Introduction
Asthma, a chronic and heterogeneous disease
affecting the lower airways, is characterized by persistent
inflammation and airway hyper-responsiveness, resulting in symptoms
such as coughing, wheezing, dyspnea and chest tightness (1,2). The
prevalence of asthma varies worldwide, ranging from 2.1% in
Indonesia to 32.2% in the United Kingdom due to environmental
differences (3). Based on
extrapolation from existing data, the World Health Organization
predicts a projected increase in the number of individuals with
asthma by an additional 100 million up to 2025(4). Therefore, the exploration of novel
therapies and therapeutic targets is imperative to enhance symptom
control and minimize exacerbations in patients with severe
asthma.
The airway epithelium functions as the primary
interface of the body with inhaled air and other substances,
establishing the initial defense barrier against exogenous
particles (5). Airway epithelial
cells constitute the frontline defense against inflammatory stimuli
and antigens, safeguarding the airways and lungs from exposure
(6). Bronchial biopsies commonly
exhibit shedding of bronchial epithelial cells, which is a
significant histological characteristic of patients with asthma
(7). A number of studies have
reported an increased incidence of apoptosis in bronchial
epithelial cells among adults with asthma (8,9).
Research has shown that nuclear factor erythroid-derived 2-related
factor 2 (Nrf2) is an essential endogenous transcription factor
with antioxidant and antiapoptotic properties (10,11).
In normal conditions, Nrf2 remains inactive in the cytoplasm while
bound to its inhibitor, Kelch-like ECH associated protein 1 (Keap1)
(12). However, exposure to
environmental stress triggers the activation of Nrf2 by separating
it from Keap1. This leads to its translocation into the nucleus and
subsequent stimulation of various genes responsible for antioxidant
activity, such as NAD(P)H quinone oxidoreductase 1 (NQO1), heme
oxygenase 1 (HO-1) and glutathione peroxidase (13). The Nrf2 signaling pathway serves a
crucial role in protecting individuals with asthma and maintaining
the integrity of bronchial epithelial barriers (14). Additionally, studies have reported
that activating Nrf2 can inhibit apoptosis in bronchial epithelial
cells, reduce airway inflammation, alleviate airway
hyper-responsiveness and mitigate oxidative stress in mouse models
of asthma (15-17).
Therefore, gaining a deeper understanding of airway epithelial
apoptosis and activation of the Nrf2 signaling pathway may uncover
novel therapeutic approaches for managing asthma.
Long-acting β2 agonists and inhaled corticosteroids
are commonly used as bronchodilators and anti-inflammatory agents
in the treatment of asthma (18).
However, the use of these drugs can lead to numerous side effects,
such as dysphonia, xerostomia, adrenal insufficiency and
osteoporosis (19). Cysteinyl
leukotrienes (CysLTs) are a group of lipid mediators that exhibit
proinflammatory activities and cause constriction of the bronchi
during allergic inflammation (20). A number of studies have reported
the presence of elevated levels of CysLTs in the urine or exhaled
air condensate from individuals with asthma (21,22).
The majority of the effects induced by CysLTs, which are relevant
to the pathophysiology of asthma, are mediated through the
activation of CysLT receptor 1 (CysLTR1) (23). This receptor was among the first
specific mediators successfully targeted for drug development
against asthma symptoms (24).
Consequently, CysLTR1 antagonists are considered alternative
medications for treating asthma effectively (25), resulting in the widespread use of
prescription drugs targeting CysLTR1 (26-28).
Montelukast sodium, a CysLTR1-specific antagonist, has shown
efficacy in reducing pulmonary fibrosis, airway
hyper-responsiveness and inflammation in mouse models of asthma
(29,30). However, the effects of CysLTR1
blockade on bronchial epithelial cell apoptosis and the Nrf2
signaling pathway during asthma progression are currently poorly
understood.
In the present study, an ovalbumin (OVA)-induced
asthmatic rat model was established. The effects of different doses
of montelukast sodium on bronchial epithelial cell apoptosis and
the Nrf2 signaling pathway in asthma progression were investigated.
These results may further clarify the role of CysLTR1 on the
progression of asthma and expand our understanding of the
protective mechanism of CysLTR1 antagonists in asthma
pathogenesis.
Materials and methods
Reagents
Montelukast sodium, OVA (grade V) and bovine serum
albumin (BSA) were purchased from MilliporeSigma. Aluminum
hydroxide gels were purchased from Thermo Fisher Scientific, Inc.
The alcian blue & periodic acid-Schiff (AB-PAS) staining kit
and TUNEL cell apoptosis kit were purchased from Solarbio Science
& Technology Co., Ltd. The Masson's trichrome staining kit was
purchased from Maxim Biotech, Inc. The hematoxylin & eosin
(H&E) staining kit and Wright-Giemsa stain kit were purchased
from Abcam. Primary antibodies targeting CysLTR1 (cat. no.
27372-1-AP), Nrf2 (cat. no. 16396-1-AP) and GAPDH (cat. no.
60004-1-Ig) were purchased from Proteintech Group. Inc. The RIPA
lysis buffer was purchased from Wuhan Boster Biological Technology,
Ltd. The ECL reagent was purchased from Tanon Science and
Technology Co., Ltd. and the BCA reagent was purchased from Thermo
Fisher Scientific, Inc. The Rat IgE ELISA kit (cat. no. EKF58258)
was purchased from Biomatik. The Rat IL-17 (cat. no. KTE9005) and
IL-4 (cat. no. KTE9003) ELISA kits as well as HRP-conjugated
secondary antibodies (cat. nos. A21020 and A21010) were purchased
from Abbkine Scientific Co., Ltd. The reduced glutathione
(GSH)/oxidized glutathione (GSSG) Ratio Fluorometric Detection
Assay Kit (cat. no. 50120ES70) was purchased from Shanghai Yeasen
Biotechnology Co., Ltd. The total RNA extraction kit (cat. no.
LS1040) was purchased from Promega Corporation. The First Strand
Kit and QuantiFast SYBR® Green PCR Kit were purchased
from Qiagen GmbH.
Animal grouping and treatment
A total of 30 Sprague-Dawley male rats (age, 8-10
weeks) weighing 240±5 g, were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. All rats were housed in
specific pathogen free cages under standard laboratory conditions,
which included a temperature of 22-25˚C, a relative humidity of
40-55% and a 12/12 h light/dark cycle with free access to water and
food. Following acclimation, the rats were randomly assigned to
five treatment groups: i) The control (Group I); ii) model (Group
II); iii) low-dose (1 mg/kg) montelukast sodium (Group III); iv)
medium-dose (4 mg/kg) montelukast sodium (Group IV); and v)
high-dose (30 mg/kg) montelukast sodium (Group V) groups, with 6
rats/group. OVA was dissolved in 4% aluminum hydroxide gels to
prepare an OVA solution (2 mg/ml). The OVA-induced asthmatic rat
model was established as previously described (31,32)
with some amendments. On days 0 and 14, rats in Groups II-V were
sensitized with an intraperitoneal injection of OVA solution (0.5
ml/rat). Rats in Group I were administered an equal volume of
saline. From the 15th day, all rats apart from the control group,
were administered with inhaled OVA aerosol (10 mg/ml; dissolved in
saline; 30 min/day) for 3 weeks. Before the OVA challenge (from the
15th day onwards), rats in Group I and II were given 10 ml/kg/day
of saline by gavage, while rats in Groups III-V were given
montelukast sodium by gavage at doses of 1, 4 and 30 mg/kg/day,
respectively. The gavage procedures were continuously conducted
until sample collection. The experiment duration was 5 weeks. The
heart rate of the animals was monitored each day using a
polyethylene cannula (PE 50) filled with heparinized saline (100
IU/ml) inserted into the right carotid artery. The cannula was
connected to a transducer, and the signal was amplified by
bioamplifier and an acquisition data system (AD Instruments Pvt.
Ltd. with software LabChart 7.3; AD Instrument Pvt. Ltd). Body
weight was monitored each week. Throughout the experiment, the aim
was to minimize the utilization of animals and alleviate their
distress as much as possible. According to the analgesic methods
described in previous studies, intraperitoneal injection of 5 mg/kg
tramadol has been reported to be a safe and effective analgesic in
rats and mice (33,34). In preliminary experiments, 5 mg/kg
tramadol was found to effectively alleviate pain in rats without
any side effects or mortality (data not shown). Therefore, 5 weeks
later, all animals received an intraperitoneal injection of
tramadol (5 mg/kg) as an analgesic method to minimize pain,
suffering and distress. The rats were housed individually in a
polycarbonate cage and allowed to recover on a heating pad to
maintain a body temperature of 37.5±0.5˚C. In addition, the rats
were monitored for any signs of fatigue and stress. Researchers
were trained to apply the humane endpoints, if any animal exhibited
features of a compromised welfare. The humane endpoints included
rapid weight loss (>20% of normal body weight) and/or rapid or
labored breathing. No animals died naturally during the experiments
and all of the rats were euthanatized by an intraperitoneal
injection of pentobarbital sodium overdose (200 mg/kg) on day 35.
Death was confirmed by cardiac and respiratory arrest and a lack of
response to tail clamping. The bronchoalveolar fluid lavage (BALF)
was obtained by washing the lungs and subsequent analysis involved
the collection of lung tissues and airway tissues. To analyze the
level of OVA-specific IgE in serum, blood samples (300 µl) were
collected from rats by cardiac puncture. Whole blood was collected
and left to coagulate at room temperature for at least 30 min, and
then centrifuged at 1,000 x g for 10 min at 4˚C. The serum samples
were stored at -20˚C until use. The experiments in the present
study were carried out by three skilled technicians who were
unaware of the experimental design and purpose. Animal experiments
followed the guidelines provided by the National Institutes of
Health Guide for the Care and Use of Laboratory Animals and
received approval from the Ethics Committee of Jinhua Polytechnic
(approval no. 20221221; Jinhua, China).
Histopathologic examination
The airway tissues were fixed using 4%
paraformaldehyde (48 h; 4˚C), embedded in paraffin and cut into
5-µm sections. Subsequently, sections were stained with hematoxylin
for 5 min and eosin for 2 min at room temperature using a H&E
staining kit. Masson's staining was performed using a Masson's
trichrome staining kit in accordance with the manufacturer's
protocol to observe collagen deposition at room temperature for a
total of 15 min (Wiegert's iron hematoxylin, 8 min; Biebrich
scarlet, 5 min; aniline blue, 2 min) at room temperature. Based on
the manufacturer's protocol of the AB-PAS staining kit, sections
were stained with alcian blue for 30 min and periodic acid Schiff
for 15 min at room temperature. The pathological structure of
airway tissues was observed using a BX53 light microscope (Olympus
Corporation).
ELISA and biochemical assays
According to the manufacturer's instructions, the
GSH/GSSG ratio, levels of IL-4 and IL-17 in lung tissues and serum
IgE concentration were determined using corresponding commercial
kits (35).
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from lung tissues was extracted using a
total RNA extraction kit. The concentration of total RNA was
measured using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Total RNA (500 ng) was reverse transcribed into
cDNA at 42˚C for 45 min using a First Strand Kit and RT-qPCR was
performed using the QuantiFast SYBR® Green PCR Kit,
according to the manufacturer's instructions. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 3 min; followed by 40 cycles of
denaturation at 95˚C for 15 sec, annealing at 60˚C for 30 sec and
elongation at 72˚C for 1 min, as well as a final extension at 72˚C
for 5 min. To determine gene expression levels, the
2-ΔΔCq method was used (36) and results were normalized to GAPDH
as a reference gene (37). The
primer sequences used are presented in Table I.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| Cysteinyl
leukotriene receptor 1 | F:
CAAATGTGCCATGCCCTGAC |
| | R:
GGTCCACTCCATTCACAGGG |
| NAD(P)H quinone
oxidoreductase 1 | F:
AGCGCTTGACACTACGATCC |
| | R:
TCTGCGTGGGCCAATACAAT |
| Heme oxygenase
1 | F:
ATGCCCCACTCTACTTCCCT |
| | R:
TACGTAGTGCTGTGTGGCTG |
| GAPDH | F:
ACTCCCATTCTTCCACCTTTG |
| | R:
CCCTGTTGCTGTAGCCATATT |
BALF analysis
The BALF samples were stained using a Wright-Giemsa
stain kit, and the eosinophil, lymphocyte and macrophage counts
were recorded under a light microscope (Olympus Corporation) and
analyzed using Image-Pro-Plus (version 6.0; Media Cybernetics). The
inhibitory activity (%) was evaluated as the following formula:
(1-A/B) x100%. A represents the number of inflammatory cells in
different groups of montelukast sodium; B represents the number of
inflammatory cells in the model group.
Immunofluorescence assays
Lung tissue sections (5 µm) underwent
deparaffinization in xylene for 10 min at room temperature and
rehydration with descending concentrations of ethanol (100, 95 and
70% for 3-5 min each), followed by antigen retrieval in heated
citrate buffer (10 mM; pH 6.0) at 80˚C for 25 min. Subsequently,
the samples were washed three times with PBS before being
permeabilized using 0.5% TritonX-100 in PBS. The sections were then
blocked with 5% BSA at room temperature for 1 h. Next, the samples
were incubated with primary antibodies against Nrf2 (1:50)
overnight at 4˚C and the corresponding secondary antibodies (1:200)
for 2 h at room temperature. Finally, after staining with DAPI (1
µg/ml) at room temperature for 15 min, the samples were imaged
using a fluorescence microscope (Olympus Corporation).
Image-Pro-Plus (version 6.0; Media Cybernetics) was adopted to
analyze the fluorescence intensity.
TUNEL assay
Apoptosis of lung tissues was determined in
accordance with the experimental procedures outlined in the
manufacturer's guidelines for the TUNEL kit. In brief, the lung
tissues were fixed using 4% paraformaldehyde (48 h; 4˚C), embedded
in paraffin and cut into 5-µm sections. The deparaffinized tissue
sections were incubated with 3% hydrogen peroxide in methanol for
10 min at 25˚C in the dark, washed three times with PBS and
incubated with 0.1% Triton X-100 in freshly prepared 0.01% sodium
citrate for 8 min at 25˚C. Tissue sections were then incubated with
proteinase K working solution for 25 min at 37˚C and washed three
times with PBS (pH 7.4) for 5 min each. A total of 50 µl TUNEL
reagent was added to each sample and incubated at 37˚C for 60 min.
The sections were washed three times with PBS (pH 7.4) and then
cell nuclei were counterstained with 2 µg/ml DAPI solution at room
temperature for 10 min in the dark and mounted with 50 µl anti-fade
mounting medium. TUNEL-positive cells were observed in five
randomly-selected fields using a fluorescence microscope (Olympus
Corporation) and analyzed using Image-Pro-Plus (version 6.0; Media
Cybernetics).
Western blot analysis
The RIPA lysis buffer was employed for the
extraction of total proteins from lung tissues, followed by
quantification using a BCA kit. Subsequently, a total of 50 µg of
protein/lane was separated by 10% SDS-PAGE and proteins then were
transferred to polyvinylidene fluoride membranes. Following
blocking with 5% nonfat milk for 2 h at 25˚C, membranes were
incubated with primary antibodies against CysLTR1 (1:4,000), Nrf2
(1:7,000) and GAPDH (1:50,000) overnight at 4˚C. Then,
tris-buffered saline with 0.05% Tween-20 was used to wash the
membranes three times. Subsequently, at room temperature, the
HRP-conjugated secondary antibodies (1:10,000) were incubated with
samples for 1 h. The signals were detected using an ECL kit
(Beyotime Institute of Biotechnology) and blots were quantified
under a Gel-Proanalyzer (version 4.0; Media Cybernetics). GAPDH was
used as the loading control (38).
Statistical analysis
Data were analyzed using one-way ANOVA, followed by
Tukey's post-hoc test. Data analysis was performed using SPSS
software (version 22.0; IBM Corp.). The data are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blockade of CysLTR1 alleviates
inflammation in asthmatic rats through Nrf2
The heart rate and body weight of rats were
monitored throughout the study. The results demonstrated a
significant decrease in the heart rate of the model group compared
with that in the control group (Fig.
1A; P<0.001). Administration of montelukast sodium
significantly increased the heart rate of rats at all doses tested,
compared with that in the model group (P<0.001). From day 21, a
significant decrease in body weight was observed in the model group
compared with the control group (Fig.
1B; P<0.05); however, administration of montelukast sodium
significantly restored the body weight of asthmatic rats from the
28th day onwards (P<0.05).
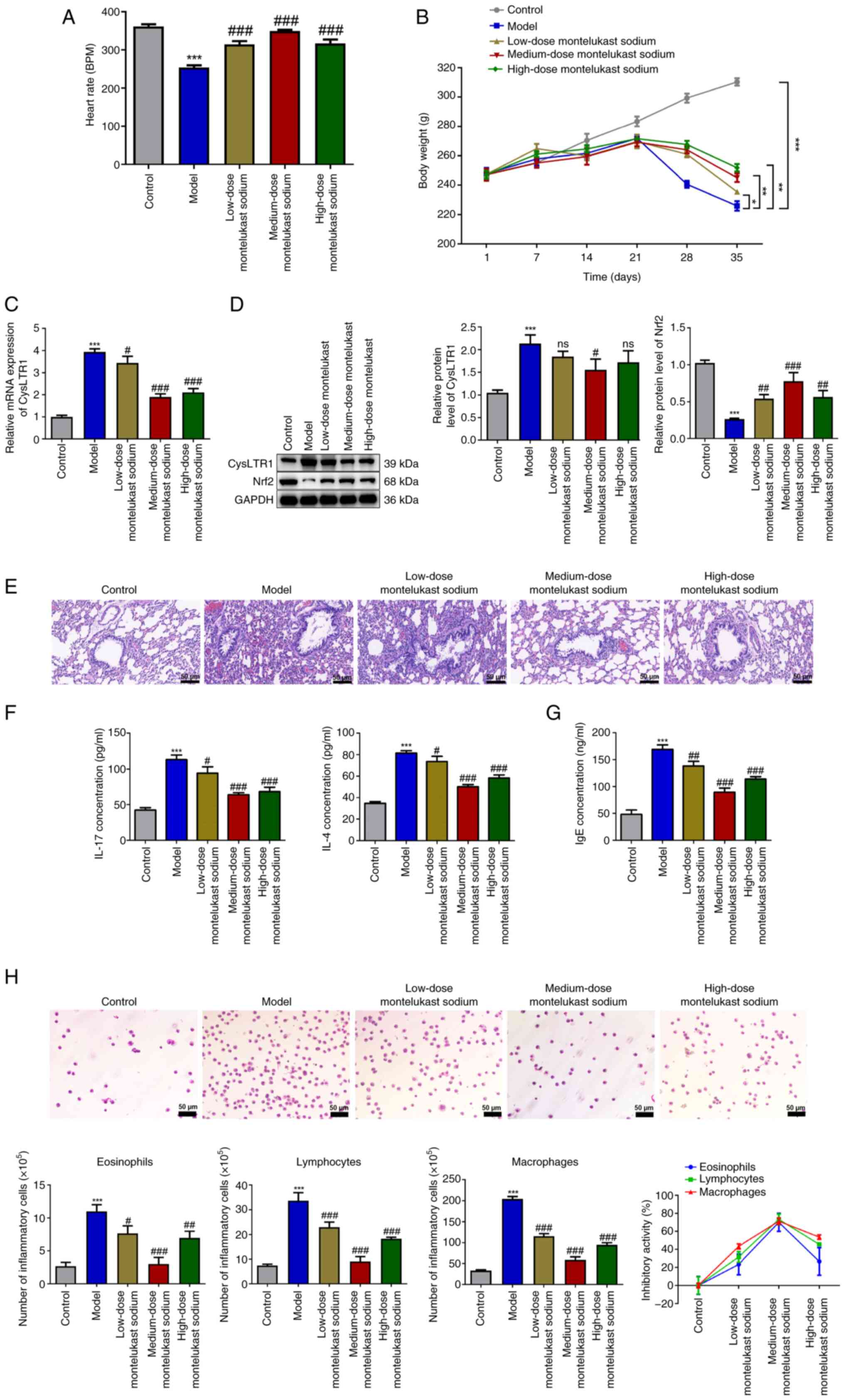 | Figure 1Blockade of CysLTR1 alleviates
inflammation in asthmatic rats. (A) Heart rate and (B) body weight
of different groups of rats treated with montelukast sodium.
*P<0.05, **P<0.01,
***P<0.001. (C) mRNA expression levels of CysLTR1 in
lung tissues were determined using reverse
transcription-quantitative PCR. (D) Protein expression levels of
CysLTR1 and Nrf2 in lung tissues were determined by western
blotting. (E) The histopathological changes in lung tissues from
different groups of rats treated with montelukast sodium were
assessed by hematoxylin and eosin staining (scale bar, 50 µm). (F)
Expression levels of IL-17 and IL-4 in the lung and (G) serum IgE
were determined by ELISA. (H) Numbers of inflammatory cells in
bronchoalveolar lavage fluid were measured by Wright-Giemsa
staining and the association between the dose of montelukast sodium
and changes in the number of lymphocytes, eosinophils and
macrophages was assessed (scale bar, 50 µm).
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. model. ns,
not significant; BPM, beats per minute; Nrf2, nuclear factor
erythroid-derived 2-related factor 2; CysLTR1, cysteinyl
leukotriene receptor 1. |
Following administration of different doses of
montelukast sodium, the mRNA and protein expression levels of
CysLTR1 in lung tissues were measured. The model group demonstrated
a significant increase in both the mRNA and protein expression
levels of CysLTR1 compared with that of the control group (Fig. 1C; P<0.001). Moreover, it was
demonstrated that, compared with the model group, administration of
montelukast sodium significantly reduced the mRNA expression level
of CysLTR1 to varying degrees (P<0.05); however, only
administration of the medium-dose montelukast sodium decreased the
protein expression level of CysLTR1 significantly compared with
that in the model group (Fig. 1D;
P<0.05). On the contrary, a significant decrease in the protein
expression level of Nrf2 was observed in the model group compared
with that in the control group (P<0.001), while all doses of
montelukast sodium significantly increased the Nrf2 protein
expression level, with the higher increase being observed at a
dosage of 4 mg/kg (P<0.001).
H&E staining was performed to assess the impact
of CysLTR1 blockade on inflammation in rat lung tissues. No
apparent inflammatory cell infiltration was observed in the control
group, while the model group demonstrated a noticeable infiltration
of inflammatory cells and visible thickening of smooth muscle
layers compared with the control group (Fig. 1E). Compared with the model group,
the administration of montelukast sodium, particularly at a dosage
of 4 mg/kg, reduced inflammatory infiltration and airway
remodeling. Upon OVA challenge, a significant increase in IL-17 and
IL-4 levels in the model group were observed compared with those in
the control group, which was consistent with the H&E staining
data (Fig. 1F; P<0.001).
Furthermore, treatment with montelukast sodium significantly
mitigated the proinflammatory effects induced by OVA challenge
(P<0.05). IgE is reported to have evolved in mammals as a
primary defense mechanism against pathogens, and increased IgE
levels are considered indicative of an increased susceptibility to
the development of asthma (39).
It was demonstrated that IgE concentration was significantly
increased in the model group compared with that in the control
group (Fig. 1G; P<0.001), while
the administration of montelukast sodium was effective in
decreasing IgE levels (P<0.01). Additionally, due to the strong
association of eosinophils, lymphocytes and macrophages with
inflammatory processes in asthma (40), cell count analysis was performed on
BALF samples from asthmatic rats. The results of Wright-Giemsa
staining demonstrated that the model group exhibited a significant
increase in the numbers of eosinophils, lymphocytes and
macrophages, compared with those in the control group (Fig. 1H; P<0.001). Administration of
montelukast sodium significantly decreased the elevated cell counts
in the BALF of asthmatic rats induced by OVA challenge (P<0.05).
The association between the dose of montelukast sodium and changes
in the number of these inflammatory cells was then assessed. It was
observed that varying dosages of montelukast sodium exhibited the
lowest inhibitory activity on eosinophils and the highest
inhibitory activity on macrophages. Moreover, among the three doses
of montelukast sodium tested, the 4 mg/kg dose demonstrated the
largest inhibitory efficacy across all three types of inflammatory
cells.
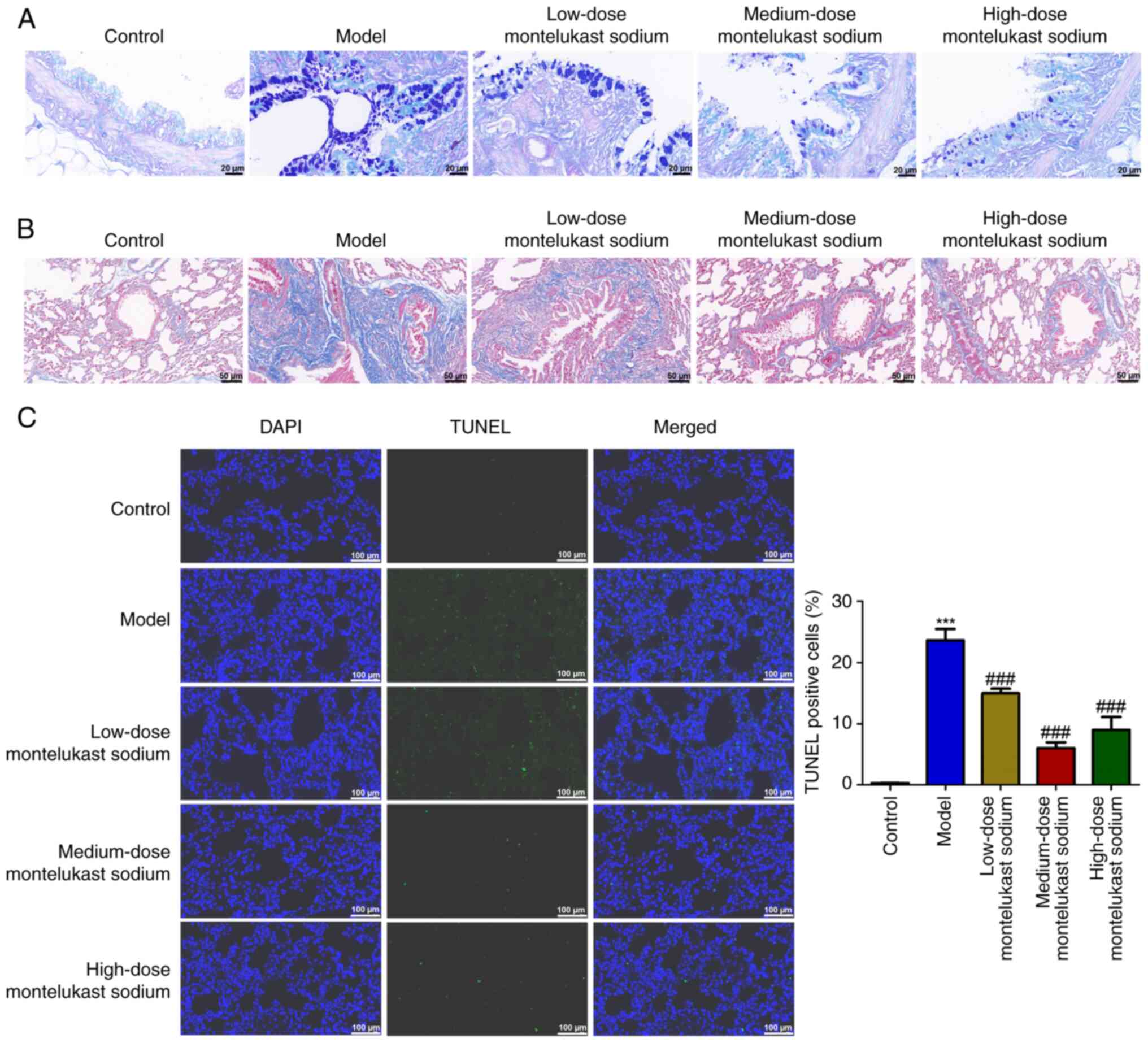
Blockade of CysLTR1 attenuates airway
remodeling in asthmatic rats
The hyperplasia of goblet cells and the deposition
of collagen in the lungs are crucial indicators for the progression
of asthma (41,42). The model group of rats demonstrated
marked goblet cell hyperplasia in comparison with the control group
(Fig. 2A). However, the
montelukast sodium-treated groups showed a decreased degree of
goblet cell hyperplasia compared with that in the model group
(Fig. 2A). Additionally, the
asthmatic rat model exhibited an exacerbation of OVA-induced
collagen deposition in the lung tissue; however, treatment with
montelukast sodium mitigated these changes. A TUNEL assay was used
to examine the impact of montelukast sodium on bronchial epithelial
cell apoptosis. The number of TUNEL-positive cells in the model
group was significantly increased compared with that in the control
group (Fig. 2C; P<0.001),
whereas the number of TUNEL-positive cells was significantly
reduced by the administration of montelukast sodium, compared with
that in the model group (P<0.001).
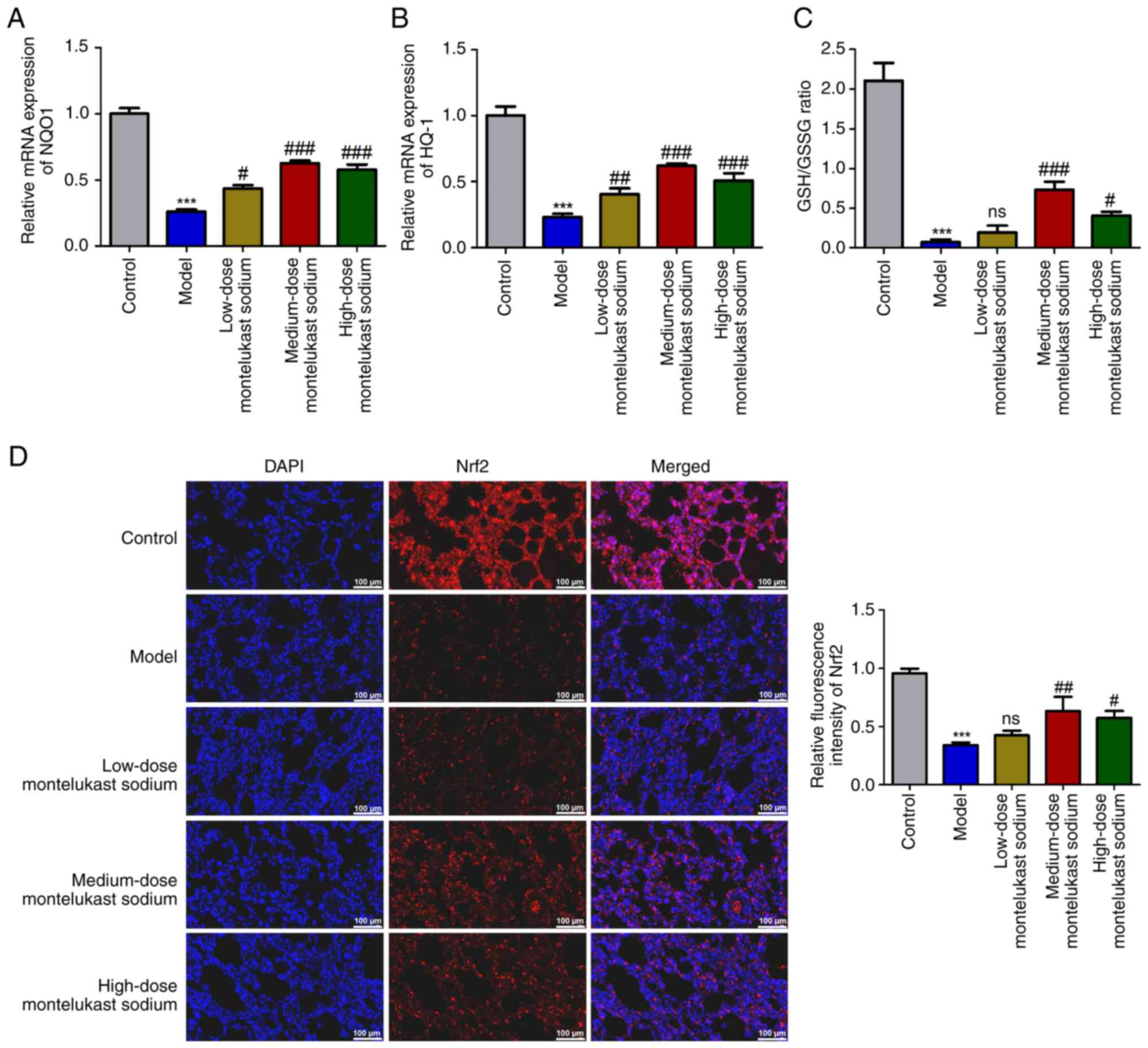
Blockade of CysLTR1 inhibits oxidative
stress and activates Nrf2 in asthmatic rats
The expression levels of antioxidant genes NQO1 and
HO-1 were measured to investigate the involvement of CysLTR1
blockade in oxidative stress. Additionally, the GSH/GSSG ratio was
calculated, due to its reported role in scavenging free radicals
(43). A significant decrease in
the mRNA expression levels of NQO1 and HO-1 was demonstrated in the
model group compared with those in the control group (Fig. 3A and B; P<0.001). Additionally, a
significant reduction in the GSH/GSSG ratio was also demonstrated
(Fig. 3C; P<0.001). These
inhibitory effects induced by OVA challenge were reversed by
treatment with montelukast sodium, particularly at a dosage of 4
mg/kg (Fig. 3A-C; P<0.001). The
transcription factor Nrf2, which is responsible for regulating
cellular redox balance and initiating protective antioxidant
responses in mammals, has been reported to be an important
therapeutic target for mitigating oxidative stress injury in asthma
(44). Immunofluorescence
microscopy demonstrated a significant decrease in Nrf2 fluorescence
intensity in the model group compared with that in the control
group (Fig. 3D; P<0.001), while
there was a significant increase following treatment with 4 or 30
mg/kg montelukast sodium (Fig. 3D;
P<0.05).
Discussion
The prevalence of asthma has increased over recent
decades, concomitant with the process of urbanization and
industrialization (45). Despite
strict adherence to prescribed anti-asthma medication, certain
patients with asthma continue to experience uncontrolled clinical
symptoms, indicating an ongoing need for effective management
(46). Medical care and excessive
absenteeism related to asthma lead to substantial healthcare
expenditures exceeding $80 billion annually, including $50.3
billion in direct medical costs, $29 billion in asthma-related
mortality and $3 billion in absenteeism (47). Therefore, despite the current
availability of innovative therapies and evidence-based care,
asthma continues to pose a significant public health challenge. The
present study aimed to further elucidate the mechanism of action of
CysLTR1 in the progression of asthma in vivo, and the
results indicated that blockade of CysLTR1 may mitigate asthma
progression in an asthmatic rat model by inhibiting bronchial
epithelial cell apoptosis and activating Nrf2 signaling.
OVA challenge is a sensitization method used to
induce asthma in murine models (48). In the present study, the
pathological tissue of asthmatic rats exhibited noticeable
infiltration of inflammatory cells and visible thickening of smooth
muscle layers. Furthermore, elevated levels of inflammatory
cytokines IL-17 and IL-4 were also observed in asthmatic rats.
Inflammatory cells, such as macrophages, lymphocytes and
eosinophils, have previously been reported to be closely associated
with the inflammatory processes in asthma (40,49).
Eosinophilic airway inflammation is a defining characteristic of
disease severity in specific subsets of individuals with severe
asthma, and there is a direct association between eosinophil count
and the frequency of asthma exacerbation (50-52).
In the present study, a significant increase in the cell counts of
macrophages, lymphocytes and eosinophils in the BALF of asthma rats
were demonstrated. High levels of IgE are considered a biological
indicator of increased susceptibility to the development of asthma
(39). Furthermore, a previous
study showed significant specific IgE sensitization in a number of
patients, particularly among young individuals with severe forms of
asthma (53). Additionally, the
relative importance of IgE compared with eosinophils in severe
asthma has also been reported, indicating that IgE is the main
cause of allergic asthma, while eosinophilia is a consequence of
the overall process (54).
Similarly, the present study demonstrated a significant increase in
IgE levels in asthmatic rats compared with control rats. The
present results were consistent with the inflammatory
characteristics of asthma, indicating the successful establishment
of an asthmatic model in rats.
CysLTs, a crucial group of inflammatory mediators in
the pathophysiology of asthma, are generated by activated
macrophages, basophils, eosinophils, myeloid dendritic cells and
mast cells (55). The above immune
cells exert proinflammatory effects by specifically binding to
CysLTR1(56). Thus, antagonism of
their actions produces anti-inflammatory properties. In the present
study, montelukast sodium, a specific CysLTR1 antagonist, was used
to block CysLTR1 and the role of CysLTR1 blockade on inflammatory
responses during the progression of asthma was explored. Previous
studies have reported that when the dosage of montelukast sodium is
<1 mg/kg, there is no significant improvement in inflammation
(57,58); however, when the dosage is ≥3
mg/kg, it can significantly reduce airway remodeling and
inflammation (59,60). Additionally, it has also been
reported that a single high dose of montelukast sodium (≥30 mg/kg)
can alleviate inflammatory symptoms in animal models of asthma
(61). Therefore, in the present
study, low (1 mg/kg), medium (4 mg/kg) and high doses (30 mg/kg) of
montelukast sodium were administered to rat models with asthma.
Blockade of CysLTR1, particularly at the dose of 4 mg/kg of
montelukast sodium, significantly attenuated the inflammatory
symptoms and inflammatory cytokine levels in asthmatic rats.
Additionally, blockade of CysLTR1 also suppressed the number of
eosinophils, lymphocytes and macrophages in the BALF of asthmatic
rats. Among the three dosages of montelukast sodium tested, the 4
mg/kg dose demonstrated the highest inhibitory capacity across all
three types of inflammatory cells. These results suggested that
blockade of CysLTR1 may confer protection against inflammatory
infiltration in asthma progression. The specific binding of CysLTs
to CysLTR1 not only mediates the inflammatory response in the
progression of asthma, but also exerts a significant impact on
airway remodeling (62). The
present study demonstrated that the blockade of CysLTR1
significantly attenuated OVA-induced goblet cell hyperplasia and
collagen deposition, which indicated its potential role in
alleviating airway remodeling in asthmatic rats. It has been
reported that the apoptosis and shedding of bronchial epithelial
cells are significant histological characteristics in the
development of asthma (63).
Therefore, the present study investigated the role of CysLTR1
blockade on bronchial epithelial cell apoptosis through the TUNEL
assay. These results demonstrated that the blockade of CysLTR1
decreased the percentage of TUNEL-positive cells in asthmatic rats,
indicating the potential suppressive role of CysLTR1 blockade on
the apoptosis of bronchial epithelial cells. However, there are a
number of limitations in measuring the rate of apoptosis. First,
other techniques, such as flow cytometry and transmission electron
microscopy, are also available for measuring apoptosis. Second,
previous studies have reported that in allergies with an
inflammatory component, apoptosis is induced by proinflammatory
mediators, such as TNF-α, produced by inflammatory cells (64) and TNF-α expression is strongly
associated with the number of inflammatory cells present in
inflammatory reactions (65).
Moreover, TNF-α-induced cells have been shown by transmission
electron microscopy to exhibit features characteristic of the early
and advanced stages of apoptotic cell death, such as condensation
of chromatin at the nuclear periphery, fragmentation of nuclei and
formation of apoptotic bodies (66). Therefore, the expression levels of
TNF-α and the associations between TNF-α and inflammatory cell
numbers should be assessed in future studies. Collectively,
blockade of CysLTR1 could potentially alleviate the development of
asthma through inhibiting inflammation, airway remodeling and
bronchial epithelial cell apoptosis.
It has been reported that the dysregulation of
antioxidant and oxidant systems can expedite the exacerbation of
asthma (67). Nrf2 regulates the
encoding of antioxidant proteins through its interaction with
antioxidant response elements, making it the foremost endogenous
pathway for combating oxidative stress reported to date. HO-1 and
NQO1 are the two most important antioxidant genes downstream of the
Nrf2 pathway (68). HO-1
safeguards cellular integrity by suppressing oxidative stress and
maintaining mitochondrial function, while NQO1 directly scavenges
superoxide and contributes to the production of antioxidant forms
(69,70). Thus, the present study determined
the expression levels of Nrf2, HO-1 and NQO1 in asthmatic rats. The
oxidative stress damage during asthma development led to the
inhibition of the antioxidant genes Nrf2, HO-1 and NQO1 in
asthmatic rats. Furthermore, the GSH/GSSG ratio, which is another
major determinant of oxidative stress, was also suppressed
following OVA challenge. Additionally, blockade of CysLTR1,
particularly at a dosage of 4 mg/kg, restored the expression levels
of Nrf2, NQO1 and HO-1 and the GSG/GSSG ratio in asthmatic rats.
These findings suggested that blockade of CysLTR1 could activate
the Nrf2 signaling pathway to inhibit oxidative stress and affect
asthma progression.
In conclusion, the present study analysed the
potential of CysLTR1 blockade in asthma pathogenesis, indicating
that it could effectively suppress inflammation, airway remodeling,
bronchial epithelial cell apoptosis and oxidative stress to improve
asthma, potentially by activating the Nrf2 signaling pathway. This
may provide valuable insights for future potential clinical
therapeutic interventions for asthma.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Plan Project in Jinhua City (grant nos. 2021-3-153 and
2022-4-028) and the Applied Research of Public Welfare Technology
Foundation of Zhejiang Province (grant no. LGF21H010002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL made substantial contributions to the conception
and design of the study. XW, YC and SC made substantial
contributions to the acquisition, analysis and interpretation of
the data. XW drafted the manuscript. All authors critically revised
the manuscript for intellectual content. XW, YC and SC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental procedures were conducted in
compliance with the Guidelines for Care and Use of Laboratory
Animals of the National Institutes of Health and were approved by
the Ethics Committee of Jinhua Polytechnic (approval no. 20221221;
Jinhua, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cevhertas L, Ogulur I, Maurer DJ, Burla D,
Ding M, Jansen K, Koch J, Liu C, Ma S, Mitamura Y, et al: Advances
and recent developments in asthma in 2020. Allergy. 75:3124–3146.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Poon AH and Hamid Q: Severe Asthma: Have
we made progress? Ann Am Thorac Soc. 13 (Suppl 1):S68–S77.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stern J, Pier J and Litonjua AA: Asthma
epidemiology and risk factors. Semin Immunopathol. 42:5–15.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
GBD 2019 Chronic Respiratory Diseases
Collaborators. Global burden of chronic respiratory diseases and
risk factors, 1990-2019: An update from the Global Burden of
Disease Study 2019. EClinicalMedicine. 59(101936)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weinstock J, Chen XX, Nino G, Koumbourlis
A and Rastogi D: The interplay between airway epithelium and the
immune system-A primer for the respiratory clinician. Paediatr
Respir Rev. 38:2–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Miller RL, Grayson MH and Strothman K:
Advances in asthma: New understandings of asthma's natural history,
risk factors, underlying mechanisms, and clinical management. J
Allergy Clin Immunol. 148:1430–1441. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y, Jia M, Ou Y, Adcock IM and Yao X:
Mechanisms and biomarkers of airway epithelial cell damage in
asthma: A review. Clin Respir J. 15:1027–1045. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yuan X, Wang E, Xiao X, Wang J, Yang X,
Yang P, Li G and Liu Z: The role of IL-25 in the reduction of
oxidative stress and the apoptosis of airway epithelial cells with
specific immunotherapy in an asthma mouse model. Am J Transl Res.
9:4137–4148. 2017.PubMed/NCBI
|
|
9
|
Potaczek DP, Miethe S, Schindler V,
Alhamdan F and Garn H: Role of airway epithelial cells in the
development of different asthma phenotypes. Cell Signal.
69(109523)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ngo V and Duennwald ML: Nrf2 and oxidative
stress: A general overview of mechanisms and implications in human
disease. Antioxidants (Basel). 11(2345)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ahmed SA and Mohammed WI: Carvedilol
induces the antiapoptotic proteins Nrf(2) and Bcl(2) and inhibits
cellular apoptosis in aluminum-induced testicular toxicity in male
Wistar rats. Biomed Pharmacother. 139(111594)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Q, Gao Y and Ci X: Role of Nrf2 and
its activators in respiratory diseases. Oxid Med Cell Longev.
2019(7090534)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Helou DG, Noel B, Gaudin F, Groux H, El
Ali Z, Pallardy M, Chollet-Martin S and Kerdine-Römer S: Cutting
Edge: Nrf2 Regulates neutrophil recruitment and accumulation in
skin during contact hypersensitivity. J Immunol. 202:2189–2194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shintani Y, Maruoka S, Gon Y, Koyama D,
Yoshida A, Kozu Y, Kuroda K, Takeshita I, Tsuboi E, Soda K and
Hashimoto S: Nuclear factor erythroid 2-related factor 2 (Nrf2)
regulates airway epithelial barrier integrity. Allergol Int. 64
(Suppl):S54–S63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu J, Wang J, Li C and Shang Y:
Fructose-1,6-bisphosphatase aggravates oxidative stress-induced
apoptosis in asthma by suppressing the Nrf2 pathway. J Cell Mol
Med. 25:5001–5014. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sussan TE, Gajghate S, Chatterjee S,
Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB,
Sidhaye VK and Biswal S: Nrf2 reduces allergic asthma in mice
through enhanced airway epithelial cytoprotective function. Am J
Physiol Lung Cell Mol Physiol. 309:L27–L36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang JH, Yang X, Chen YP, Zhang JF and Li
CQ: Nrf2 Activator RTA-408 Protects Against Ozone-Induced Acute
Asthma Exacerbation by Suppressing ROS and үδT17 Cells.
Inflammation. 42:1843–1856. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamamoto T, Miyata J, Arita M, Fukunaga K
and Kawana A: Current state and future prospect of the therapeutic
strategy targeting cysteinyl leukotriene metabolism in asthma.
Respir Investig. 57:534–543. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Burg N, Stenberg H, Bjermer L,
Diamant Z and Tufvesson E: Cysteinyl-leukotriene and prostaglandin
pathways in bronchial versus alveolar lavage in allergic
asthmatics. Allergy. 77:2549–2551. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ban GY, Kim SH and Park HS: Persistent
eosinophilic inflammation in adult asthmatics with high serum and
urine levels of leukotriene E(4). J Asthma Allergy. 14:1219–1230.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Polomska J, Bar K and Sozanska B: Exhaled
Breath Condensate-A non-invasive approach for diagnostic methods in
asthma. J Clin Med. 10(2697)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
da Cunha AA, Silveira JS, Antunes GL,
Abreu da Silveira K, Benedetti Gassen R, Vaz Breda R and Márcio
Pitrez P: Cysteinyl leukotriene induces eosinophil extracellular
trap formation via cysteinyl leukotriene 1 receptor in a murine
model of asthma. Exp Lung Res. 47:355–367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Israel E, Chervinsky PS, Friedman B, Van
Bavel J, Skalky CS, Ghannam AF, Bird SR and Edelman JM: Effects of
montelukast and beclomethasone on airway function and asthma
control. J Allergy Clin Immunol. 110:847–854. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Quaranta VN, Dragonieri S, Crimi N, Crimi
C, Santus P, Menzella F, Pelaia C, Scioscia G, Caruso C, Bargagli
E, et al: Can leukotriene receptor antagonist therapy improve the
control of patients with severe asthma on biological therapy and
coexisting bronchiectasis? A Pilot Study. J Clin Med.
11(4702)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Miyata J, Fukunaga K, Kawashima Y, Ohara O
and Arita M: Cysteinyl leukotriene metabolism of human eosinophils
in allergic disease. Allergol Int. 69:28–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meshram D, Bhardwaj K, Rathod C, Mahady GB
and Soni KK: The role of leukotrienes inhibitors in the management
of chronic inflammatory diseases. Recent Pat Inflamm Allergy Drug
Discov. 14:15–31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dholia N, Sethi GS, Naura AS and Yadav
UCS: Cysteinyl leukotriene D(4) (LTD(4)) promotes airway epithelial
cell inflammation and remodelling. Inflamm Res. 70:109–126.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen X, Peng W, Zhou R, Zhang Z and Xu J:
Montelukast improves bronchopulmonary dysplasia by inhibiting
epithelial-mesenchymal transition via inactivating the TGF-β1/Smads
signaling pathway. Mol Med Rep. 22:2564–2572. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Du C, Zhang Q, Wang L, Wang M, Li J and
Zhao Q: Effect of montelukast sodium and graphene oxide
nanomaterials on mouse asthma model. J Nanosci Nanotechnol.
21:1161–1168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fei X, Zhang X, Zhang GQ, Bao WP, Zhang
YY, Zhang M and Zhou X: Cordycepin inhibits airway remodeling in a
rat model of chronic asthma. Biomed Pharmacother. 88:335–341.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bai F, Fang L, Hu H, Yang Y, Feng X and
Sun D: Vanillic acid mitigates the ovalbumin (OVA)-induced asthma
in rat model through prevention of airway inflammation. Biosci
Biotechnol Biochem. 83:531–537. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bianchi M and Panerai AE:
Anti-hyperalgesic effects of tramadol in the rat. Brain Res.
797:163–166. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Costa PPC, Waller SB, Dos Santos GR,
Gondim FL, Serra DS, Cavalcante FSÁ, Gouveia Júnior FS, de Paula
Júnior VF, Sousa EHS, Lopes LGF, et al: Anti-asthmatic effect of
nitric oxide metallo-donor FOR811A
[cis-[Ru(bpy)2(2-MIM)(NO)](PF6)3] in the respiratory mechanics of
Swiss mice. PLoS One. 16(e0248394)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vuletic A, Konjevic G, Milanovic D,
Ruzdijic S and Jurisic V: Antiproliferative effect of
13-cis-retinoic acid is associated with granulocyte differentiation
and decrease in cyclin B1 and Bcl-2 protein levels in G0/G1
arrested HL-60 cells. Pathol Oncol Res. 16:393–401. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-alpha induced apoptosis is
accompanied with rapid CD30 and slower CD45 shedding from K-562
cells. J Membr Biol. 239:115–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thornton CA, Holloway JA, Popplewell EJ,
Shute JK, Boughton J and Warner JO: Fetal exposure to intact
immunoglobulin E occurs via the gastrointestinal tract. Clin Exp
Allergy. 33:306–311. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Alobaidi AH, Alsamarai AM and Alsamarai
MA: Inflammation in asthma pathogenesis: Role of T cells,
macrophages, epithelial cells and type 2 inflammation. Antiinflamm
Antiallergy Agents Med Chem. 20:317–332. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zeki AA, Bratt JM, Rabowsky M, Last JA and
Kenyon NJ: Simvastatin inhibits goblet cell hyperplasia and lung
arginase in a mouse model of allergic asthma: A novel treatment for
airway remodeling? Transl Res. 156:335–349. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yamauchi K and Inoue H: Airway remodeling
in asthma and irreversible airflow limitation-ECM deposition in
airway and possible therapy for remodeling. Allergol Int.
56:321–329. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Poprac P, Jomova K, Simunkova M, Kollar V,
Rhodes CJ and Valko M: Targeting free radicals in oxidative
stress-related human diseases. Trends Pharmacol Sci. 38:592–607.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pandey V, Yadav V, Singh R, Srivastava A
and Subhashini : β-Endorphin (an endogenous opioid) inhibits
inflammation, oxidative stress and apoptosis via Nrf-2 in asthmatic
murine model. Sci Rep. 13(12414)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ozdermir C, Kucuksezer UC, Ogulur I, Pat
Y, Yazici D, Ardicli S, Akdis M, Nadeau K and Akdis CA: Lifestyle
changes and industrialization in the development of allergic
diseases. Curr Allergy Asthma Rep. 24:331–345. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Agache I, Eguiluz-Gracia I, Cojanu C,
Laculiceanu A, Del Giacco S, Zemelka-Wiacek M, Kosowska A, Akdis CA
and Jutel M: Advances and highlights in asthma in 2021. Allergy.
76:3390–3407. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Patel SJ and Teach SJ: Asthma. Pediatr
Rev. 40:549–567. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kumar RK, Herbert C and Foster PS: The
‘classical’ ovalbumin challenge model of asthma in mice. Curr Drug
Targets. 9:485–494. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nakagome K and Nagata M: Involvement and
possible role of eosinophils in asthma exacerbation. Front Immunol.
9(2220)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Garcia G, Taille C, Laveneziana P, Bourdin
A, Chanez P and Humbert M: Anti-interleukin-5 therapy in severe
asthma. Eur Respir Rev. 22:251–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Price DB, Rigazio A, Campbell JD, Bleecker
ER, Corrigan CJ, Thomas M, Wenzel SE, Wilson AM, Small MB, Gopalan
G, et al: Blood eosinophil count and prospective annual asthma
disease burden: A UK cohort study. Lancet Respir Med. 3:849–858.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ying S, Meng Q, Zeibecoglou K, Robinson
DS, Macfarlane A, Humbert M and Kay AB: Eosinophil chemotactic
chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant
protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3
expression in bronchial biopsies from atopic and nonatopic
(Intrinsic) asthmatics. J Immunol. 163:6321–6329. 1999.PubMed/NCBI
|
|
53
|
Poowuttikul P, Saini S and Seth D:
Inner-City Asthma in Children. Clin Rev Allergy Immunol.
56:248–268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Matucci A, Vultaggio A, Maggi E and
Kasujee I: Is IgE or eosinophils the key player in allergic asthma
pathogenesis? Are we asking the right question? Respir Res.
19(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kanaoka Y and Boyce JA: Cysteinyl
leukotrienes and their receptors: Cellular distribution and
function in immune and inflammatory responses. J Immunol.
173:1503–1510. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhou X, Cai J, Liu W, Wu X and Gao C:
Cysteinyl leukotriene receptor type 1 (CysLT1R) antagonist
zafirlukast protects against TNF-α-induced endothelial
inflammation. Biomed Pharmacother. 111:452–459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Patel B, Gupta N and Ahsan F: Aerosolized
montelukast polymeric particles-an alternative to oral
montelukast-alleviate symptoms of asthma in a rodent model. Pharm
Res. 31:3095–3105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Basyigit I, Sahin M, Sahin D, Yildiz F,
Boyaci H, Sirvanci S and Ercan F: Anti-inflammatory effects of
montelukast on smoke-induced lung injury in rats. Multidiscip
Respir Med. 5:92–98. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu AY, Chik SC, Chan AW, Li Z, Tsang KW
and Li W: Anti-inflammatory effects of high-dose montelukast in an
animal model of acute asthma. Clin Exp Allergy. 33:359–366.
2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu Y, Zhou C, Tao J and Li S: Montelukast
prevents the decrease of interleukin-10 and inhibits NF-kappaB
activation in inflammatory airway of asthmatic guinea pigs. Can J
Physiol Pharmacol. 84:531–537. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Abdel Aziz RR, Helaly NY, Zalata KR and
Gameil NM: Influence of inhaled beclomethasone and montelukast on
airway remodeling in mice. Inflammopharmacology. 21:55–66.
2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Holgate ST, Peters-Golden M, Panettieri RA
and Henderson WR Jr: Roles of cysteinyl leukotrienes in airway
inflammation, smooth muscle function, and remodeling. J Allergy
Clin Immunol. 111 (1 Suppl):S18–S34; discussion S34-16.
2003.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Berair R, Hartley R, Mistry V, Sheshadri
A, Gupta S, Singapuri A, Gonem S, Marshall RP, Sousa AR, Shikotra
A, et al: Associations in asthma between quantitative computed
tomography and bronchial biopsy-derived airway remodelling. Eur
Respir J. 49(1601507)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Jurisic V, Bogdanovic G, Kojic V, Jakimov
D and Srdic T: Effect of TNF-alpha on Raji cells at different
cellular levels estimated by various methods. Ann Hematol.
85:86–94. 2006.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jurisic V, Bumbasirevic V, Konjevic G,
Djuricic B and Spuzic I: TNF-alpha induces changes in LDH isotype
profile following triggering of apoptosis in PBL of non-Hodgkin's
lymphomas. Ann Hematol. 83:84–91. 2004.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Michaeloudes C, Abubakar-Waziri H, Lakhdar
R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK and Chung KF:
Molecular mechanisms of oxidative stress in asthma. Mol Aspects
Med. 85(101026)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Audousset C, McGovern T and Martin JG:
Role of Nrf2 in Disease: Novel molecular mechanisms and therapeutic
approaches-pulmonary disease/asthma. Front Physiol.
12(727806)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yachie A: Heme oxygenase-1 deficiency and
oxidative stress: A review of 9 independent human cases and animal
models. Int J Mol Sci. 22(1514)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ross D and Siegel D: The diverse
functionality of NQO1 and its roles in redox control. Redox Biol.
41(101950)2021.PubMed/NCBI View Article : Google Scholar
|