Introduction
Diabetic kidney disease (DKD) is one of the most
severe chronic complications in patients with diabetes mellitus
(DM) and the leading cause of end-stage kidney disease (ESRD)
worldwide (1,2). Consequently, DKD-associated ESRD
incurs colossal human, social and financial burdens. In 2019, it
was estimated that ~463 million individuals were afflicted with DM
according to the statement of International Diabetes Federation,
the number of which is predicted to increase to 693 million in the
next 25 years (3). According to
the latest epidemiological survey in China, 34.2% of patients with
DM developed DKD (4). Therefore,
the early diagnosis and treatment of DKD are of high importance.
Since the pathophysiology of progressive microvascular decay caused
by DM and its association with inflammatory response was first
proposed in 1999(5), accumulating
evidence has supported the notion that the inflammatory response
serves a vital role in the occurrence and progression of DKD
(6). Such reported inflammatory
factors include C-reactive protein (CRP), TNF-α and IL-6. A
randomized, double-blind, placebo-controlled 3x3 crossover study
previously demonstrated that angiotensin-converting enzyme
inhibitors and angiotensin receptor blockers (ARB) were able to
induce anti-inflammatory effects (7). In addition, sodium-glucose
co-transporter 2 (SGLT2) inhibitors were shown to provide renal
protection other than decreasing glucose and reducing the events of
composite cardio/kidney endpoints (8). However, SGLT2 inhibitors may also
decrease the estimated glomerular filtration rate (eGFR) and
increase the risk of genitourinary system infection and
ketoacidosis (9). A systematic
analysis of a series of chronic kidney disease (CKD) cases from
1990 to 2017 published in The Lancet concluded that the currently
existing treatments are not sufficient to prevent the deterioration
from CKD into ESRD (10).
Therefore, there are substantial challenges remaining in treating
DKD, such that more effective novel treatment methods are urgently
in demand.
Emerging advantages of Traditional Chinese Medicine
(TCM) have been garnering attention in both academic and clinical
research fields. DKD pertains to the category of ‘Shenxiao’ in TCM
(11). On the basis of the theory
of TCM, deficiency of kidney and spleen along with dampness, turbid
phlegm and blood stasis dominate the pathogenesis of DKD.
Correspondingly, the treatment principle is to tonify kidney and
spleen, replenish water and tonify qi, supplemented by activation
of blood and dissolution of stasis. Niaoduqing (NDQ) granules is a
patented TCM formulation that has the reported functions of
‘tonifying spleen’ (jian-pi), ‘free fu’ (tong-fu) and ‘eliminating
pathogens’ (jie-du). It has been applied in clinical practice in
China for ≥20 years. The NDQ granules received approval by the
China State Food and Drug Administration (National Medical Products
Administration) to treat CKD (national medicine permit no.
Z20073356) in 2000. It is composed of 16 TCM herbs (12), including rhubarb root and rhizome
(Radix et Rhizoma rhei), white paeony root (Paeoniae
Radix Alba), milkvetch root (Astragali Radix), Pinellia
ternata (Pinelliae Rhizoma), chrysanthemum flower (Flos
Chrysanthemi), Danshen root (Radix Salviae miltiorrhizae et
Rhizoma), Szechuan lovage rhizome (Chuanxiong Rhizoma),
tuber fleeceflower root (Polygoni Multiflori Radix),
Medicinal Changium root (Changii Radix), Largehead
Atractylodes Rh (Atractylodis macrocephalae rhizoma), Indian
buead (Poria), white mulberry root bark (Mori
Cortex), lightyellow sophora root (Radix Sophorae
Flavescentis), Asiatic plantain herb (Plantaginis
Herba), Chinese Thorowax root (Bupleuri Radix) and
liquorice root (Glycyrrhizae Radix et Rhizoma). NDQ granules
have been used to treat patients with CKD according to the
‘Clinical Application Guide of Chinese Patent Medicine in The
Treatment of CKD’ (13). Over the
past few years, various clinical trials have attempted to assess
the effects of treatment with NDQ granules on DKD, and its
effectiveness has been initially verified. Evidence from these
clinical studies suggested that the NDQ granules are not only able
to improve kidney function, but may also reduce the severity of the
inflammatory response by adjusting the micro-inflammatory state
compared with basic treatment or placebo (14,15).
However, the sample size of available clinical trial data on NDQ
granules in terms of its anti-inflammatory effects is relatively
small, rendering the evidence inconclusive. Therefore, the present
systematic review and meta-analysis was performed to assess the
effects of NDQ granules on the inflammatory response to provide
additional information on the effects of TCM treatment on DKD.
Materials and methods
Database and search strategies
The present systematic review and meta-analysis was
performed in accordance with the Preferred Reporting Items for
Systematic Reviews and Meta-analyses statement (16), and has been registered to PROSPERO
(https://www.crd.york.ac.uk/PROSPERO/;
registration no. CRD42022340017). However, it should be noted that
the final search date was extended to 31st of May 2023 to include
the most up-to-date clinical studies. In total, seven electronic
databases, namely the China National Knowledge Infrastructure
database (https://www.cnki.net), China Science and
Technology Journal Database (www.cqvip.com), Wanfang Database (https://www.wanfangdata.com.cn), the Chinese
Biomedicine Database (http://www.sinomed.ac.cn/), PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(www.webofscience.com) and Cochrane
Library (https://www.cochranelibrary.com) were searched
comprehensively for literature from their inception to 31st of May
2023. Only papers published in Chinese and English were selected.
The search terms used were as follows: ‘NDQ’, ‘NDQ granules’, ‘NDQ
particle’, ‘uremic clearance granules’, ‘diabetic kidney disease’,
‘diabetic nephropathy’, ‘nephropathy, diabetic’, ‘kidney disease,
diabetic’, ‘randomized controlled trial’ (RCT), ‘RCT’ and ‘random’.
The combination of subject words and free words was adopted. In
addition, eligible literature was also obtained by examining the
reference lists of relevant reviews and included studies
manually.
Inclusion criteria
Studies were selected based on the following
conditions: i) Participating patients were ≥18 years old, diagnosed
with DKD based on Chinese clinical practice guidelines, expert
consensus of DKD or the World Health Organization (WHO) diagnostic
criteria of DM (17-19)
and the staging criteria based on the internationally recognized
Mogensen staging system or those produced by the WHO (5,20),
regardless of sex or the presence of primary diseases, such as
hypertension, hyperlipidemia or hyperuricemia; ii) intervention was
carried out with the NDQ granules combined with the therapeutic
regimen in the control group; iii) the control group received basic
treatment, including management of hyperglycemia, hypertension,
hyperlipidemia, anti-infection, correction of anemia, electrolyte,
acid-basic balance and renal replacement therapy; iv) primary
outcomes comprised inflammatory factors, such as CRP, TNF-α and
IL-6, with or without secondary outcomes, including kidney
function, proteinuria, clinical effective rate and adverse reaction
rate; and v) the study design was that of an RCT regardless of
blinding, protocol or bias.
Exclusion criteria
Studies were excluded based on the following
conditions: i) NDQ granules treatment was combined with other
Western, Chinese medicine or ‘characteristically Chinese’
therapies, including acupuncture or Chinese medicine enema in the
intervention group; ii) case reports, reviews, observational
studies, experiments on animals, experience summaries, theoretical
explorations, academic papers and conference literature; iii)
incomplete papers; iv) published in a language that is not English
or Chinese; v) duplicate publications; vi) non-RCT; and vii) the
NDQ granules used without the supplier or doses explicitly
stated.
Data extraction
In total, the electronic databases were separately
searched and eligible studies were selected in terms of the
aforementioned inclusion and exclusion criteria. Duplicated
publications were eliminated by Endnote 20 (Clarivate), before the
eligible studies were logged into Microsoft Excel 16 (Microsoft
Corporation) and checked against each other. Any divergence in
opinion would be solved through discussion between two of the
authors or by another researcher (PZ, ZH and WX). The extracted
data from each study were the following: Author, publication year,
sex, age, course of DM, intervention measures, treatment duration
and outcomes of inflammatory factors.
Quality evaluation
The ‘risk of bias’ evaluation tool provided by the
Cochrane Collaboration Handbook for Systematic Reviews of
Intervention was applied for the assessment of methodological
quality (21). The following seven
domains were used for systematic and comprehensive evaluation: i)
Methods of generating random sequence; ii) hidden distribution;
iii) use of the double blind technique for participants and
personnel; iv) blinding of outcome assessors; v) data integrality
of outcome; vi) outcome reporting with bias; and vii) other sources
of bias. ‘High risk’, ‘low risk’ and ‘unclear risk’ were the three
levels of bias designated for evaluating each of the studies
included in the present analysis in the aforementioned domains.
Statistical analysis
The Review Manager software (version 5.3; Cochrane)
was applied for logging data and conducting data analysis. The
diverse effect measures were assigned to different variables,
whereas the standardized mean difference (SMD) was used for
assessing continuous variables and the risk ratio (RR) was used for
dichotomous variables. In addition, the 95% confidence interval
(CI) was used for statistical analysis. The tau-squared
(Tau2), inconsistency index (I2) and
chi-squared (χ2) test were used to measure the
heterogeneity of study results. The random-effects model was
applied for the analysis of heterogeneity (I2>50%)
and P<0.05 was considered to indicate a statistically
significant difference; otherwise, the fixed-effects model would be
chosen. Simultaneously, sensitivity analysis was performed by
sequentially deleting one study at a time to confirm the robustness
of the present findings, whereas subgroup analyses were performed
to explore the impact of heterogeneity on the overall findings. If
>10 studies were included in the same analysis, the assessment
of publication biases was performed using funnel plots.
Results
Search results
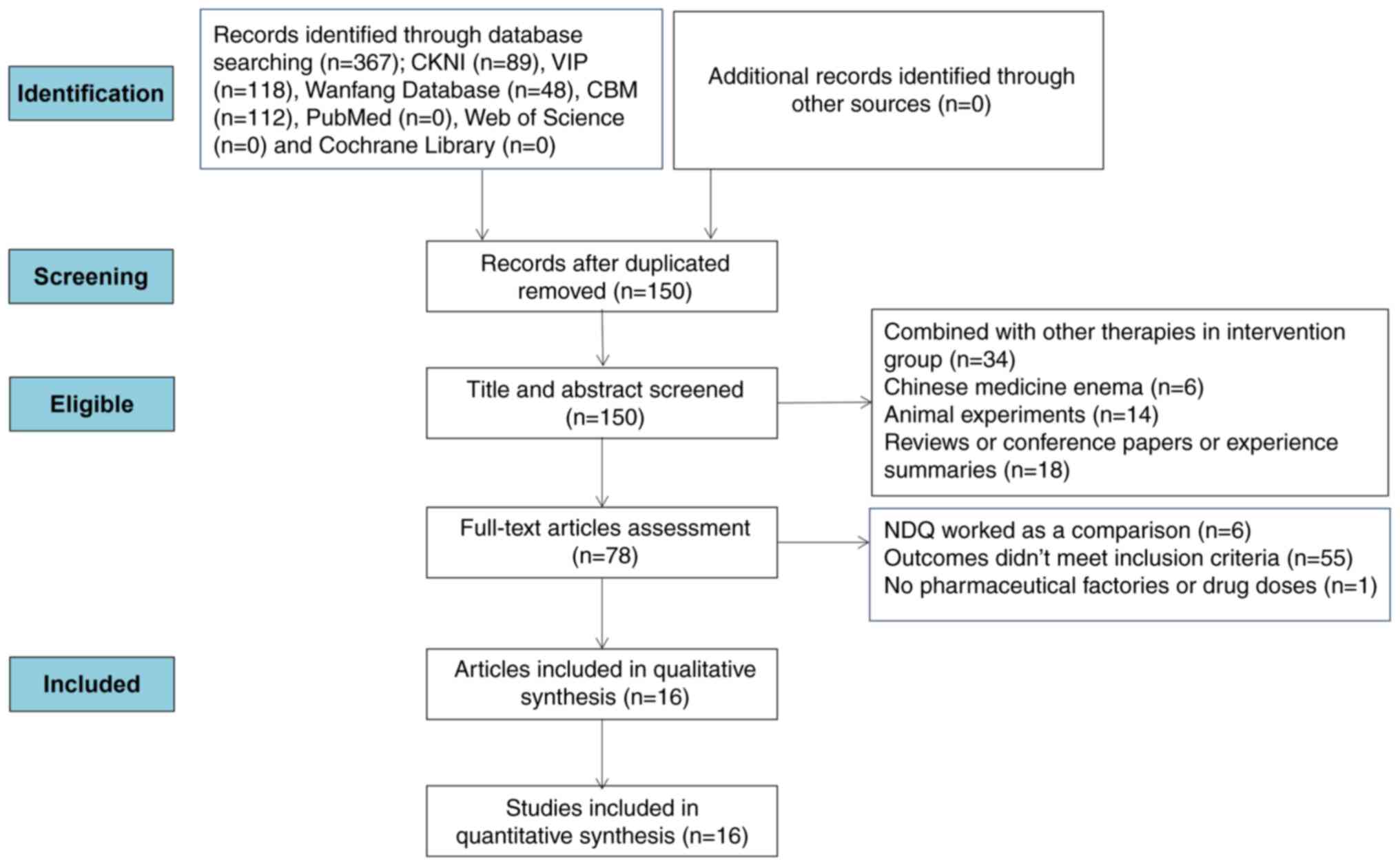
A total of 367 potentially relevant entries were
retrieved from the aforementioned databases and 217 duplicated
articles were eliminated by Endnote. After the titles and abstracts
were examined, 72 studies were eliminated due to the combination of
treatment with NDQ granules with other therapies in the
intervention group, treatment with Chinese medicine enema, animal
experiments, conference and academic papers, reviews or experience
summaries. In addition, 62 studies were eliminated after the
assessment of full-text articles, where NDQ granules was used as a
comparison in six studies, 55 studies were rendered illegible
according to the inclusion criteria, and one study was removed due
to lack of supplier details and drug doses. Ultimately, 16 articles
were deemed eligible for the present systematic review and
meta-analysis (22-37).
A summary of this screening process is depicted in Fig. 1.
Study characteristics
The 16 articles included in the present analysis
comprised 1,526 patients, 765 from the intervention group and 761
from the control group, with a sex ratio of men to women of 1:0.75
(783 males and 587 females). However, one study did not mention the
specific sex distribution (30).
The sample size range of the individual studies was 40-220. The
average age was 57.69±9.74 years and the average course of DM was
7.79 years, while three studies (27,33,36)
did not specify the course. The treatment duration range was 1-6
months. The control group received basic treatment and other
conventional drugs, such as α-ketoacid, insulin and ARB. The
intervention group received treatment with the NDQ granules
combined with the treatment of the control group. These detailed
characteristics of the studies included are summarized in Table I.
 | Table IDetails of studies included in the
present meta-analysis. |
Table I
Details of studies included in the
present meta-analysis.
| Author, year | Sex, n (M/F) | Age, years, mean ±
SD | Course of diabetes
mellitus, years, mean ± SD | Intervention
measures | Treatment
duration | Outcomes | (Refs.) |
|---|
| Liang, 2021 | | | | | 4 weeks | CRP, TNF-α, BUN,
Scr, 24-h UPE, CER, ADR | (22) |
|
Intervention
group | 49 (27/22) | 50.05±12.99 | 4.17±0.82 | CON + NDQ 25
g/d | | | |
|
Control
group | 48 (26/22) | 50.01±13.01 | 4.14±0.74 | Basic treatment +
compound α ketoacid | | | |
| Zhang, 2011 | | | | | 60 days | CRP, BUN, Scr, 24-h
UPE | (23) |
|
Intervention
group | 40 (49/31) | 54.6±7.5 | 11±2.5 | CON + NDQ 30
g/d | | | |
|
Control
group | 40 (49/31) | 54.6±7.5 | 11±2.5 | Basic
treatment | | | |
| Zhang, 2014 | | | | | 12 weeks | CRP, TNF-α,
IL-6, | (24) |
|
Intervention
group | 52 (34/18) | 68.2±7.6 | 8.1±3.4 | CON + NDQ 20
g/d | | | |
|
Control
group | 50 (31/19) | 67.3±7.4 | 7.8±3.7 | Basic
treatment | | | |
| He, 2015 | | | | | 2 months | CRP, TNF-α, BUN,
Scr, 24-h UPE, CER | (25) |
|
Intervention
group | 24 (13/11) | 47.15±3.53 | 6.87±1.61 | CON + NDQ 25
g/d | | | |
|
Control
group | 24 (14/10) | 46.75±3.26 | 6.97±1.51 | Basic
treatment | | | |
| He, 2021 | | | | | 3 months | TNF-α, IL-6, BUN,
Scr, 24-h UPE, CER, ADR | (26) |
|
Intervention
group | 44 (23/21) | 62.2±8.5 | 6.5±1.4 | CON + NDQ 20
g/d | | | |
|
Control
group | 43 (24/19) | 61.5±8.3 | 6.4±1.2 | Basic treatment +
Valsartan | | | |
| Yang, 2010 | | | | | 8 weeks | IL-6, BUN, Scr | (27) |
|
Intervention
group | 20 (13/7) | 48.5±12.9 | Not mentioned | CON + NDQ 40
g/d | | | |
|
Control
group | 20 (13/7) | 41.8±11.2 | Not mentioned | basic
treatment | | | |
| Wu, 2021 | | | | | 1 month | CRP, TNF-α, BUN,
Scr, ADR | (28) |
|
Intervention
group | 41 (27/14) | 59.87±3.69 | 8.37±1.58 | CON + NDQ 20
g/d | | | |
|
Control
group | 41 (26/15) | 59.84±3.67 | 8.36±1.57 | Basic treatment +
Valsartan | | | |
| Li, 2015 | | | | | 60 days | CRP, BUN, Scr, 24-h
UPE | (29) |
|
Intervention
group | 45(22/23) | 52.2±6.3 | 9.4±3.3 | CON + NDQ 30
g/d | | | |
|
Control
group | 45(22/23) | 52.2±6.3 | 9.4±3.3 | basic
treatment | | | |
| Liu, 2020 | | | | | 6 months | CRP, TNF-α, IL-6,
BUN, Scr, CER, ADR | (30) |
|
Intervention
group | 78 | 67.20±9.42 | 10.02±1.49 | CON + NDQ 25
g/d | | | |
|
Control
group | 78 | 65.19±7.31 | 9.85±2.04 | Basic treatment +
insulin | | | |
| Li, 2021 | | | | | 3 months | CRP, TNF-α, BUN,
Scr, CER, ADR | (31) |
|
Intervention
group | 40 (24/16) | 63.24±11.36 | 9.47±3.21 | CON + NDQ 25
g/d | | | |
|
Control
group | 40 (27/13) | 64.55±11.24 | 10.13±3.75 | Basic treatment +
Irbesartan | | | |
| Chen, 2021 | | | | | 60 days | CRP, TNF-α, BUN,
Scr, 24-h UPE, CER, ADR | (32) |
|
Intervention
group | 41 (26/15) | 51.40±1.38 | 8.40±1.18 | CON + NDQ 20
g/d | | | |
|
Control
group | 41 (25/16) | 51.38±2.46 | 8.43±1.20 | Basic treatment +
insulin | | | |
| Zheng, 2019 | | | | | 3 months | CRP, TNF-α,
IL-6 | (33) |
|
Intervention
group | 53 (31/22) | 55.84±8.33 | Not mentioned | CON + NDQ 20
g/d | | | |
|
Control
group | 53 (29/24) | 56.38±8.42 | | Basic treatment +
artificial kidney | | | |
| Wang, 2017 | | | | | 12 weeks | CRP, TNF-α, IL-6,
CER | (34) |
|
Intervention
group | 47 (25/22) | 63.54±4.16 | 4.63±1.82 | CON + NDQ 20
g/d | | | |
|
Control
group | 47 (24/23) | 62.71±5.43 | 4.13±1.62 | Basic treatment +
Atorvastatin | | | |
| Gong, 2013 | | | | | 3 months | CRP, BUN, Scr,
ADR | (35) |
|
Intervention
group | 31 (15/16) | 59.4±7.5 | 10.3±3.7 | CON + NDQ 20
g/d | | | |
|
Control
group | 31 (17/14) | 60.0±7.3 | 10.7±3.5 | Basic treatment +
Irbesatan | | | |
| Tong, 2022 | | | | | 4 weeks | TNF-α, IL-6, BUN,
Scr, 24-h UPE, ADR | (36) |
|
Intervention
group | 110 (58/52) | 57.1±5.4 | Not mentioned | CON + NDQ 25
g/d | | | |
|
Control
group | 110 (56/54) | 55.8±5.4 | Not mentioned | basic treatment +
insulin, Dapagliflozin | | | |
| Yu, 2022 | | | | | 3 months | CRP, IL-6, BUN,
Scr, CER, ADR | (37) |
|
Intervention
group | 50 (32/18) | 52.47±3.17 | 4.37±1.61 | CON + NDQ
15g/d | | | |
|
Control
group | 50 (30/20) | 51.54±3.68 | 4.14±1.57 | Basic treatment +
aspirin | | | |
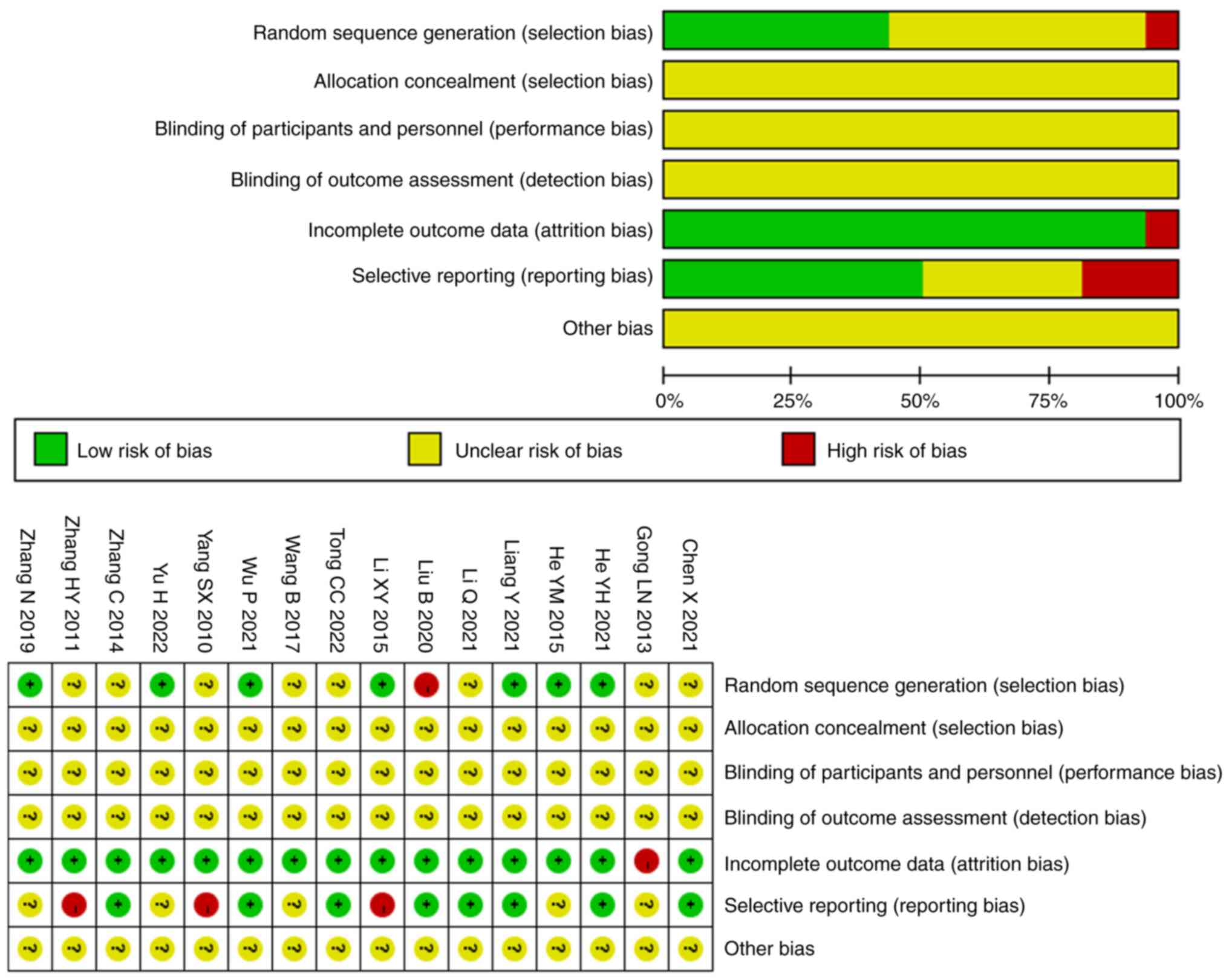
Quality assessment of included
studies
All the included studies were RCTs, seven of which
(22,25,26,28,29,33,37)
specifically used random table methods, and one study (30) used non-standard randomization
methods. None of the studies mentioned hidden allocation, triple
blinding or other bias. Of note, one study (35) mentioned that incomplete outcome
data was the reason for severe adverse reactions. However, a total
of eight studies (22,24,26,28,30-32,36,38)
briefly stated approval by the Ethics Committee of the subordinate
hospital and had obtained informed consent from the patients and
their family members, five studies (25,33-35,37)
only mentioned informed consent and three studies (23,26,29)
mentioned neither. Fig. 2 presents
the results of the quality assessment of all included studies in
the present review and meta-analysis.
Primary outcomes
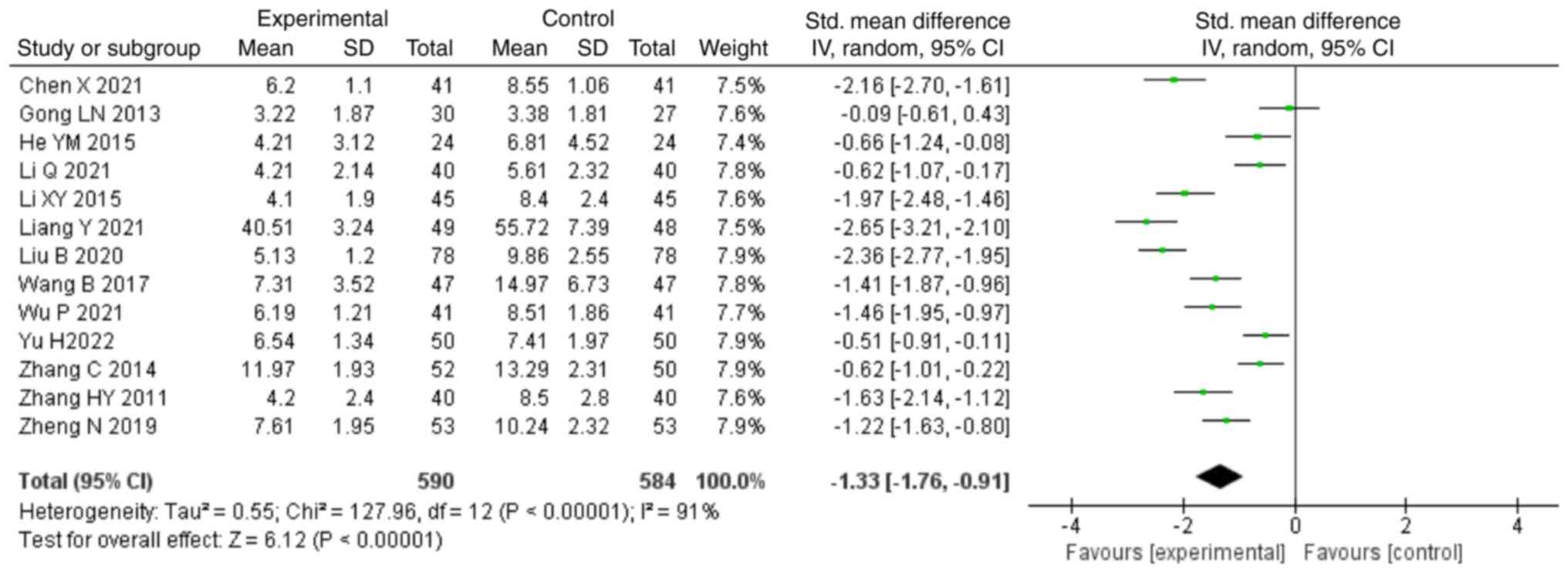
For the outcome of CRP, 13 studies (22-25,38-35,37)
were included in the analysis, consisting of 1,174 patients. Due to
the high heterogeneity noted (P<0.00001; I2=91%), the
random-effects model was used to analyze the data. The combination
of NDQ granules with conventional treatment was able to decrease
levels of CRP compared with those in the control group (SMD, -1.33;
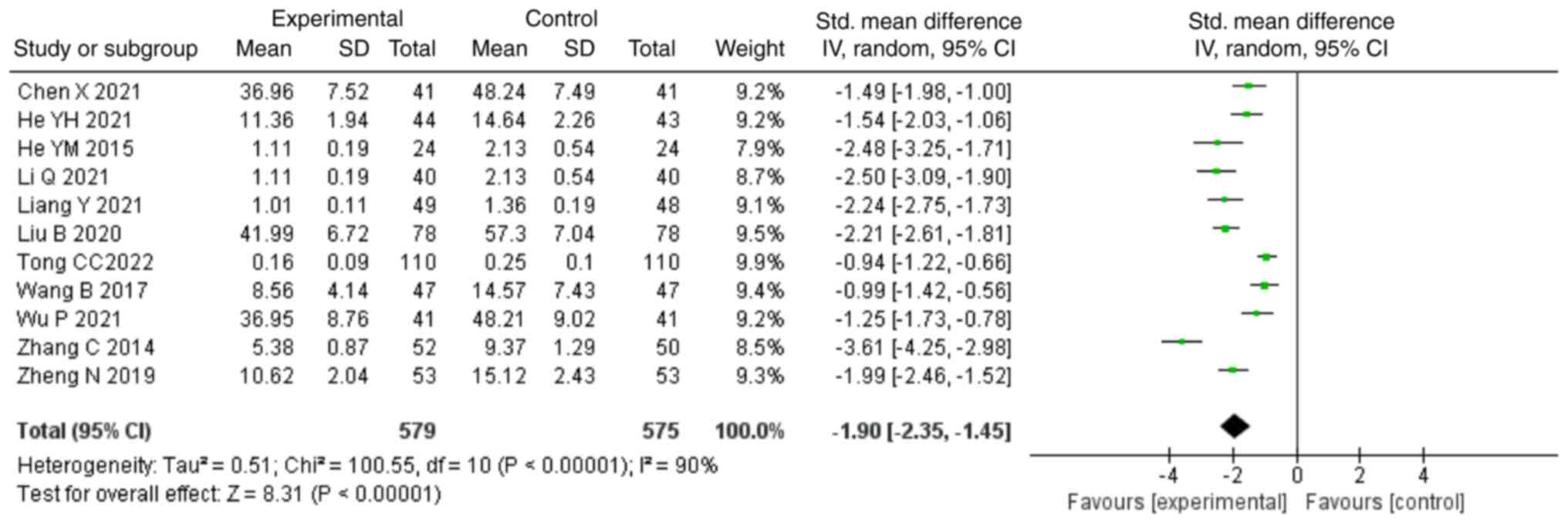
95% CI, -1.76,-0.91; P<0.00001; Fig. 3). In addition, 11 studies (22,24-26,28,30-34,36)
reported TNF-α levels as an outcome, comprising 1,154 patients. A
random-effects model was chosen due to high heterogeneity
(P<0.00001; I2=90%). The combination of NDQ granules
and conventional treatment could decrease the levels of TNF-α
compared with those in the control group (SMD, -1.90; 95% CI,
-2.35,-1.45; P<0.00001; Fig.
4). A total of 8 studies (24,26,27,30,33,34,36,37)
reported IL-6 as an outcome, including 905 patients. The
heterogeneity remained high (P<0.00001; I2=96%),
justifying the use of the random-effects model. The data revealed
that the combination of NDQ granules and conventional treatment
could decrease the levels of IL-6 compared with those in the
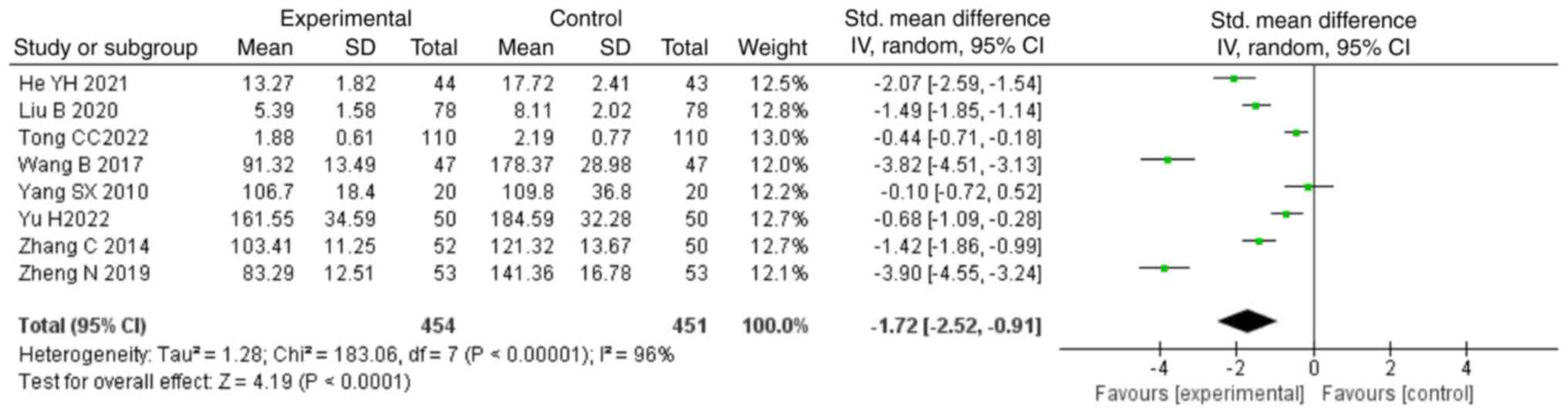
control group (SMD, -1.72; 95% CI, -2.52,-0.91; P<0.0001;
Fig. 5).
Secondary outcomes. Kidney
function
In total, 13 studies (22,23,25-32,35-37)
contributed to the analysis of blood urea nitrogen (BUN) and these
included a total of 1,219 patients. The data were analyzed using a
random-effects model because of high heterogeneity (P<0.00001;
I2=82%). The meta-analysis showed that the combination
of NDQ granules and conventional treatment decreased the levels of
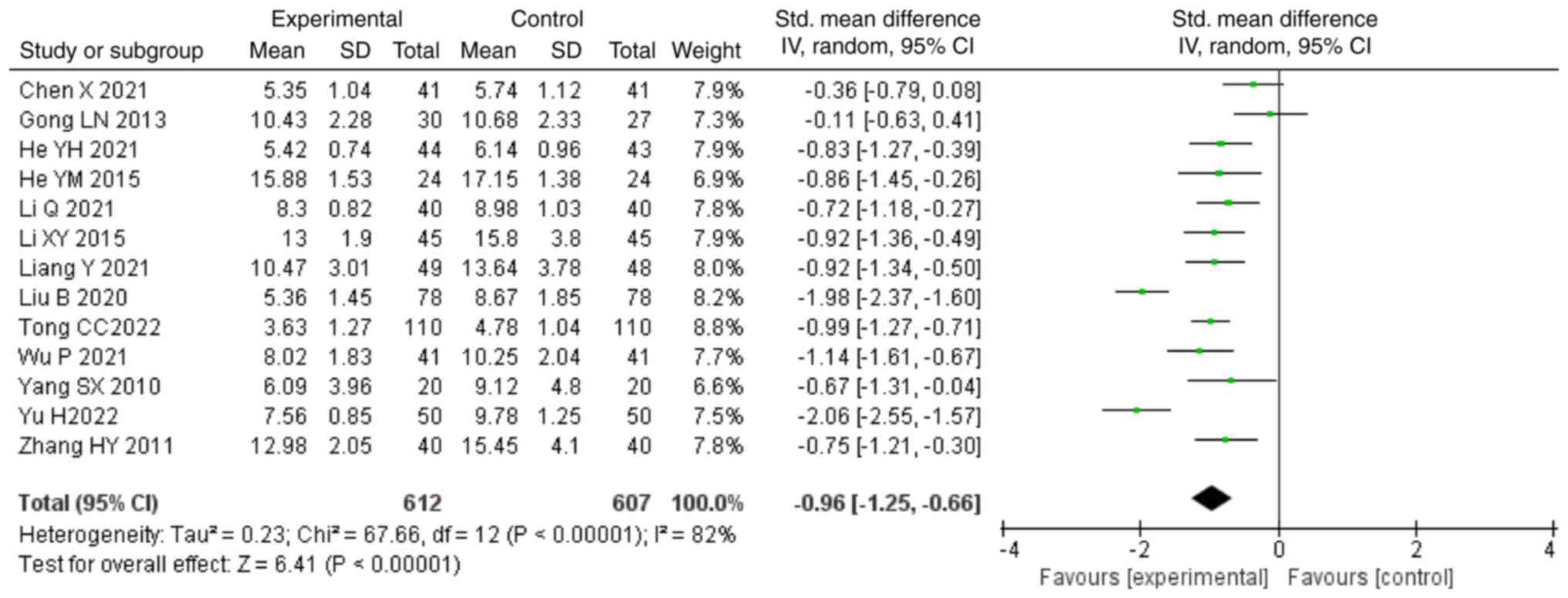
BUN compared with those in the control group (SMD, -0.96; 95% CI,
-1.25,-0.66; P<0.00001; Fig.
6). Regarding serum creatinine (SCr) used as an outcome, 13
studies (22,23,25-32,35-37)
were included in the analysis, and heterogeneity was found
(P<0.00001; I2=90%). The data revealed that the
combination of treatment with NDQ granules and conventional
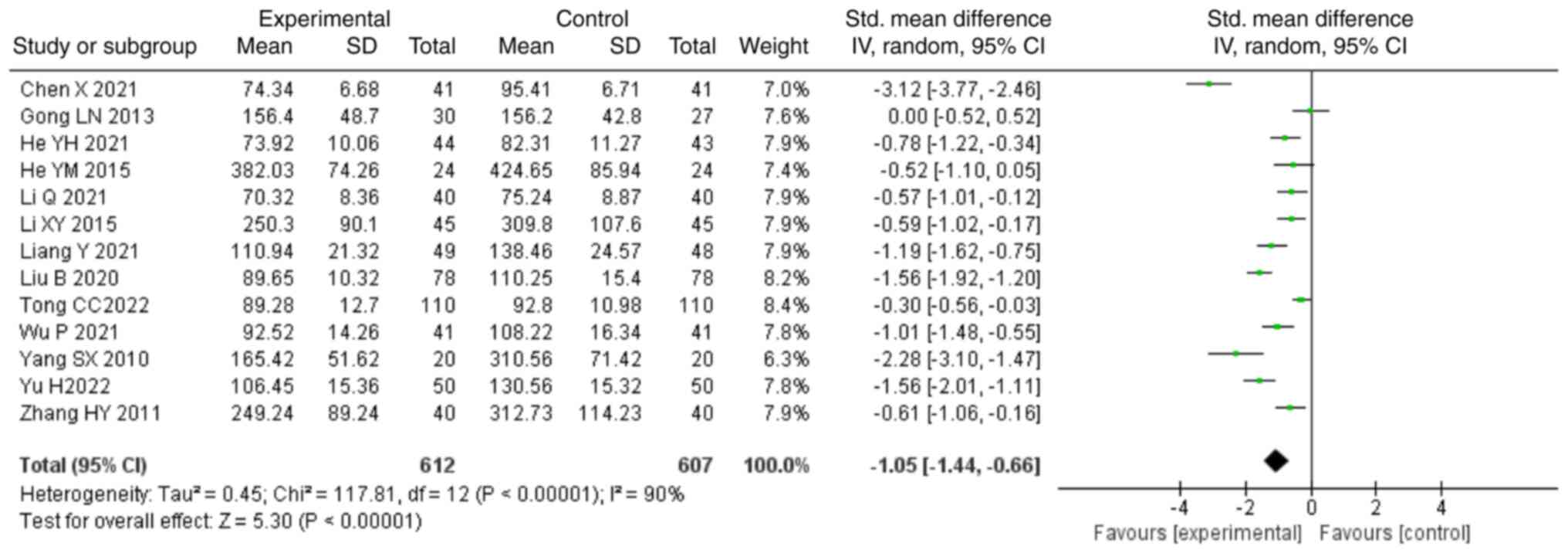
treatment decreased the levels of SCr compared with those in the
control group (SMD, -1.05; 95% CI, -1.44,-0.66; P<0.00001;
Fig. 7). In total, seven studies
(22,23,25,26,29,32,36)
reported 24-h urinary protein excretion (24-h UPE), covering a
total of 704 patients. Due to the high heterogeneity P<0.00001;
I2=88%), a random-effects model was used to analyze the
data. The combination of treatment with NDQ granules and
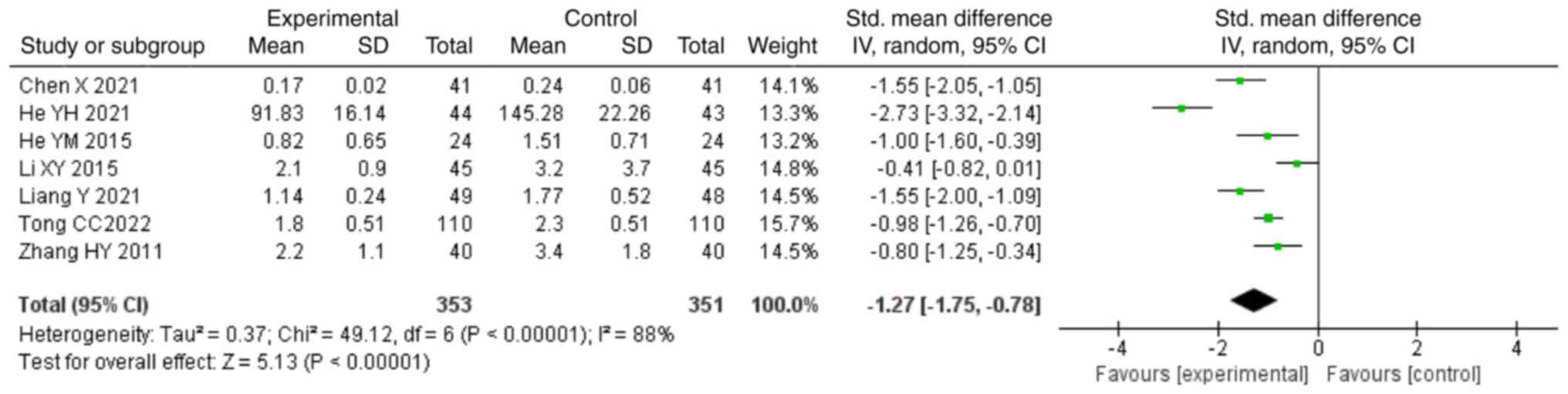
conventional treatment was able to decrease the levels of 24-h UPE
compared with those in the control group (SMD, -1.27; 95% CI,
-1.75,-0.78; P<0.00001; Fig.
8).
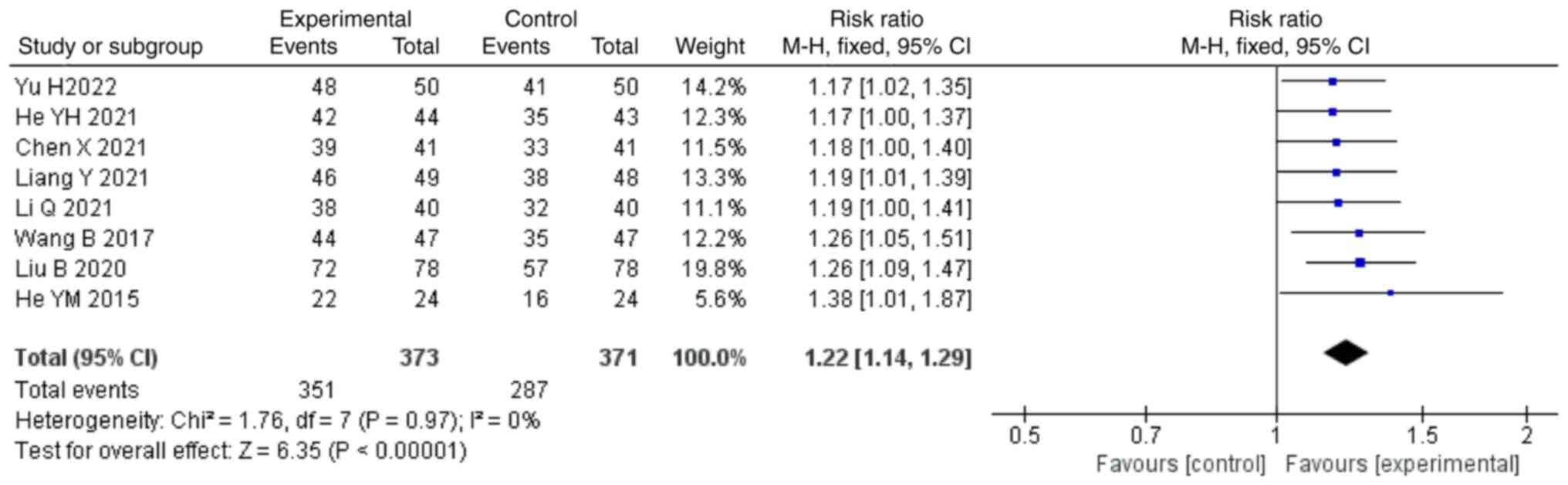
Clinical effective rate. A total of eight
studies (22,25,26,30-32,34,37)
reported the clinical effective rate as an outcome, including a
total of 744 patients. No significant heterogeneity (P=0.97;
I2=0%) was noted among them, and thus, a fixed-effects
model was applied for the analysis. The meta-analysis indicated
that the combination of treatment with NDQ granules and
conventional treatment could increase the clinical effective rate
compared with those in the control group (RR, 1.22; 95% CI,
1.14,1.29; P<0.00001; Fig.
9).
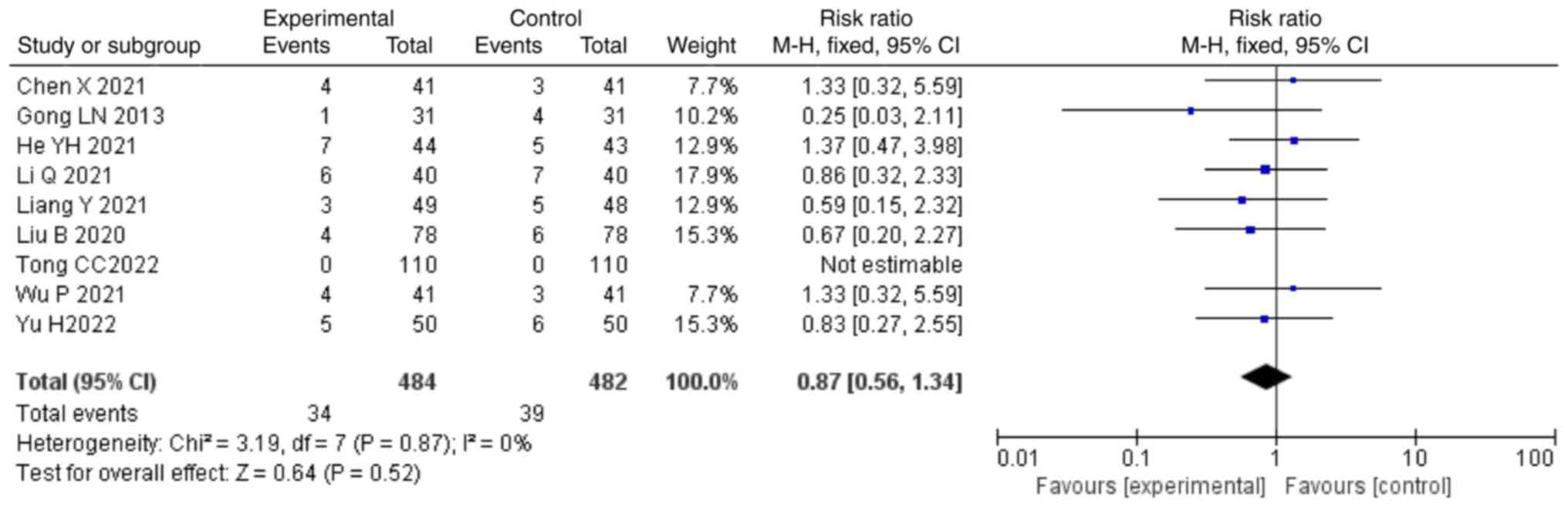
Safety. A total of nine studies (22,26,28,30,35-37)
mentioned the adverse reaction rate as an outcome, including a
total of 966 patients. Due to the low heterogeneity (P=0.87;
I2=0%), a fixed-effects model was used for the analysis.
The data indicated that the combination of treatment with NDQ
granules and conventional treatment had an incidence of adverse
reactions that was not significantly different compared with that
in the control group (RR, 0.87; 95% CI, 0.56,1.34; P=0.52; Fig. 10). A total of 34 patients in the
control group developed adverse reaction events and 39 patients in
the intervention group. Particularly, nearly half of them had
gastrointestinal reactions, accounting for 15 and 14 separately.,
Statistics regarding the adverse reaction events are summarized in
Table II.
 | Table IIStatistics of adverse reaction events
in the intervention group (n=484) and in the control group
(n=482). |
Table II
Statistics of adverse reaction events
in the intervention group (n=484) and in the control group
(n=482).
| Adverse reaction
events | Intervention group,
n (%) | Control group, n
(%) |
|---|
| Gastrointestinal
reactions | 15 (3.10) | 14 (2.90) |
| Dizziness | 3 (0.62) | 5 (1.04) |
| Hypoglycemia | 5 (1.03) | 6 (1.24) |
| Asthenia | 6 (1.24) | 2 (0.41) |
| Cough | 0 (0.00) | 1 (0.21) |
| Rash | 1 (0.21) | 1 (0.21) |
| Allergy | 1 (0.21) | 1 (0.21) |
| Hyperkalemia | 2 (0.41) | 4 (0.83) |
| High
creatinine | 0 (0.00) | 2 (0.41) |
| Hypercalcemia | 1 (0.21) | 3 (0.62) |
| Total | 34 (7.02) | 39 (8.09) |
Sensitivity analysis
Sensitivity analyses of all the outcomes were
conducted to confirm the stability of the meta-analysis results.
Each of the studies was removed one by one and the meta-analyses
were re-performed with the remaining studies. The obtained results
were then compared with the previous ones. However, no significant
changes in heterogeneity could be found (data not shown),
suggesting that the meta-analysis results were stable.
Subgroup analysis
Due to the persistence of high heterogeneity and a
sufficient number of studies, subgroup analyses were performed to
explore the sources of heterogeneity based on the dosage of NDQ
granules (<30 or ≥30 g). The results revealed that the
heterogeneity in the subgroup analysis of the dosage of NDQ
granules of ≥30 g regarding CRP, BUN and 24-h UPE was significantly
reduced (I2<50%). However, the rest of the studies
remained to be considerably heterogeneous. This finding suggests
that the dosage of NDQ granules may be one of the sources of
heterogeneity of the CRP, BUN and 24-h UPE data. In addition, none
of the included studies regarding TNF-α could be classified because
all studies describing TNF-α were using NDQ granules <30 g.
There was no significance on IL-6 for the dosage of NDQ granules
≥30 g (SMD, -0.10; 95% CI, -0.72,0.52; P=0.74). The results of the
subgroup analysis are presented in Table III and Fig. S1, Fig. S2, Fig. S3, Fig. S4 and Fig. S5.
 | Table IIISubgroup analyses of C-reactive
protein, IL-6, blood urea nitrogen, serum creatinine and 24 h
urinary protein excretion based on the dosage of NDQ granules. |
Table III
Subgroup analyses of C-reactive
protein, IL-6, blood urea nitrogen, serum creatinine and 24 h
urinary protein excretion based on the dosage of NDQ granules.
| Outcome/dosage of
NDQ granule, g | n | MD/SMD (95%
confidence interval) | I2,
% | Z | P-value |
|---|
| C-reactive
protein | | | | | |
|
<30 | 11 | -1.25
(-1.73,-0.77) | 92 | 5.08 | <0.00001 |
|
≥30 | 2 | -1.80
(-2.16,-1.44) | 0 | 9.83 | <0.00001 |
| IL-6 | | | | | |
|
<30 | 5 | -1.94
(-2.80,-1.08) | 96 | 4.41 | <0.0001 |
|
≥30 | 1 | -0.10
(-0.72,0.52) | - | 0.33 | 0.74 |
| Blood urea
nitrogen | | | | | |
|
<30 | 10 | -1.00
(-1.38,-0.63) | 86 | 5.22 | <0.00001 |
|
≥30 | 3 | -0.81
(-1.09,-0.53) | 0 | 5.63 | <0.00001 |
| Serum
creatinine | | | | | |
|
<30 | 10 | -1.04
(-1.51,-0.58) | 91 | 4.41 | <0.0001 |
|
≥30 | 3 | -1.08
(-1.91,-0.26) | 86 | 2.58 | 0.01 |
| 24-h urinary
protein excretion | | | | | |
|
<30 | 5 | -1.54
(-2.11,-0.97) | 87 | 5.31 | <0.00001 |
|
≥30 | 2 | -0.59
(-0.97,-0.21) | 35 | 3.02 | 0.003 |
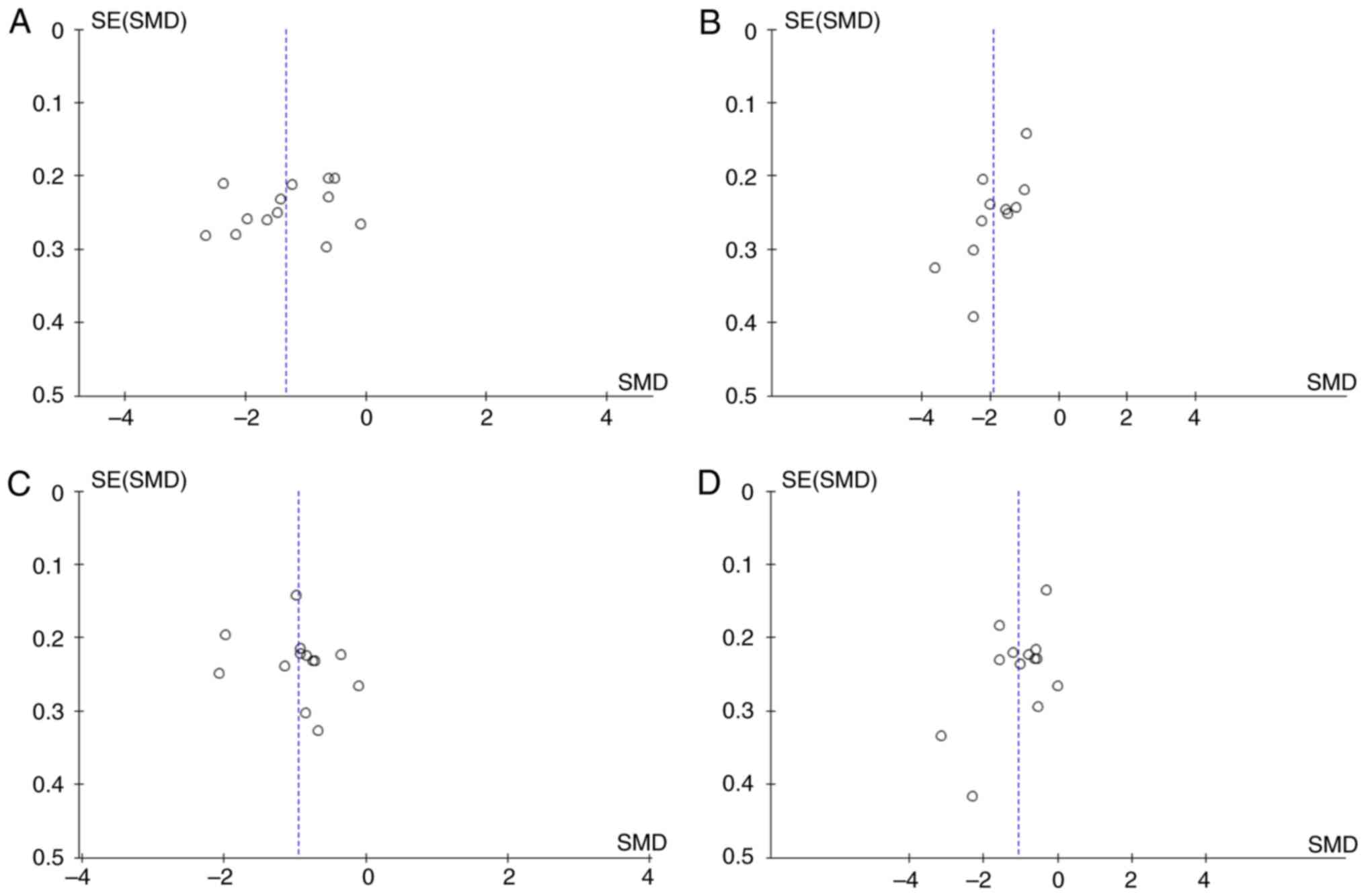
Publication bias
As the numbers of the included studies in the four
meta-analyses performed were ~10, a publication bias analysis of
the outcomes of CRP, TNF-α, BUN and SCr was performed. Visual
inspection of the data in the funnel plots revealed an asymmetrical
and sparse distribution, indicating that publication biases likely
exist in the included studies. One reason for this may be the small
sample size and the other is likely associated with the majority of
the studies being of medium or low quality (Fig. 11).
Discussion
The present meta-analysis incorporated 16 studies,
comprising a total of 1,526 patients. In comparison to basic
treatment, it was discovered that the combination of NDQ with
western medicine could further decrease CRP, TNF-α, IL-6, BUN, SCr
and 24-h UPE. Although the clinical effective rate in the
intervention group was superior compared with that in the control
group, the included studies had no consensus criteria. In addition,
the results of the present review revealed that the incidence of
adverse reactions was not different between the intervention and
the control group, suggesting that NDQ granules is likely to be
safe for clinical use. Although the sample sizes of all outcomes
selected in the present study are relatively small to fully allay
the remaining ambiguity, these results provide evidence based on
the clinical practice of NDQ granules for treating the inflammatory
response caused by DKD.
Surrogate endpoints rather than clinical endpoints
are typically selected in small-scale clinical studies due to the
latter requiring larger sample sizes and longer observation periods
(38). Furthermore, the effects of
treatment with the NDQ granules on the inflammatory response caused
by DKD are typically observed at early stages, which provides
insufficient evidence. Therefore, the outcomes identified in the
included studies in the present systematic analysis were all
surrogate endpoints. Although they could not offer direct evidence
for clinical prognosis, they potentially lay a foundation for
future clinical trials.
CRP is a sensitive and non-specific biomarker and
the circulating concentration is solely determined by its synthesis
rate (39). At present, CRP is
generally recognized as a gold standard for evaluating the degree
of inflammation, which is also independently associated with the
development of DKD (40). A study
discovered that lower concentrations of CRP are associated with
endothelial dysfunction in patients with DM (41). A previous animal study reported
that CRP can be a therapeutic target for the treatment of DKD by
improving the epithelial-mesenchymal transition process through
Wnt/β-catenin and ERK1/2 signaling (42).
TNF-α is a potent indicator of inflammation,
together with its receptors TNF receptor (TNFR)1 and TNFR2. In DKD,
it has been documented to be involved in the synthesis of cytokines
and the mediation of a variety of cytotoxic effects on renal cells
(43,44). Previous data have suggested that
inflammatory biomarkers, including TNF-α and IL-6, rather than CRP,
were independently associated with CKD (45). Another study indicated that the
concentration of TNF-α in DKD was higher compared with that in DM,
suggesting that patients with DKD had more severe inflammation
(46). In animal models of DKD,
early rises in renal TNF-α levels were found to precede the
detection of urinary albumin, suggesting that TNF-α may be used as
a predictive factor for early DKD (47).
The IL family of cytokines, including IL-1β, IL-6
and IL-18, is secreted by a variety of cells (such as endocytes,
machrophages and fibroblasts) and serves a pivotal role in
inflammation, particularly in the progression of DKD (6). IL-6 was found to be elevated in the
serum and urine samples of patients with DKD (48). A prospective cohort study
previously demonstrated that IL-6 polymorphisms were associated
with DKD and the morbidity rate of DKD was increased as the levels
of IL-6 also increased (49).
Therefore, CRP, TNF-α and IL-6 were selected to be the main
outcomes assessed in the present systematic review. The results
showed that NDQ granules could decrease the levels of these
inflammatory factors, which are consistent with the subgroup
analysis of different dosages. Simultaneously, due to the
significant reduction of heterogeneity in the subgroup analysis of
dosage of NDQ granules ≥30 g on CRP, the dosage was likely to be a
source of heterogeneity. This suggests that further in-depth
studies are required for future verification. Because the dosage of
treatment with NDQ granules in all of the studies reporting on
TNF-α was <30 g, a subgroup analysis on TNF-α could not be
performed. The effect of IL-6 on the dosage of NDQ granules ≥30 g
showed no obvious significance as the sample size was too small,
therefore, larger sample sizes are required for future studies.
Parameters such as BUN, SCr and urinary protein
excretion are important indicators for assessing the extent of
renal damage associated with DKD. A previous study reported that
high variability in BUN and SCr levels in patients with CKD can
predict the risk of subsequent mortality from non-cardiac causes
(50). It was recommended by the
Chinese clinical practice guidelines of DKD that the urinary
protein levels and eGFR be measured to assist in the early
diagnosis of DKD (51). In the
present analysis, the effect of the NDQ granules on BUN, SCr and
24-h UPE were explored, where the meta-analysis results showed that
the inclusion of NDQ granules decreased BUN, SCr and 24-h UPE. The
heterogeneity of BUN and 24-h UPE in the subgroup analysis of
dosages of ≥30 g was significantly decreased, suggesting that the
dosage of NDQ may be the cause of heterogeneity. However, a high
degree of heterogeneity persisted in the subgroup analysis based on
dosage, indicating that the heterogeneity may result from other
causes. It is possible that publication bias or small sample sizes
are the contenders of heterogeneity, which should be investigated
further in the future.
The mechanism of DKD is complex and involves
alterations in kidney hemodynamics, metabolic changes, oxidative
stress and genetic factors. In particular, inflammatory responses
may participate in the occurrence and progression of DKD (52). DKD is likely caused by
microvascular inflammation in a manner that is independent of
pathogenic microorganism infection (53). In addition, it has been reported
that, apart from hemodynamic changes, metabolic disorder can also
activate proinflammatory pathways and aggravate kidney disease
progression by elevating the intraglomerular pressure, mesangial
proliferation or damaging podocyte and tubular cells (6,54). A
previous study proposed that inflammation-associated indices should
be explored as possible biomarkers, therapeutic targets or
prognostic factors (55). Various
trials of commonly used hypoglycemic agents, including SGLT2
inhibitors, dipeptidyl-peptidase-4 and glucagon-like peptide-1,
have also been shown to alleviate inflammatory effects (56,57).
However, under the circumstance of unsatisfactory curative effects
and high rates of morbidity, TCM herbs are under consideration as
an alternative choice for treating DKD. Previous pharmacological
studies have found that NDQ granules was able to alleviate
inflammatory responses and oxidative stress, and improve renal
dysfunction and tubular interstitial fibrosis in DKD (12,58).
In terms of safety, no significance could be found between the
intervention and control group, consistently with a previous review
(59). Therefore, it is suggested
that NDQ granules has potential in suppressing inflammation and
preserving renal function under DKD. However, the underlying
mechanism remains poorly understood, which requires further
study.
To the best of our knowledge, the present study was
the first to evaluate the extent of inflammation after treatment
with NDQ granules in patients with DKD. There have been two
previous systematic reviews of the effects of NDQ granules on DKD
(60,61), but differences exist compared with
the present analysis. The key difference is that the outcomes
selected in the two previous reviews were mainly the urinary
albumin excretion rate, SCr clearance, total cholesterol and
fasting blood-glucose, whilst inflammatory factors, such as CRP,
TNF-α and IL-6, were lacking. Of note, the present meta-analysis
also had limitations. The quality of the included studies was not
high, as the allocation concealment, blinding methods and other
aspects were not reported in detail and, therefore, selection,
performance, detection and other bias affected their credibility.
In addition, all of the included studies had small sample sizes and
none of them were large-sample, multi-center international RCTs. No
placebo groups were included in the control group and conventional
therapy was used according to the different conditions, with
adjustments of blood pressure and glucose, and acid-base balance
used for the treatment of patients with DKD. To a certain extent,
it reduced the reliability of the conclusions of the present
meta-analysis. Furthermore, a number of included studies did not
record adverse reactions, meaning that safety could not be assessed
comprehensively. None of the included studies described the
follow-up conditions, resulting in the influence of NDQ granules on
the prognosis of patients with DKD not being verified.
According to the results of the present analysis,
future clinical trials of TCM herbs are required to follow the
Consolidated Standards of Reporting Trials guidelines (62), where the evaluation of long-term
prognosis should be emphasized. In this regard, more high-quality,
large-sample, multi-center and double-blinded RCTs are needed to
provide sufficient information on the effects of treatment with NDQ
granules on DKD.
In conclusion, current evidence indicates that NDQ
granules may be effective for the improvement of inflammation
caused by DKD when used in combination with conventional treatment.
However, caution should be taken when considering the present
meta-analysis results due to the inclusion of low-quality studies,
deficient placebo control and large heterogeneity among different
studies. In addition, the safety of NDQ granules remains vague,
meaning further assessment through high-quality studies is required
in the future.
Supplementary Material
Subgroup analysis based on dosage of
Niaoduqing granule for C-reactive protein. CI, confidence interval;
df, degrees of freedom; IV, inverse variance; Std.,
standardized.
Subgroup analysis based on dosage of
Niaoduqing granule for IL-6. CI, confidence interval; df, degrees
of freedom; IV, inverse variance; Std., standardized.
Subgroup analysis based on dosage of
Niaoduqing granule for blood urea. CI, confidence interval; df,
degrees of freedom; IV, inverse variance; Std., standardized.
Subgroup analysis based on dosage of
Niaoduqing granule for serum creatinine. CI, confidence interval;
df, degrees of freedom; IV, inverse variance; Std.,
standardized.
Subgroup analysis based on dosage of
Niaoduqing granule for 24 h-urea protein excretion. CI, confidence
interval; df, degrees of freedom; IV, inverse variance; Std.,
standardized.
Sensitivity analysis by deleting the
study one by one.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82174293) and Jiangsu
Province Postgraduate Research and Innovation Program (grant no.
KYCX23_2173).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and JY conceived the study and designed the
protocol for the systematic review. PZ, ZH and WX conducted the
literature screening and data extraction. ZH and WX performed the
statistical analysis. PZ and ZH drafted the manuscript. PZ and JY
inspected all aspects of this systematic review. PZ and JY confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Satirapoj B and Adler SG: Prevalence and
management of diabetic nephropathy in western countries. Kidney Dis
(Basel). 1:61–70. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang L, Long J, Jiang W, Shi Y, He X,
Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, et al: Trends in
chronic kidney disease in China. N Engl J Med. 375:905–906.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cho NH, Shaw JE, Karuranga S, Huang Y, da
Rocha Fernandes JD, Ohlrogge AW and Malanda B: IDF diabetes atlas:
Global estimates of diabetes prevalence for 2017 and projections
for 2045. Diabetes Res Clin Pract. 138:271–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Luk AOY, Hui EMT, Sin MC, Yeung CY, Chow
WS, Ho AYY, Hung HF, Kan E, Ng CM, So WY, et al: Declining trends
of cardiovascular-renal complications and mortality in type 2
diabetes: The Hong Kong diabetes database. Diabetes Care.
40:928–935. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schmidt MI, Duncan BB, Sharrett AR,
Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP and
Heiss G: Markers of inflammation and prediction of diabetes
mellitus in adults (Atherosclerosis Risk in Communities study): A
cohort study. Lancet. 353:1649–1652. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rayego-Mateos S, Morgado-Pascual JL,
Opazo-Ríos L, Guerrero-Hue M, García-Caballero C, Vázquez-Carballo
C, Mas S, Sanz AB, Herencia C, Mezzano S, et al: Pathogenic
pathways and therapeutic approaches targeting inflammation in
diabetic nephropathy. Int J Mol Sci. 21(3798)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gamboa JL, Pretorius M, Todd-Tzanetos DR,
Luther JM, Yu C, Ikizler TA and Brown NJ: Comparative effects of
angiotensin-converting enzyme inhibition and angiotensin-receptor
blockade on inflammation during hemodialysis. J Am Soc Nephrol.
23:334–342. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perkovic V, Jardine MJ, Neal B, Bompoint
S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull
S, et al: Canagliflozin and renal outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 380:2295–2306. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lupsa BC and Inzucchi SE: Use of SGLT2
inhibitors in type 2 diabetes: Weighing the risks and benefits.
Diabetologia. 61:2118–2125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
GBD Chronic Kidney Disease Collaboration.
Global, regional, and national burden of chronic kidney disease,
1990-2017: A systematic analysis for the global burden of disease
study 2017. Lancet. 395:709–733. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao JX, Deng DQ and Li Q: Identification
of Chinese medicine diseases related to diabetic nephropathy. J
Nanjing Univ Traditional Chin Med. 288-289:2005.
|
|
12
|
Huang YR, Wei QX, Wan YG, Sun W, Mao ZM,
Chen HL, Meng XJ, Shi XM, Tu Y and Zhu Q: Ureic clearance granule,
alleviates renal dysfunction and tubulointerstitial fibrosis by
promoting extracellular matrix degradation in renal failure rats,
compared with enalapril. J Ethnopharmacol. 155:1541–1552.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Disease SPGoTCAGotCMftToC: The clinical
application guide of chinese patent medicine in the treatment of
chronic kidney disease stage 3-5 (Non Dialysis) (2020 Version).
Chin J Integrated Traditional and Western Medicine. 41:261–272.
2021.
|
|
14
|
Zheng Y, Cai GY, He LQ, Lin HL, Cheng XH,
Wang NS, Jian GH, Liu XS, Liu YN, Ni ZH, et al: Efficacy and safety
of niaoduqing particles for delaying moderate-to-severe renal
dysfunction: A randomized, double-blind, placebo-controlled,
multicenter clinical study. Chin Med J (Engl). 130:2402–2409.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang YX, Yu GQ, Su XY, Chen J and Zhuang
YZ: The ameliorating effect of uremic clearance granule played on
micro-inflammatory state for patients in early and mild stage of
chronic renal insufficiency. Chin J Int Trad Western Nephrol.
17:1050–1052. 2016.
|
|
16
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. BMJ. 339(b2535)2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
The MCGoCDA: Expert consensus of
prevention and treatment of diabetic kidney disease (2014 version).
Chin J Diab Mellitus. 6:792–801. 2014.
|
|
18
|
The MCGoCDA: Chinese clinical practice
guideline of diabetic kidney disease. Chin J Diab Mellitus.
11:15–28. 2019.
|
|
19
|
Tong GY and Zhu DL: Interpretation of
clinical practice guidelines and expert consensuses for the
evaluation and management of diabetic kidney disease at home and
abroad. Chin J Pract Int Med. 37:211–216. 2017.
|
|
20
|
Mogensen CE, Schmitz A and Christensen CK:
Comparative renal pathophysiology relevant to IDDM and NIDDM
patients. Diabetes Metab Rev. 4:453–483. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deyno S, Eneyew K, Seyfe S, Tuyiringire N,
Peter EL, Muluye RA, Tolo CU and Ogwang PE: Efficacy and safety of
cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A
meta-analysis and meta-regression. Diabetes Res Clin Pract.
156(107815)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang Y: Clinical effIcacy of compound
alpha ketoacid combined with Niaoduqing granules in treatment of
patients with stage IV diabetic kidney disease. Med Diet Health.
19:66–67. 2021.
|
|
23
|
Zhang HY, Zhang HX, Xiao Y and Feng XZ:
Effects of Niaoduqing granules on renal function and serum hs-CRP
levels in DN patients with chronic renal insufficiency. Shand Med
J. 51:77–78. 2011.
|
|
24
|
Zhang C, Wang ML, Ma H, Xia XB and Ma J:
The effects of Niaoduqing granules on micro-inflammatory state and
vascular endothelial growth factor in aged patients with diabetic
nephropathy. Chin J Diff Compl Cases. 13:697–699, 703. 2014.
|
|
25
|
He YM and Ding XQ: Clinical research of
Niaoduqing granules on inflammation factors in patients with early
and middle diabetic nephropathy. Pharmacol Clin Chin Mat Med.
31:160–162. 2015.
|
|
26
|
He YH: Effect of niaoduqing granules
combined with valsartan on kidney function, inflammatory factors
and immune function in patients with early diabetic nephropathy.
New Chin Med. 53:60–63. 2021.
|
|
27
|
Yang SX and Chen BP: Changes of Niaoduqing
granules on IL-6 levels in treatment of early renal failure of type
2 diabetic nephropathy. Chi Comm Doct. 12(161)2010.
|
|
28
|
Wu P: Effects of Niaoduqing granules
combined with Valsartan on kidney function in patients of early
diabetic nephropathy. Chron Pathematol J. 22:474–475+478. 2021.
|
|
29
|
Li XY: Changes of renal function and serum
hs-CRP levels in diabetes patients with chronic renal insufficiency
before and after taking Niaoduqing particle. Chin J Pract Med.
42:41–42. 2015.
|
|
30
|
Liu B and Yang SL: Effect of niaoduqing
combined with insulin detemir on inflammation, oxidative stress and
curative effect in patients with diabetic nephropathy. Diab New
World. 23:13–17. 2020.
|
|
31
|
Li Q, Cao M, Li K and Li J: Observation on
the effect of niaoduqing granules combined with irbesartan in the
treatment of early diabetic nephropathy. Med Innov China. 18:16–20.
2021.
|
|
32
|
Chen X, Luo HY, Gao J and Qi C: Clinical
efficacy of insulin degludec and insulin aspart combined with
Niaoduqing in treatment of patients with early diabetic
nephropathy. Chin J Integrated Trad West Med Int Crit Care.
28:404–408. 2021.
|
|
33
|
Zheng N, An Z and Liu HG: Effects of
combination of Niaoduqing granules and artificial kidney on blood
lipid metabolism inflammatory response and cellular immune function
in patients with diabetic nephropathy undergoing maintenance
hemodialysis. Mod J Int Trad Chin West Med. 28:2208–2211. 2019.
|
|
34
|
Wang B: Effect of Atorvastatin and
Niaoduqing in diabetic nephropathy patients with maintenance
hemodialysis and influence of micro inflammatory. J Med Forum.
38:34–35+38. 2017.
|
|
35
|
Gong LN: Clinical efficacy of compound
Niaoduqing particles and irbesartan in patients with stage Ⅳ
diabetic nephropathy. J Front Med. 115–116. 2013.
|
|
36
|
Tong CC, Chen HC, Cong ZY, Wang W, Li Q,
Zhao XJ and Zheng Y: Effects of Dapagliflozin combined with
Niaoduqing granules in the treatment of diabetic nephropathy.
Modern Journal of Integrated Traditional Chinese and Western
Medicine. 31:3310–3314. 2022.
|
|
37
|
Yu H, Liang CD, Hu XH and Yu Y: Effects of
Niaoduqing granules on oxidative/antioxidant balance and serum
LncRNA KCNQ1OT1 and LncRNA Malat1 in the adjuvant treatment of
early diabetic nephropathy. Lab Med Clin. 19:3334–3338. 2022.
|
|
38
|
Ciani O, Manyara A and Taylor RS: Need for
better reporting of trials with surrogate endpoints:
SPIRIT|CONSORT-SURROGATE extensions. J Epidemiol Community Health.
76:769–770. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sinha SK, Nicholas SB, Sung JH, Correa A,
Rajavashisth TB, Norris KC and Lee JE: hs-CRP is associated with
incident diabetic nephropathy: Findings from the Jackson heart
study. Diabetes Care. 42:2083–2089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Balamir I, Ates I, Topcuoglu C and Turhan
T: Association of endocan, ischemia-modified albumin, and hsCRP
levels with endothelial dysfunction in type 2 diabetes mellitus.
Angiology. 69:609–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang L, Shen ZY, Wang K, Li W, Shi JM,
Osoro EK, Ullah N, Zhou Y and Ji SR: C-reactive protein exacerbates
epithelial-mesenchymal transition through Wnt/β-catenin and ERK
signaling in streptozocin-induced diabetic nephropathy. FASEB J.
33:6551–6563. 2019.
|
|
43
|
Navarro JF and Mora-Fernández C: The role
of TNF-alpha in diabetic nephropathy: Pathogenic and therapeutic
implications. Cytokine Growth Factor Rev. 17:441–450.
2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Niewczas MA, Gohda T, Skupien J, Smiles
AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas
TN, et al: Circulating TNF receptors 1 and 2 predict ESRD in type 2
diabetes. J Am Soc Nephrol. 23:507–515. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee BT, Ahmed FA, Hamm LL, Teran FJ, Chen
CS, Liu Y, Shah K, Rifai N, Batuman V, Simon EE, et al: Association
of C-reactive protein, tumor necrosis factor-alpha, and
interleukin-6 with chronic kidney disease. BMC Nephrol.
16(77)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Fathy SA, Mohamed MR, Ali M, El-Helaly AE
and Alattar AT: Influence of IL-6, IL-10, IFN-γ and TNF-α genetic
variants on susceptibility to diabetic kidney disease in type 2
diabetes mellitus patients. Biomarkers. 24:43–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kalantarinia K, Awad AS and Siragy HM:
Urinary and renal interstitial concentrations of TNF-alpha increase
prior to the rise in albuminuria in diabetic rats. Kidney Int.
64:1208–1213. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shikano M, Sobajima H, Yoshikawa H, Toba
T, Kushimoto H, Katsumata H, Tomita M and Kawashima S: Usefulness
of a highly sensitive urinary and serum IL-6 assay in patients with
diabetic nephropathy. Nephron. 85:81–85. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chang WT, Huang MC, Chung HF, Chiu YF,
Chen PS, Chen FP, Lee CY, Shin SJ, Hwang SJ, Huang YF and Hsu CC:
Interleukin-6 gene polymorphisms correlate with the progression of
nephropathy in Chinese patients with type 2 diabetes: A prospective
cohort study. Diabetes Res Clin Pract. 120:15–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nakazato Y, Kurane R, Hirose S, Watanabe A
and Shimoyama H: Variability of laboratory parameters is associated
with frailty markers and predicts non-cardiac mortality in
hemodialysis patients. Clin Exp Nephrol. 19:1165–1178.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Association TMCGoCD: Chinese clinical
practice guideline of diabetic kidney disease. Chin J Diab.
11:2019.
|
|
52
|
Tang S and Yiu WH: Innate immunity in
diabetic kidney disease. Nat Rev Nephrol. 16:206–222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kaysen GA: The microinflammatory state in
uremia: Causes and potential consequences. J Am Soc Nephrol.
12:1549–1557. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lytvyn Y, Bjornstad P, van Raalte DH,
Heerspink HL and Cherney DZ: The new biology of diabetic kidney
disease-mechanisms and therapeutic implications. Endocr Rev.
41:202–231. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Niewczas MA, Pavkov ME, Skupien J, Smiles
A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ,
et al: A signature of circulating inflammatory proteins and
development of end-stage renal disease in diabetes. Nat Med.
25:805–813. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Donate-Correa J, Ferri CM,
Sánchez-Quintana F, Pérez-Castro A, González-Luis A, Martín-Núñez
E, Mora-Fernández C and Navarro-González JF: Inflammatory cytokines
in diabetic kidney disease: Pathophysiologic and therapeutic
implications. Front Med (Lausanne). 7(628289)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rayego-Mateos S, Rodrigues-Diez RR,
Fernandez-Fernandez B, Mora-Fernández C, Marchant V, Donate-Correa
J, Navarro-González JF, Ortiz A and Ruiz-Ortega M: Targeting
inflammation to treat diabetic kidney disease: the road to 2030.
Kidney Int. 103:282–296. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tan F, Sheng YH and He Y: Effect of uremic
granule on renal anti-inflammatory and antioxidant protection of
diabetic nephropathy rats and on TGF-β1/p38MAPK signaling pathway.
Trad Chin Drug Res Clin Pharmacol. 30:117–122. 2019.
|
|
59
|
Fu B, Shang Z, Song S, Xu Y, Wei L, Li G
and Yang H: Adverse reactions of Niaoduqing granules: A systematic
review and meta-analysis. Phytomedicine. 109(154535)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang J, Li J, Zhang HL, Qu HS, Zhang XZ,
Cheng YQ, Zhang ZM and Huang JQ: Meta-analysis of niaoduqing
granules combined with ACEI/ARB in the treatment of diabetic
nephropathy. World Chin Med. 16:274–283. 2021.
|
|
61
|
Luan W, Yin H and Gu J: Systematic review
on assisted treatment for diabetic nephropathy with niaoduqing
granule. J Liaoning Univ Trad Chin Med. 17:131–135. 2015.
|
|
62
|
Patrick D: Reporting of patient-reported
outcomes in randomized trials: The CONSORT PRO extension. Value
Health. 16:455–456. 2013.PubMed/NCBI View Article : Google Scholar
|