Introduction
Angiomyxoma (AM) is a rare soft-tissue neoplasm that
commonly occurs in the pelvis and perineum in females of
reproductive age, while it is uncommon in males, with a
female-to-male ratio of approximately 6:1(1). AM is a mesenchymal tumor that is
histologically composed of myxoid stroma and vasculature, and is
distinguished by its propensity for numerous local recurrences and
lack of metastatic potential. Reports of this disease are limited
(1), and it occurs less frequently
in the liver. This pathological entity does not have any unique
clinical, biological or imaging characteristics that allow
differentiation. Therefore, patients are not clearly diagnosed
preoperatively and must wait until the postoperative pathological
diagnosis has been confirmed. AM characteristically grows slowly
and insidiously and carries a risk of local recurrence and distant
metastases in patients with AM (2). This aggressive behavior needs to be
taken into account, and long-term close follow-up is
recommended.
Focal nodular hyperplasia (FNH) is recognized as the
second most prevalent benign hepatic mass (3). Although the causes of FNH are
considered to be associated with local vascular abnormalities, the
exact etiology and pathological mechanism is still poorly
understood. The imaging presentation of typical FNH commonly
involves a stellate scar located at the center of the lesion, as
observed through contrast-enhanced computed tomography (CT) and
magnetic resonance imaging (MRI). The majority of FNH cases can be
diagnosed through the use of imaging techniques. To the best of our
knowledge, this is the first case of AM arising from the liver
combined with FNH. This study aims to provide a new approach for
clinical physicians to diagnose patients with AM combined with
FNH.
Case report
A 56-year-old woman without any specific symptoms
and no history of viral hepatitis or a family history of cancer was
admitted to the Affiliated Tumor Hospital of Xinjiang Medical
University (Urumqi, China) in March 2023 due to two intrahepatic
lesions found on a routine physical examination. On physical
examination, no other abnormalities were detected. The
α-fetoprotein and carcinoembryonic antigen levels were 1.68 ng/ml
(normal range, 0-13.4 ng/ml) and 2.76 µg/l (normal range, 0-5
µg/l), respectively. Ultrasound suggested two solid nodules in the
liver, with clear borders and no blood flow signals.
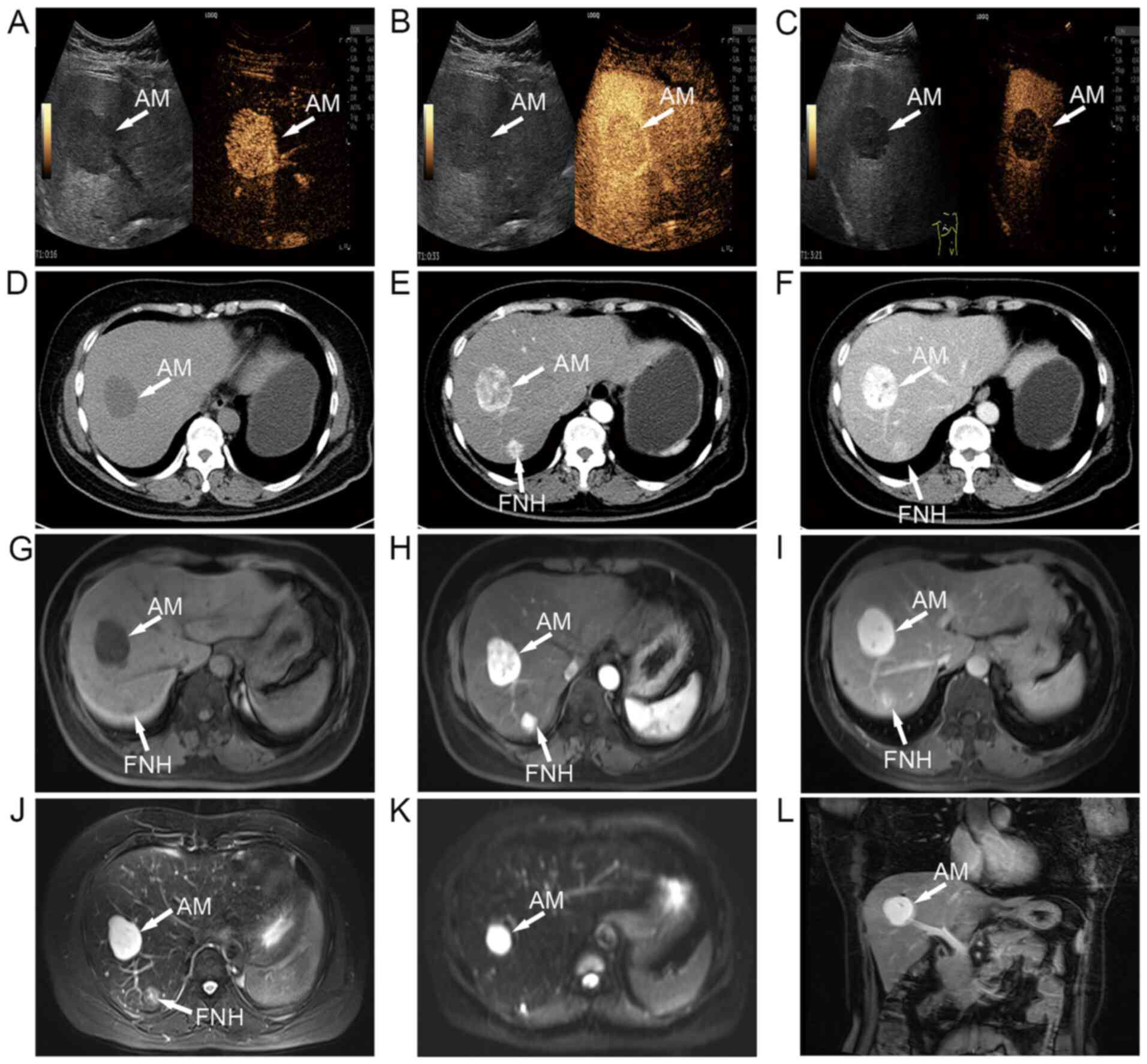
Contrast-enhanced ultrasound revealed a solid, hypoechoic tumor
that was relatively well-circumscribed, with rapidly intensified
enhancement in segment VIII of the liver in the arterial phase (16
sec post-contrast injection; Fig.
1A), gradually decreased enhancement in the venous phase (33
sec post-contrast injection; Fig.
1B) and further decreased enhancement in the delayed phase (3
min and 21 sec post-contrast injection; Fig. 1C). A CT scan showed a regular,
lobular, low-density mass that contained of a pool of highly
viscous liquid (Fig. 1D). Next,
contrast-enhanced CT of the abdomen revealed uneven enhancement
during the arterial phase (Fig.
1E), continuous enhancement in the venous phase and irregular
enhancement in the delayed phase (Figs. 1F and S1A). An MRI plain scan displayed
T1-weighted low signal nodules (Fig. 1G). Moreover, it was observed that
the mass was obviously hypointense on T1-weighted images
in the arterial phase (Fig. 1H)
and slightly less hypointense in the delayed phase (Fig. 1I). MRI showed a T2
hyperintensity in segment VIII and mild-moderate T2
hyperintensity in segment VII (Fig.
1J). Diffusion-weighted imaging illustrated the diffuse high
signal intensity and restricted diffusion of the lesion (Fig. 1K). Coronal MRI confirmed that the
AM nodule was located in segment VIII (Fig. 1L).
Subsequently, a wedge liver resection was performed
on the patient. Gross specimens of the masses after surgical
removal revealed two separate, differently sized and well-defined
masses (Fig. S1B). The resected
specimen were fixed overnight in 10% formalin at room temperature,
embedded in paraffin, and sliced into 5-µm thick sections that were
mounted on slides. Macroscopic examination revealed a white,
rubbery mass with a soft and smooth texture, and myxoid components
located in segment VIII of the liver. In the context of hematoxylin
and eosin (HE) staining, tissue sections underwent a sequential
process that involved incubation in hematoxylin for 2 min,
de-staining in 1% hydrochloric acid in 70% ethanol for 5 sec,
rinsing in 80% ethanol for 1 min, a brief exposure to eosin for 1
sec, dehydration in ethanol (95 and 100%, three times) and clearing
in xylene (three times) at room temperature. HE staining was
analyzed using an optical microscope (BX43; Olympus Corporation).
The nodule in segment VII was tough, greyish and lustreless, with a
marked spoke-wheel scar in the centre (Fig. 2A). HE staining revealed variably
sized thick-walled blood vessels in mucinous degeneration in
segment VIII of the liver. The nodule in segment VII was composed
of disorganizing hepatocyte cords with distinct fibrous septa of
varying widths, and hyperplastic small bile ducts, thick-walled
vessels and lymphocytic infiltrates were observed within the
central stellate fibrous scar tissue (Fig. 2A). The results of
immunohistochemical staining (Data
S1) in segment VIII showed positive staining for CD34 and
smooth muscle actin, and no staining for Desmin, epithelial
membrane antigen, human melanoma black-45, pan-cytokeratin
(AE1/AE3), estrogen receptor (ER), progesterone receptor (PR),
proto-oncogene c-Kit (c-Kit) and anoctamin-1 (DOG1) (Figs. 2B and S1C). Immunohistochemical staining of the
lesion in segment VII was positive for cytokeratin CAM5.2, CD34 and
CK7 markers, but negative for glypican 3, c-Kit and DOG1 markers
(Figs. 2C and S1C). Based on the aforementioned
results, the mass in segment VIII was identified as an AM, and the
nodule in segment VII was diagnosed as FNH. Based on the risk of
recurrence in AM, the patient was required to come to the hospital
for follow-up every 3-6 months in the first year. To date, there
have been no signs of recurrence. Within the next 3 years, the
follow-up will be conducted semi-annually, with subsequent
follow-ups for 5 years annually.
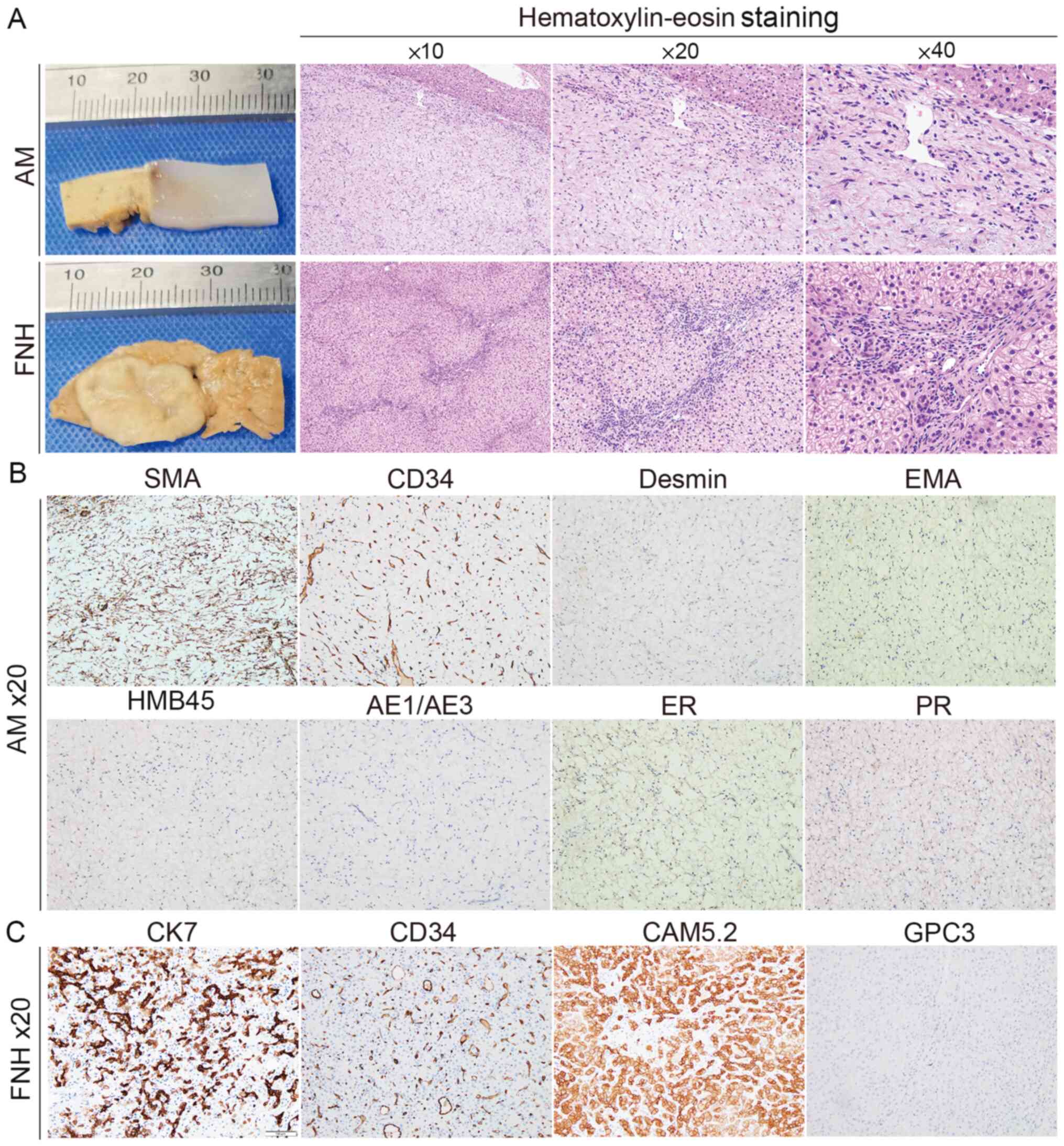 | Figure 2Histopathological analysis of the
resected lesions. (A) Formalin was used to fix the AM and FNH
lesions. The pathological features of AM and FNH were analyzed by
hematoxylin and eosin staining. Immunohistochemistry was
implemented to evaluate the markers in AM (B) including SMA, CD34,
Desmin, EMA, HMB45, AE1/AE3, ER and PR, and the markers in FNH (C)
including CAM5.2, CD34, CK7 and GPC3. AM, angiomyxoma; FNH, focal
nodular hyperplasia; EMA, epithelial membrane antigen; ER, estrogen
receptor; PR, progesterone receptor; CAM5.2, cytokeratin CAM5.2;
AE1/AE3, pan-cytokeratin; CK, cytokeratin; HMB45, human melanoma
black-45; SMA, smooth muscle actin; GPC3, glypican 3. |
Discussion
AM is a distinct mesenchymal tumor with mucinous and
vascular components that was first described and reported by
Steeper and Rosai in 1983(4).
Currently, AM can be divided into two types. The first type is
superficial angiomyxoma, which arises in superficial areas such as
the head, neck, trunk and lower limb regions. The other type is
deep angiomyxoma, also known as aggressive angiomyxoma (AAM), which
develops more frequently in deep tissues such as the pelvis. Apart
from the difference in the location of the occurrence, AAMs have
aggressive, locally infiltrative and recurrent characteristics. To
the best of our knowledge, only four cases of AM have occurred in
the liver (5-8),
all of which were reported in female patients aged between 30 and
50 years. However, no cases of coexisting FNH were recorded. A
summary of reported cases of AM of the liver is presented in
Table I.
 | Table ICurrently reported cases of
angiomyxoma of the liver. |
Table I
Currently reported cases of
angiomyxoma of the liver.
| |
Immunohistochemistry | |
|---|
| First author,
year | Age, years | Sex | Size, cm | Positive | Negative | Treatment method | Complementary
treatment; prognosis | (Refs.) |
|---|
| Qi et al,
2015 | 50 | F | 2.0x2.0x1.0 | Vimentin, CD34,
SMA | Desmin, S-100, Ki-67,
EMA, ER, PR, CD99, CD10, CAM5.2, CK19 | Surgical
excision | None; no
recurrence | (5) |
| Sato et al,
2017 | 33 | F | 8.0x7.5 | Vimentin, Desmin,
CD34, ER, PR | S-100, EMA, CK19,
CD99, HMB45, SMA | S8 sub-segmental
resection | None; no
recurrence | (6) |
| Malik et al,
2018 | 46 | F | 1.3x6.0 | SMA | CD31, CD34, CD117,
DOG1, Desmin, S100, AE1/AE3, AFP | Right
hemihepatecto-my | None; no
recurrence | (7) |
| Sun et al,
2019 | 45 | F | 2.2x1.8x0.9 | CD34, SMA, Ki-67
(2%) | Desmin, S100, CK,
ER | Left lateral hepatic
lobectomy | None; no
recurrence | (8) |
Several studies have been conducted to examine the
imaging characteristics of this rare entity. Detection of
intratumor and peritumor blood flow by color Doppler imaging can
reveal a number of specific imaging features, including the
‘layered’ or ‘swirled’ sign of internal echogenicity and
finger-like growth patterns (9).
On MRI, AM shows a low signal intensity on T1-weighted
imaging and high-intensity signals on T2-weighted
imaging (10), with a laminar and
vortex-like arrangement of low-signal stripes. In the present
patient, the imaging features were in agreement with the previous
reports.
Myxomatous tumors with thin-walled blood vessels
occur infrequently in the liver. Angiomyofibroblastoma (AMFB) is
most likely to be confused with AM. AMFBs and AMs are both enriched
in blood vessels and mucus, so they are easily confused on
microscopic examination; however, AMs show more distinctive myxoid
degeneration and more numerous blood vessels than AMFBs. AMFBs
generally exhibit much greater cellularity, and their tumor cells
are ovoid, numerous and clustered around blood vessels in the form
of bundles or nests; by contrast, AM cells are sparsely and
diffusely distributed (11). AMFBs
are characterized by the expression of vimentin, desmin and CD34.
Desmin, in particular, is considered to be the most specific marker
for identification. The expression of desmin plays a crucial role
in distinguishing AMFB and AM. However, it should be noted that
positive desmin expression has been observed in previously
documented cases of AM in the liver (5,7-8).
Desmin expression in AM may be partially or completely absent,
whereas desmin and vimentin expression in AMFB is diffusely
positive (12). It is imperative
to distinguish myxoid neurofibroma and myxoid liposarcoma from AM
in clinical practice. In myxoid neurofibroma, the majority of
neoplastic cells are positive for S-100, with a minority of cells
expressing CD34(13). The tumor
cells are heterogeneous and show lipoblasts of varying degrees of
differentiation, and the interstitial vessels tend to clump or
branch. The tumor cells of myxoid liposarcoma are heterogeneous and
show lipoblasts of varying degrees of differentiation, and the
interstitial vessels tend to be clumped or branched (14). Primary gastrointestinal stromal
tumors of the liver have specific histological features and
cytological manifestations, including positive expression of c-Kit,
CD117 and CD34(15). Based on the
aforementioned findings, AM can be distinguished by pathological
and immunohistochemical analysis.
The coexistence of AM and FNH was difficult to
identify in the present case. However, ultrasonography of typical
FNH is distinctive and reveals a central location of stellate
scarring with spoke-like enhancement in the arterial phase.
Hepatocellular hyperplastic nodules, abnormal blood vessel growth
in the central scar, bile duct hyperplasia and fibrous septa are
typical histological features of FNH (16). The present study suggests that both
AM and FNH are common conditions among women. AM tumor cells are
mostly positive for ER, PR and androgen receptor, which suggests
that hormones may manipulate the occurrence and/or development of
AM (17). Additionally, exposure
to oral contraceptive pills and endogenous hormones is considered
to play a role in the development of FNH (18). In summary, further research is
needed to determine the potential involvement of hormone exposure
in the underlying mechanism of the co-occurrence of AM and FNH.
According to a previous case report, FNH formation
has been proposed to occur as a result of a hyperplastic reaction
to a vascular anomaly (19).
Common microscopic characteristics of AM include a multitude of
irregularly shaped, thin-walled blood vessels. Vascular anomalies
are common pathological features of both conditions; therefore, we
hypothesize that an underlying pathological change or genetic
alteration causes the common histogenesis of abnormal angiogenesis.
However, there are no reports in the literature on the possible
common aetiology of the two diseases, and perhaps they occur
independently of each other, with no pathogenetic link between
them. There were some limitations to the present study. Most
important of all, there was no macroscopic image containing both
lesions, for the reason that the subject would benefit from
preserving as much of the normal liver tissue as possible.
Therefore, the lesions were resected separately during the surgical
procedure.
It has been widely postulated that employing a
surgical technique involving a broad incision effectively mitigates
the likelihood of postoperative recurrence. However, some studies
have shown that there is no significant correlation between
surgical margins and postoperative recurrence (20). The recurrence factors of AAM are
unknown, and there is a lack of good indicators for predicting the
possibility of recurrence. The gold standard for diagnosing AM is
postoperative pathology. In the present case, the main microscopic
features of the AM included numerous irregularly organized walled
vessels and scattered small spindles, ovoids or astrocytes on a
loose mucus background. Immunohistochemical analysis indicated that
the tumor tissue, which exhibited characteristic differentiation
towards fibroblasts and myofibroblasts, likely originated from the
mesenchyme.
Gonadotropin-releasing hormone analog therapy is
regarded as an effective therapeutic approach for patients whose
specimens are positive for ER and/or PR (21). Due to the negative staining result
for ER and PR in the present study, the patient was not
administered any other adjuvant therapy after the operation. It is
possible that distant metastasis and local recurrence could still
develop; however, the patient was regularly reviewed, with no signs
of recurrence or metastasis as of the 16-month follow-up.
In conclusion, the present study reports a case in
which AM of the liver coexisted with FNH. The simultaneous
occurrence of AM and FNH in the liver poses diagnostic challenges,
and differentiating them from other benign and malignant liver
tumors represents a novel and previously unreported challenge. AM
is extremely rare clinically and often confused with other
diseases. To diagnose AM, a medical history, CT, MRI and
cytological examinations, and preoperative and postoperative
pathological tests are needed. The preferred treatment is surgery
with complete resection, and patients with AM are advised to
undergo annual postoperative follow-up examinations.
Supplementary Material
Immunohistochemical staining
CT and immunohistochemical results of
AM and FNH. (A) CT images of AM and FNH in the delayed phase. (B)
Images showing the gross tumor after excision. (C) The expression
level of c-Kit and DOG1 in AM and FNH was evaluated via
immunohistochemistry. CT, computed tomography; AM, angiomyxoma;
FNH, focal nodular hyperplasia; DOG1, anoctamin 1; c-Kit,
proto-oncogene c Kit.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the National Natural Science
Foundation of China (grant no. 82160571).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XL and BQW made substantial contributions to study
conception and design, acquisition of data, and analysis and
interpretation of data. XL and KX were involved in drafting the
manuscript and revising it critically for important intellectual
content. WHL, CL and CY managed the patient. XXL reported the
pathological findings. WHL, CL, JTT and BQW performed the surgery.
KX, YZ and ZYJ obtained medical images. WHL, JTT and BQW advised on
patient treatment and analyzed the patient data. XL, KX and BQW
analyzed the patient data. XL and BQW confirm the authenticity of
all the raw data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for the case to
be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun J, Lian PH, Ye ZX, Dong X, Ji ZG, Wen
J and Li HZ: Aggressive angiomyxoma in the scrotum: A case series
and literature review. Front Surg. 9(762212)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li W, Chen J, Zhang E, Chen W, Hu Y, Miao
C and Luo C: Characteristics and outcomes of patients with primary
abdominopelvic aggressive angiomyxoma: A retrospective review of 12
consecutive cases from a sarcoma referral center. BMC Surg.
23(88)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ding Z, Lin K, Fu J, Huang Q, Fang G, Tang
Y, You W, Lin Z, Lin Z, Pan X and Zeng Y: An MR-based radiomics
model for differentiation between hepatocellular carcinoma and
focal nodular hyperplasia in non-cirrhotic liver. World J Surg
Oncol. 19(181)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Steeper TA and Rosai J: Aggressive
angiomyxoma of the female pelvis and perineum. Report of nine cases
of a distinctive type of gynecologic soft-tissue neoplasm. Am J
Surg Pathol. 7:463–475. 1983.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qi S, Li B, Peng J, Wang P, Li W, Chen Y,
Cui X, Liu C and Li F: Aggressive angiomyxoma of the liver: A case
report. Int J Clin Exp Med. 8:15862–15865. 2015.PubMed/NCBI
|
|
6
|
Sato K, Ohira M, Shimizu S, Kuroda S, Ide
K, Ishiyama K, Kobayashi T, Tahara H, Shiroma N, Arihiro K, et al:
Aggressive angiomyxoma of the liver: A case report and literature
review. Surg Case Rep. 3(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Malik AK, Filobbos R, Manoharan A, Harvey
N, O'Reilly DA and de Liguori Carino N: A case report of an
angiomyxoma in the liver. Ann R Coll Surg Engl. 100:e81–e84.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun PJ, Yu YH and Cui XJ: Large aggressive
angiomyxoma of the liver: A case report and brief review of the
literature. Front Oncol. 9(133)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao CY, Su N, Jiang YX and Yang M:
Application of ultrasound in aggressive angiomyxoma: Eight case
reports and review of literature. World J Clin Cases. 6:811–819.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kumar N, Goyal A, Manchanda S, Sharma R,
Kumar A and Bansal VK: Aggressive pelvic angiomyxoma and its
mimics: Can imaging be the guiding light? Br J Radiol.
93(20200255)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiu P, Wang Z, Li Y and Cui G: Giant
pelvic angiomyofibroblastoma: Case report and literature review.
Diagn Pathol. 9(106)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang YF, Qian HL and Jin HM: Local
recurrent vaginal aggressive angiomyxoma misdiagnosed as cellular
angiomyofibroblastoma: A case report. Exp Ther Med. 11:1893–1895.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shaker N, Iwenofu H, Shaker N, Tynski Z,
Sangueza OP and Abid A: Myxoid neurofibroma masquerading as
lymphatic-venous malformation and poses a diagnostic challenge on
fine needle aspiration biopsy. Diagn Cytopathol. 52:E111–E115.
2024.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
De Vita A, Mercatali L, Recine F, Pieri F,
Riva N, Bongiovanni A, Liverani C, Spadazzi C, Miserocchi G,
Amadori D and Ibrahim T: Current classification, treatment options,
and new perspectives in the management of adipocytic sarcomas. Onco
Targets Ther. 9:6233–6246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qian XH, Yan YC, Gao BQ and Wang WL:
Prevalence, diagnosis, and treatment of primary hepatic
gastrointestinal stromal tumors. World J Gastroenterol.
26:6195–6206. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wakui N, Takayama R, Kamiyama N, Kobayashi
K, Matsui D, Matsukiyo Y, Kanekawa T, Ikehara T, Ishii K and Sumino
Y: Arrival time parametric imaging using Sonazoid-enhanced
ultrasonography is useful for the detection of spoke-wheel patterns
of focal nodular hyperplasia smaller than 3 cm. Exp Ther Med.
5:1551–1554. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lin XM, Wang L and Wang Q: Aggressive
angiomyxoma of pelvis: A case report and literature review.
Medicine (Baltimore). 101(e31617)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fukahori S, Kawano T, Obase Y, Umeyama Y,
Sugasaki N, Kinoshita A, Fukushima C, Yamakawa M, Omagari K and
Mukae H: Fluctuation of hepatic focal nodular hyperplasia size with
oral contraceptives use. Am J Case Rep. 20:1124–1127.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
LeGout JD, Bolan CW, Bowman AW, Caserta
MP, Chen FK, Cox KL, Sanyal R, Toskich BB, Lewis JT and Alexander
LF: Focal nodular hyperplasia and focal nodular hyperplasia-like
lesions. Radiographics. 42:1043–1061. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gay F, Champigneulle J, Tortuyaux JM, Cuny
T, Régent D and Laurent-Croisé V: Aggressive angiomyxoma. Diagn
Interv Imaging. 94:657–661. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gorgulu G, Kole M, Ayaz D, Kuru O, Gokcu M
and Sanci M: Aggressive Angiomyxoma. A case series of eight years
of experience. Ann Ital Chir. 93:562–565. 2022.PubMed/NCBI
|