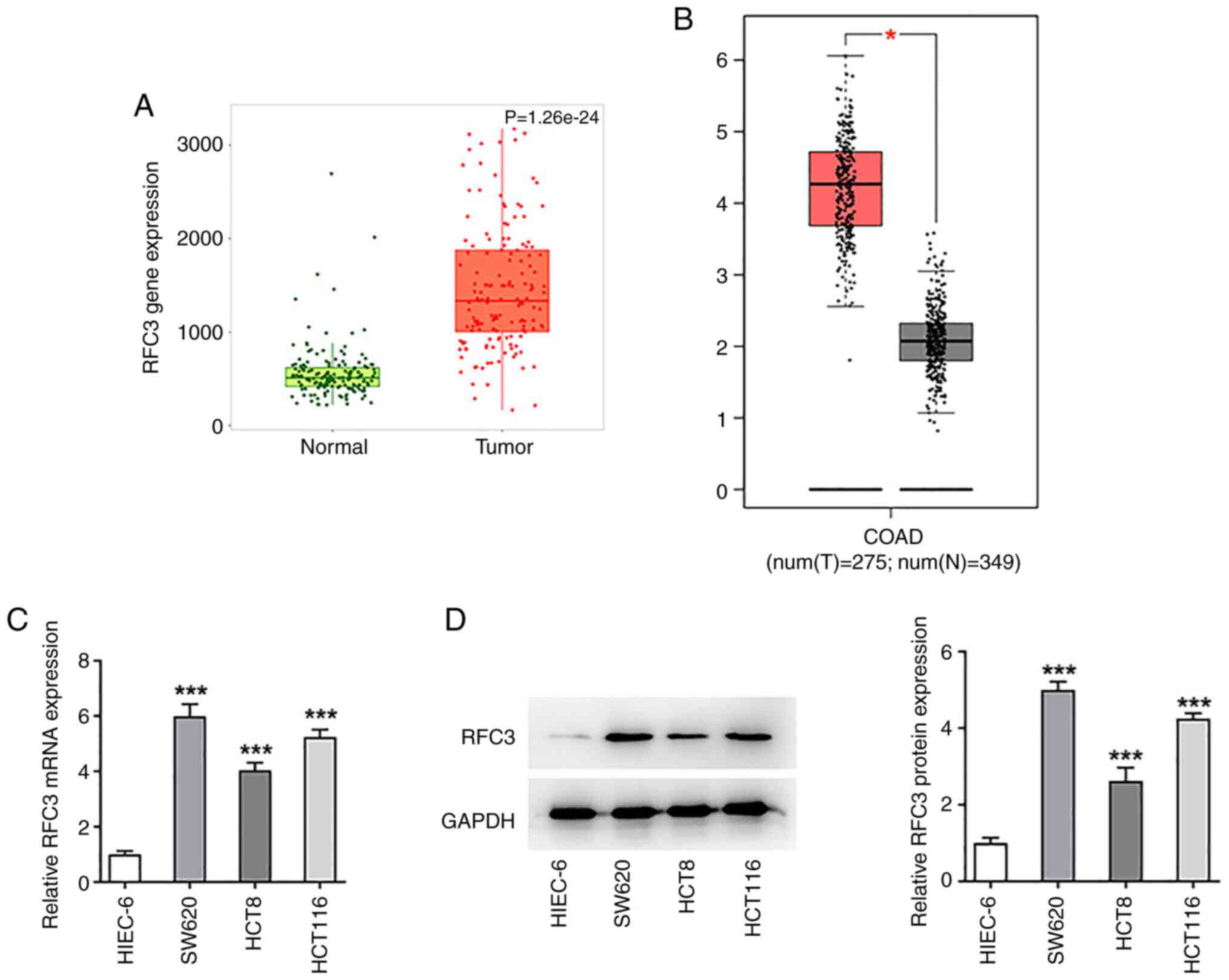
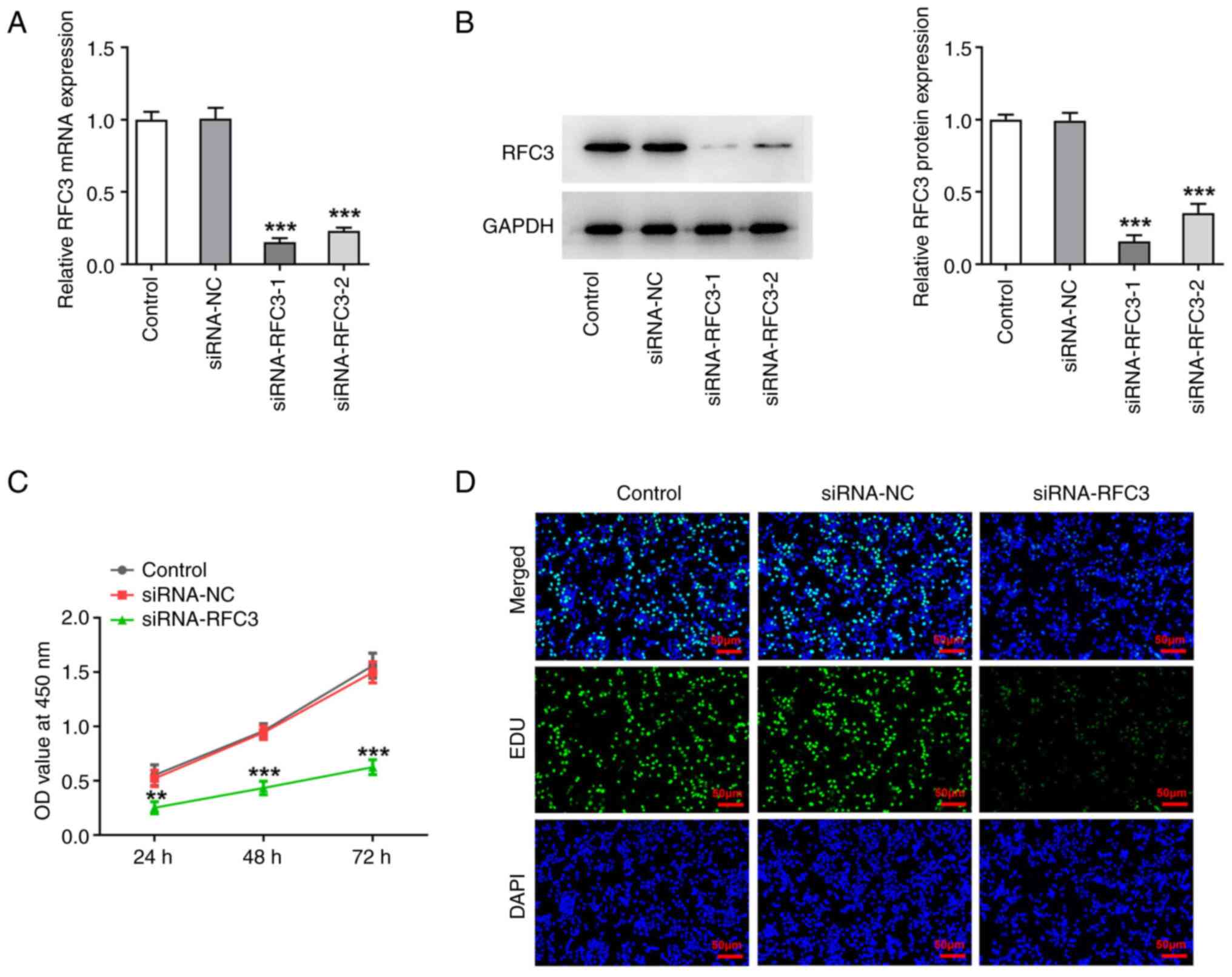
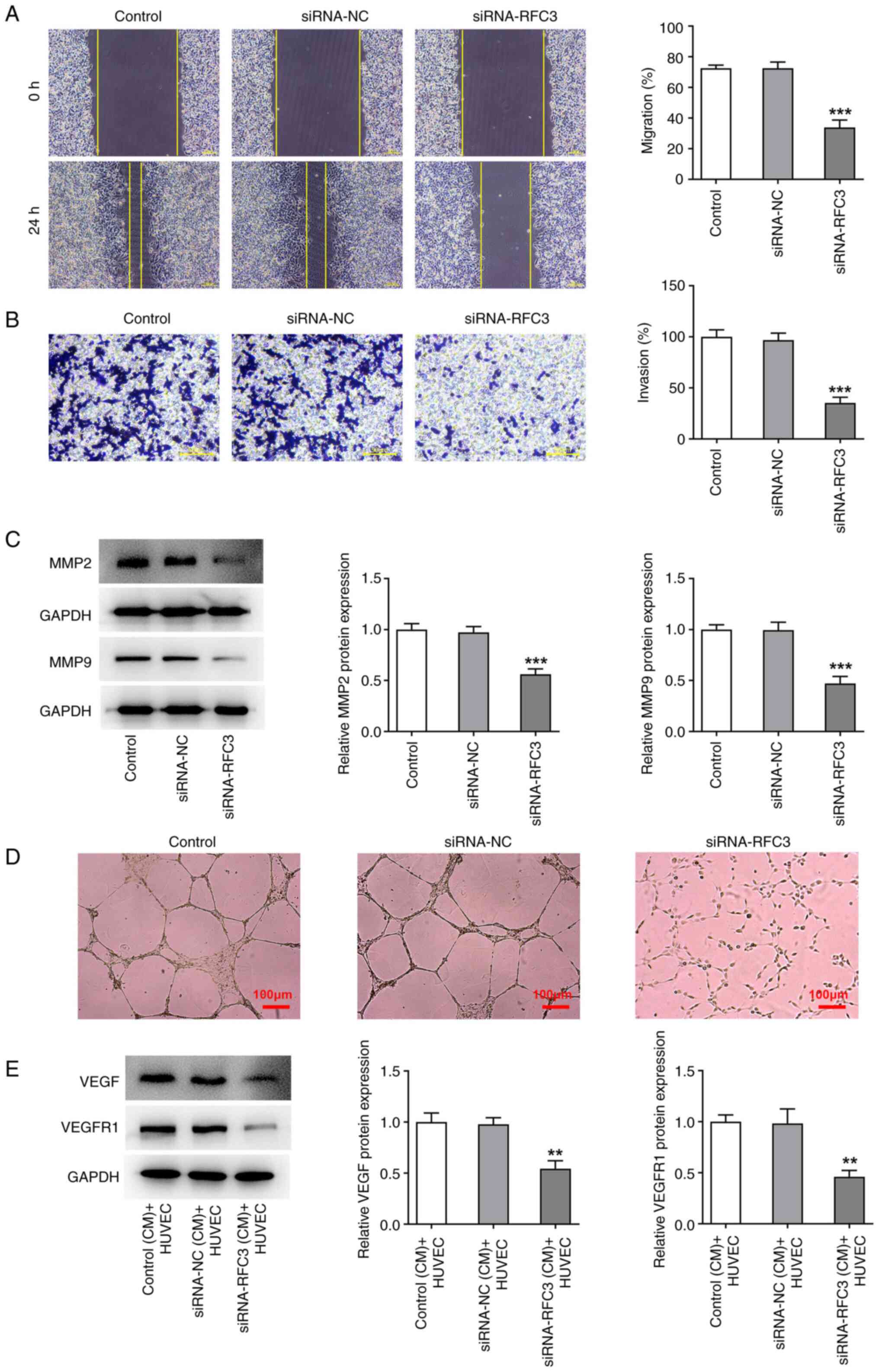
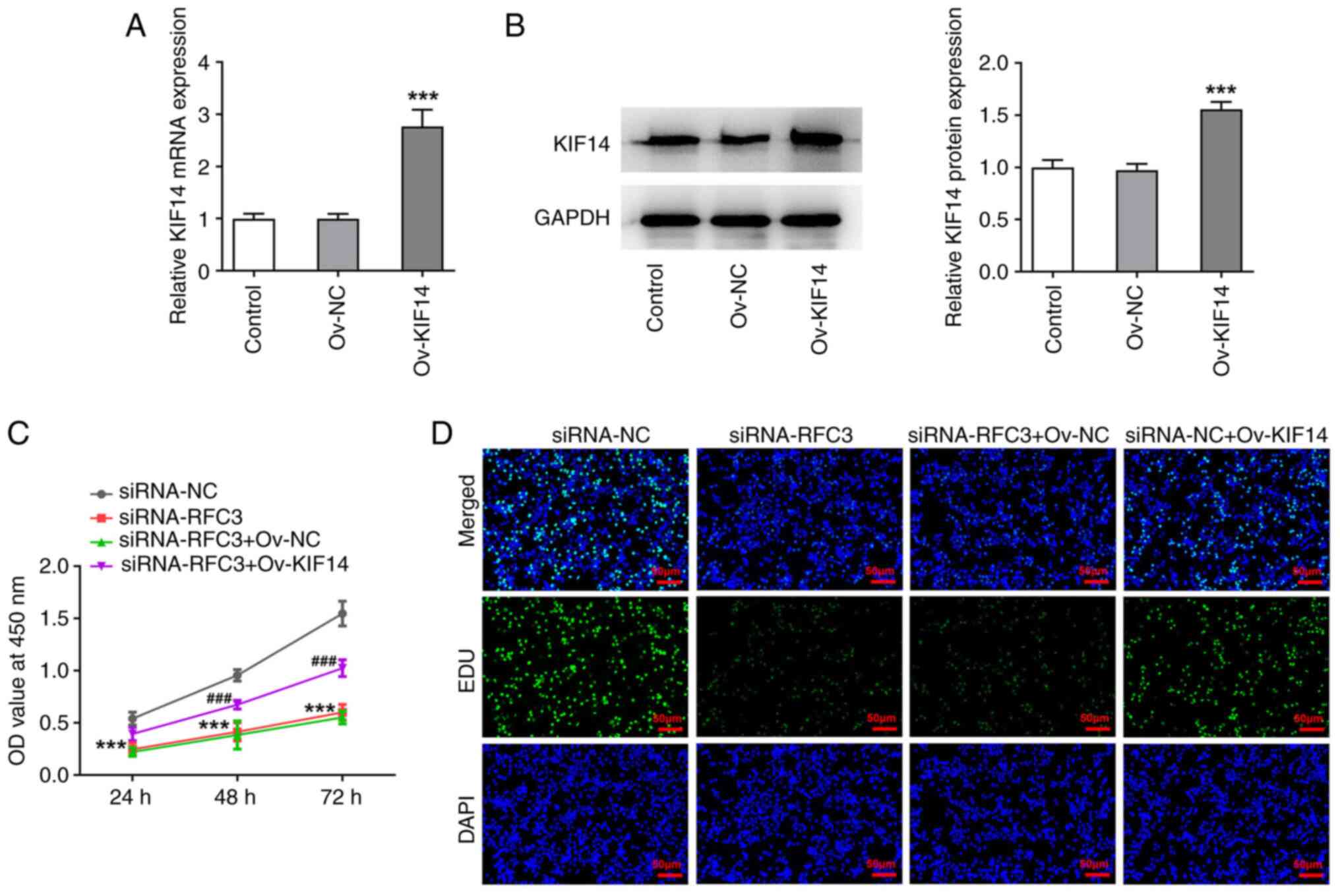
|
1
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Simard J, Kamath S and Kircher S:
Survivorship guidance for patients with colorectal cancer. Curr
Treat Options Oncol. 20(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jung G, Hernández-Illán E, Moreira L,
Balaguer F and Goel A: Epigenetics of colorectal cancer: Biomarker
and therapeutic potential. Nat Rev Gastroenterol Hepatol.
17:111–130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Essa HYS, Kusaf G, Yuruker O and Kalkan R:
Epigenetic alteration in colorectal cancer: A biomarker for
diagnostic and therapeutic application. Glob Med Genet. 9:258–262.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sakato M, O'Donnell M and Hingorani MM: A
central swivel point in the RFC clamp loader controls PCNA opening
and loading on DNA. J Mol Biol. 416:163–175. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Majka J and Burgers PM: The PCNA-RFC
families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol
Biol. 78:227–260. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xia S, Xiao L, Gannon P and Li X: RFC3
regulates cell proliferation and pathogen resistance in
Arabidopsis. Plant Signal Behav. 5:168–170. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gong S, Qu X, Yang S, Zhou S, Li P and
Zhang Q: RFC3 induces epithelial-mesenchymal transition in lung
adenocarcinoma cells through the Wnt/β-catenin pathway and
possesses prognostic value in lung adenocarcinoma. Int J Mol Med.
44:2276–2288. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shen H, Cai M, Zhao S, Wang H, Li M, Yao S
and Jiang N: Overexpression of RFC3 is correlated with ovarian
tumor development and poor prognosis. Tumour Biol. 35:10259–10266.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yao Z, Hu K, Huang H, Xu S, Wang Q, Zhang
P, Yang P and Liu B: shRNA-mediated silencing of the RFC3 gene
suppresses hepatocellular carcinoma cell proliferation. Int J Mol
Med. 36:1393–1399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He ZY, Wu SG, Peng F, Zhang Q, Luo Y, Chen
M and Bao Y: Up-Regulation of RFC3 promotes triple negative breast
cancer metastasis and is associated with poor prognosis via EMT.
Transl Oncol. 10:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin K, Zhu X, Luo C, Bu F, Zhu J and Zhu
Z: Data mining combined with experiments to validate CEP55 as a
prognostic biomarker in colorectal cancer. Immun Inflamm Dis.
9:167–182. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Niwa S: Kinesin superfamily proteins and
the regulation of microtubule dynamics in morphogenesis. Anat Sci
Int. 90:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Morfini G, Schmidt N, Weissmann C, Pigino
G and Kins S: Conventional kinesin: Biochemical heterogeneity and
functional implications in health and disease. Brain Res Bull.
126(Pt 3):347–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lucanus AJ and Yip GW: Kinesin
superfamily: Roles in breast cancer, patient prognosis and
therapeutics. Oncogene. 37:833–838. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang J, Buranjiang G, Mutalifu Z, Jin H
and Yao L: KIF14 affects cell cycle arrest and cell viability in
cervical cancer by regulating the p27(Kip1) pathway.
World J Surg Oncol. 20(125)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang Z, Li C, Yan C, Li J, Yan M, Liu B,
Zhu Z, Wu Y and Gu Q: KIF14 promotes tumor progression and
metastasis and is an independent predictor of poor prognosis in
human gastric cancer. Biochim Biophys Acta Mol Basis Dis.
1865:181–192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Hare M, Shadmand M, Sulaiman RS, Sishtla
K, Sakisaka T and Corson TW: Kif14 overexpression accelerates
murine retinoblastoma development. Int J Cancer. 139:1752–1758.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang ZZ, Yang J, Jiang BH, Di JB, Gao P,
Peng L and Su XQ: KIF14 promotes cell proliferation via activation
of Akt and is directly targeted by miR-200c in colorectal cancer.
Int J Oncol. 53:1939–1952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nappi A, Nasti G, Romano C, Berretta M and
Ottaiano A: Metastatic colorectal cancer: Prognostic and predictive
factors. Curr Med Chem. 27:2779–2791. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chan E: Angiogenesis in colorectal cancer:
Antibodies. Cancer J. 22:179–181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Coppedè F: The role of epigenetics in
colorectal cancer. Expert Rev Gastroenterol Hepatol. 8:935–948.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Gan S, Ren L, Yuan L, Liu J, Wang W,
Wang X, Zhang Y, Jiang J, Zhang F and Qi X: Multifaceted regulation
and functions of replication factor C family in human cancers. Am J
Cancer Res. 8:1343–1355. 2018.PubMed/NCBI
|
|
27
|
Hu T, Shen H, Li J, Yang P, Gu Q and Fu Z:
RFC2, a direct target of miR-744, modulates the cell cycle and
promotes the proliferation of CRC cells. J Cell Physiol.
235:8319–8333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Al-Ostoot FH, Salah S, Khamees HA and
Khanum SA: Tumor angiogenesis: Current challenges and therapeutic
opportunities. Cancer Treat Res Commun. 28(100422)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bielenberg DR and Zetter BR: The
contribution of angiogenesis to the process of metastasis. Cancer
J. 21:267–273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
31
|
Wang R, Ma Y, Zhan S, Zhang G, Cao L,
Zhang X, Shi T and Chen W: B7-H3 promotes colorectal cancer
angiogenesis through activating the NF-κB pathway to induce VEGFA
expression. Cell Death Dis. 11(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dakowicz D, Zajkowska M and Mroczko B:
Relationship between VEGF family members, their receptors and cell
death in the neoplastic transformation of colorectal cancer. Int J
Mol Sci. 23(3375)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Freire Valls A, Knipper K, Giannakouri E,
Sarachaga V, Hinterkopf S, Wuehrl M, Shen Y, Radhakrishnan P, Klose
J, Ulrich A, et al: VEGFR1(+) metastasis-associated macrophages
contribute to metastatic angiogenesis and influence colorectal
cancer patient outcome. Clin Cancer Res. 25:5674–5685.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ji J and Fu J: MiR-17-3p facilitates
aggressive cell phenotypes in colon cancer by targeting PLCD1
through affecting KIF14. Appl Biochem Biotechnol. 195:1723–1735.
2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao Q, Chen S and Chen L: LETM1 (leucine
zipper-EF-hand-containing transmembrane protein 1) silence reduces
the proliferation, invasion, migration and angiogenesis in
esophageal squamous cell carcinoma via KIF14 (kinesin family member
14). Bioengineered. 12:7656–7665. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu H, Zhao G, Zhang Y, Jiang H, Wang W,
Zhao D, Yu H and Qi L: Long non-coding RNA PAXIP1-AS1 facilitates
cell invasion and angiogenesis of glioma by recruiting
transcription factor ETS1 to upregulate KIF14 expression. J Exp
Clin Cancer Res. 38(486)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Neska-Długosz I, Buchholz K, Durślewicz J,
Gagat M, Grzanka D, Tojek K and Klimaszewska-Wiśniewska A:
Prognostic impact and functional annotations of KIF11 and KIF14
expression in patients with colorectal cancer. Int J Mol Sci.
22(9732)2021.PubMed/NCBI View Article : Google Scholar
|