
Ethics Humanit Med. 11:22016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McGrath JC, Drummond GB, McLachlan EM,
Kilkenny C and Wainwright CL: Guidelines for reporting experiments
involving animals: The ARRIVE guidelines. Br J Pharmacol.
160:1573–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Araujo LC, de Souza IL, Vasconcelos LH,
Brito Ade F, Queiroga FR, Silva AS, da Silva PM, Cavalcante Fde A
and da Silva BA: Chronic aerobic swimming exercise promotes
functional and morphological changes in rat ileum. Biosci Rep.
35(pii): e002592015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguiar AF, de Souza RW, Aguiar DH, Aguiar
RC, Vechetti IJ Jr and Dal-Pai-Silva M: Creatine does not promote
hypertrophy in skeletal muscle in supplemented compared with
nonsupplemented rats subjected to a similar workload. Nutr Res.
31:652–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Wu Y, Sha H, Zhang P, Jia J, Hu Y
and Zhu J: Early exercise affects mitochondrial transcription
factors expression after cerebral ischemia in rats. Int J Mol Sci.
13:1670–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piantadosi CA and Suliman HB:
Mitochondrial transcription factor A induction by redox activation
of nuclear respiratory factor 1. J Biol Chem. 281:324–333. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee N, Shin S, Chung HJ, Kim DK, Lim JM,
Park H and Oh HJ: Improved quantification of protein in vaccines
containing aluminum hydroxide by simple modification of the Lowry
method. Vaccine. 33:5031–5034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly DP and Scarpulla RC: Transcriptional
regulatory circuits controlling mitochondrial biogenesis and
function. Genes Dev. 18:357–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamel MA, Helmy MH, Hanafi MY, Mahmoud SA,
Elfetooh HA and Badr MS: Maternal obesity and malnutrition in rats
differentially affect glucose sensing in the muscles and adipose
tissues in the offspring. Int J Biochem Res Rev. 4:440–469. 2014.
View Article : Google Scholar
|
|
22
|
Martinez N, Campbell B, Franek M, Buchanan
L and Colquhoun R: The effect of acute pre-workout supplementation
on power and strength performance. J Int Soc Sports Nutr.
13:292016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gualano B, Artioli GG, Poortmans JR and
Lancha Junior AH: Exploring the therapeutic role of creatine
supplementation. Amino Acids. 38:31–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riesberg LA, Weed SA, McDonald TL,
Eckerson JM and Drescher KM: Beyond muscles: The untapped potential
of creatine. Int Immunopharmacol. 37:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finck BN and Kelly DP: PGC-1 coactivators:
Inducible regulators of energy metabolism in health and disease. J
Clin Invest. 116:615–622. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arany Z, He H, Lin J, Hoyer K, Handschin
C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al: Transcriptional
coactivator PGC-1 alpha controls the energy state and contractile
function of cardiac muscle. Cell Metab. 1:259–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Head SI, Greenaway B and Chan S:
Incubating isolated mouse EDL muscles with creatine improves force
production and twitch kinetics in fatigue due to reduction in ionic
strength. PLoS One. 6:e227422011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murphy RM, Stephenson DG and Lamb GD:
Effect of creatine on contractile force and sensitivity in
mechanically skinned single fibers from rat skeletal muscle. Am J
Physiol Cell Physiol. 287:C1589–C1595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Passaquin AC, Renard M, Kay L, Challet C,
Mokhtarian A, Wallimann T and Ruegg UT: Creatine supplementation
reduces skeletal muscle degeneration and enhances mitochondrial
function in mdx mice. Neuromuscul Disord. 12:174–182. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li-Sha G, Yi-He C, Na-Dan Z, Teng Z and
Yue-Chun L: Effects of carvedilol treatment on cardiac cAMP
response element binding protein expression and phosphorylation in
acute coxsackievirus B3-induced myocarditis. BMC Cardiovasc Disord.
13:1002013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Halling JF, Ringholm S, Olesen J, Prats C
and Pilegaard H: Exercise training protects against aging-induced
mitochondrial fragmentation in mouse skeletal muscle in a PGC-1α
dependent manner. Exp Gerontol. 96:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Drake JC, Wilson RJ and Yan Z: Molecular
mechanisms for mitochondrial adaptation to exercise training in
skeletal muscle. FASEB J. 30:13–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deminice R and Jordao AA: Creatine
supplementation reduces oxidative stress biomarkers after acute
exercise in rats. Amino Acids. 43:709–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lehman JJ, Barger PM, Kovacs A, Saffitz
JE, Medeiros DM and Kelly DP: Peroxisome proliferator-activated
receptor gamma coactivator-1 promotes cardiac mitochondrial
biogenesis. J Clin Invest. 106:847–856. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sverdlov AL, Elezaby A, Behring JB,
Bachschmid MM, Luptak I, Tu VH, Siwik DA, Miller EJ, Liesa M,
Shirihai OS, et al: High fat, high sucrose diet causes cardiac
mitochondrial dysfunction due in part to oxidative
post-translational modification of mitochondrial complex II. J Mol
Cell Cardiol. 78:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huss JM and Kelly DP: Nuclear receptor
signaling and cardiac energetics. Circ Res. 95:568–578. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Powers ME, Arnold BL, Weltman AL, Perrin
DH, Mistry D, Kahler DM, Kraemer W and Volek J: Creatine
supplementation increases total body water without altering fluid
distribution. J Athl Train. 38:44–50. 2003.PubMed/NCBI
|
|
38
|
Becque MD, Lochmann JD and Melrose DR:
Effects of oral creatine supplementation on muscular strength and
body composition. Med Sci Sports Exerc. 32:654–658. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deldicque L, Atherton P, Patel R, Theisen
D, Nielens H, Rennie MJ and Francaux M: Effects of resistance
exercise with and without creatine supplementation on gene
expression and cell signaling in human skeletal muscle. J Appl
Physiol (1985). 104:371–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Safdar A, Yardley NJ, Snow R, Melov S and
Tarnopolsky MA: Global and targeted gene expression and protein
content in skeletal muscle of young men following short-term
creatine monohydrate supplementation. Physiol Genomics. 32:219–228.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chrusch MJ, Chilibeck PD, Chad KE, Davison
KS and Burke DG: Creatine supplementation combined with resistance
training in older men. Med Sci Sports Exerc. 33:2111–2117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Neves M Jr, Gualano B, Roschel H, Lima FR,
Lúcia de Sá-Pinto A, Seguro AC, Shimizu MH, Sapienza MT, Fuller R,
Lancha AH Jr and Bonfá E: Effect of creatine supplementation on
measured glomerular filtration rate in postmenopausal women. Appl
Physiol Nutr Metab. 36:419–422. 2011. View Article : Google Scholar : PubMed/NCBI
|