Introduction
Prostate cancer (PCa) is the second most common
cause of cancer-related mortality among males in developed
countries (1). It is well known
that tissue homeostasis in the normal prostate gland (as in most
other organs) is maintained by the quantitative relationship
between the rate of cell proliferation and the rate of apoptotic
cell death (2,3). Thus, apoptosis comprises an important
role in the development of the prostate and in the normal process
of prostatic glandular self-renewal. Consistently, dysregulation of
apoptosis represents an important mechanism of prostate
carcinogenesis.
The most prominent executioners of apoptosis are
represented by the family of caspases comprising initiator
(caspase-1, -2, -4, -5, -8, -9 and -10) and effector caspases
(caspase-3, -6 and -7) (4,5). Caspases are cysteine proteases and
are synthesized as inactive proenzymes (6). On activation (cleavage), effector
caspases can cleave a broad range of intracellular targets thus
leading to apoptosis. Activation of caspases and initiation of
different apoptotic pathways depend on the cell type (7). In a few cell types active caspase-8
activates executioners such as caspase-3, -6 and -7 in a
mitochondrial-independent manner. In contrast, in other cell types
apoptosis involves mitochondrial death signals mediated by the
Bcl-2 family proteins. Here, active caspase-8 cleaves Bid into a
tBid (truncated form of Bid) that translocates to the mitochondria,
promoting the release of cytochrome c into the cytosol
(8). This, in turn, leads to the
formation of the apoptosome and subsequently to the activation of
caspase-3. Caspase-1 (known as interleukin-1β-converting enzyme) is
also required for apoptosis (9),
and caspase-9 activity is dependent on cytosolic factors (10). Cytochrome c release from
mitochondria initiates activation of caspase-9 which subsequently
activates caspase-3 as an important executioner of apoptosis
(11,12). Caspase-6, an important effector
caspase, can activate caspase-3 resulting in apoptosis. The Bcl-2
gene product is a potent inhibitor of apoptosis, since it
stabilizes the mitochondrial membrane and blocks the release of
cytochrome c. In turn, cytochrome c can bind to
caspase-9 which triggers the activation of caspase-3 (13,14).
Taken together, a variety of proteins, e.g., caspases and members
of the Bcl-2 family, are involved in tissue homeostasis.
Although expression of caspase-1 and -3 was shown to
be decreased in PCa compared to benign prostate epithelium (BPE)
(15), there is limited
information on the role of caspases in PCa. Therefore, in the
present study we investigated the expression of caspase-1 and -9,
uncleaved caspase-3 and -6, cleaved caspase-3 and -6, and Bcl-2 in
PCa.
Materials and methods
Prostate specimens
In this study, we investigated 20 primary prostate
adenocarcinomas localized at the peripheral zone of the prostate
from patients undergoing radical prostatectomy due to PCa at the
Clinic and Polyclinic of Urology, University of Greifswald. No
patient had received any therapy before surgery. Patient age ranged
from 52 to 71 years (mean age, 61.65 years). Tissue samples and
patient data were obtained and used after recommendations from the
Ethics Committee of the University of Greifswald and in accordance
with the Declaration of Helsinki. The entire prostate glands were
fixed in 4% buffered formalin (72 h, 4°C) immediately after radical
prostatectomy and subsequently embedded in paraffin. Whole mount
prostates were cut at 4 μm and mounted on precoated glass slides
(Superfrost, Menzel, Braunschweig). All sections included in this
study contained BPE glands showing benign prostate hyperplasia
(BPH) and PCa.
Histopathological evaluation
Histological diagnosis and Gleason grading (16,17)
was performed by two experienced pathologists (C.W. and G.L.) on
H&E-stained paraffin sections. Immunostaining for active and
inactive caspases was carried out in parallel on consecutive
sections.
Immunohistochemistry
Expression of apoptosis-associated proteins was
determined using primary antibodies against caspase-1 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), uncleaved and cleaved
caspase-3 and -6 (New England Biolabs, Frankfurt, Germany),
caspase-9 (Acris Antibodies, Hiddenhausen, Germany), as well as
Bcl-2 (Biosource, Nivelles, Belgium), employing the 4plus™
Universal Immunoperoxidase Detection System (Biocarta, Hamburg,
Germany). After deparaffinizing and rehydration, endogenous
peroxidase activity was blocked (Peroxydazed 1, Biocarta), and
sections were subjected to antigen-retrieval (10 mM citrate buffer,
pH 6.0) in a microwave oven (700 W for 20 min). Slides were then
allowed to cool in citrate buffer (20 min), washed in tap water (2
min), distilled water (2 min) and finally in PBS buffer (pH 7.3).
Blocking with blocking reagent (5 min; Background Erazer; Biocarta)
was followed by incubation with the respective antibody (overnight
at 4°C). Slides were washed in PBS buffer (2×5 min), incubated with
secondary antibody (10 min; Universal Link; Biocarta) and washed in
buffer (2×5 min). Slides were incubated with streptavidin-HRP
solution (10 min) and washed in PBS (2×5 min). Visualization was
achieved by incubation with 0.1% diaminobenzidine (Sigma) in
PBS/0.01% H2O2 (8 min). Slides were
counterstained with hematoxylin, dehydrated and mounted in
Neo-mount (Merck, Darmstadt, Germany). Routine pathological
observation was carried out on H&E-stained sections.
Control reactions to demonstrate specific antibody
binding were carried out by i) incubation of slides with PBS and
omitting all steps, except DAB-chromogen reaction, ii) omitting the
primary antibody and iii) competitive inhibition for cleaved
caspase-3 and -6, as previously demonstrated (18). Images were captured using an
Olympus BX 50 microscope equipped with an Olympus DP 10 digital
camera.
Immunohistochemical staining for all caspases and
Bcl-2 was scored by visual assessment of ten ×40 fields as
followed: (+) 0–30% of prostate glands or tumour tissue
immunopositive, (++) 31–70% of prostate glands or tumour tissue
immunopositive and (+++) >71% of prostate glands or tumour
tissue immunopositive.
Statistical analysis
Differences between caspase expression in benign and
cancer tissue were analysed by a two-tailed Student’s t-test.
P<0.05 was regarded as significant.
Results
Histology and grading of tumours
All prostates were evaluated by H&E staining and
revealed Gleason scores between 3 and 8 and pT stage from 2a to 3c
(Table I). During the clinical
follow-up after surgery (24 months) metastases were diagnosed in
one patient.
 | Table I.Gleason score and tumour stage of the
20 PCa samples. |
Table I.
Gleason score and tumour stage of the
20 PCa samples.
| Gleason score
| pT tumour stage
|
|---|
| 3–4 | 5–6 | 7–8 | 2a–2c | 3a–3c |
|---|
| No. of specimens | 4 | 9 | 7 | 17 | 3 |
Immunohistochemistry and statistical
analysis
Immunohistochemistry for caspase-1 showed a weak (+)
cytoplasmic staining in non-neoplastic tissue and in the tumour
tissue of all samples investigated. For caspase-9, immunostaining
was observed in the cytoplasm of some prostate basal cells as well
as in some tumour cells of all samples (+) (Table II, figures not shown). Caspase-3
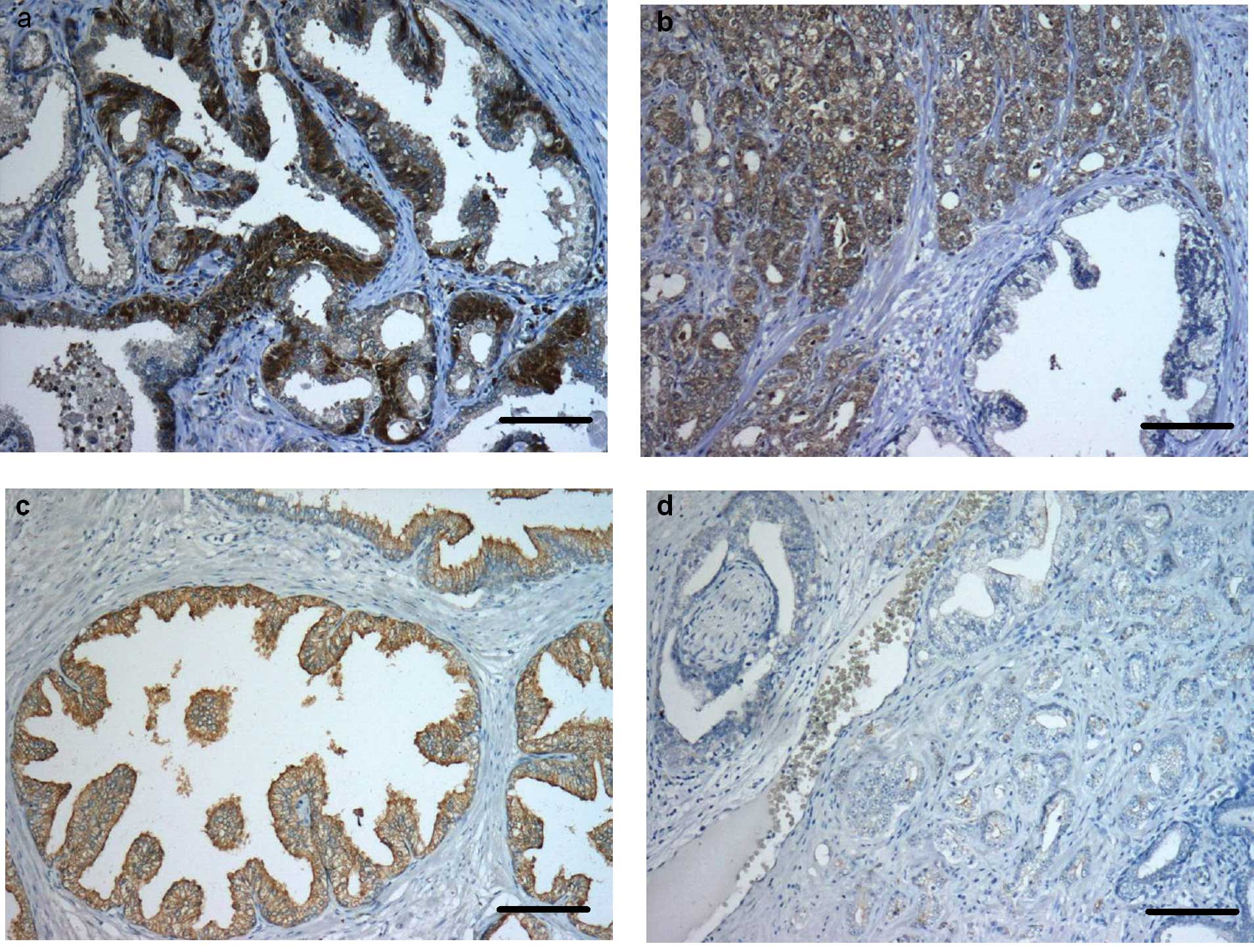
immunoreactivity was predominantly seen in the cytoplasm of basal
cells of normal prostate glands (++). In most of the apical cells
no immunostaining was evident (Fig.
1a). In the prostate tumour tissue of all of the patients,
>50% of tumour cells were caspase-3-positive (++) (Figs. 1b and 4a). Otherwise cleaved caspase-3 was
strongly expressed in apical cells of the non-neoplastic tissue
(+++), whereas only a few tumour cells were immunopositive for the
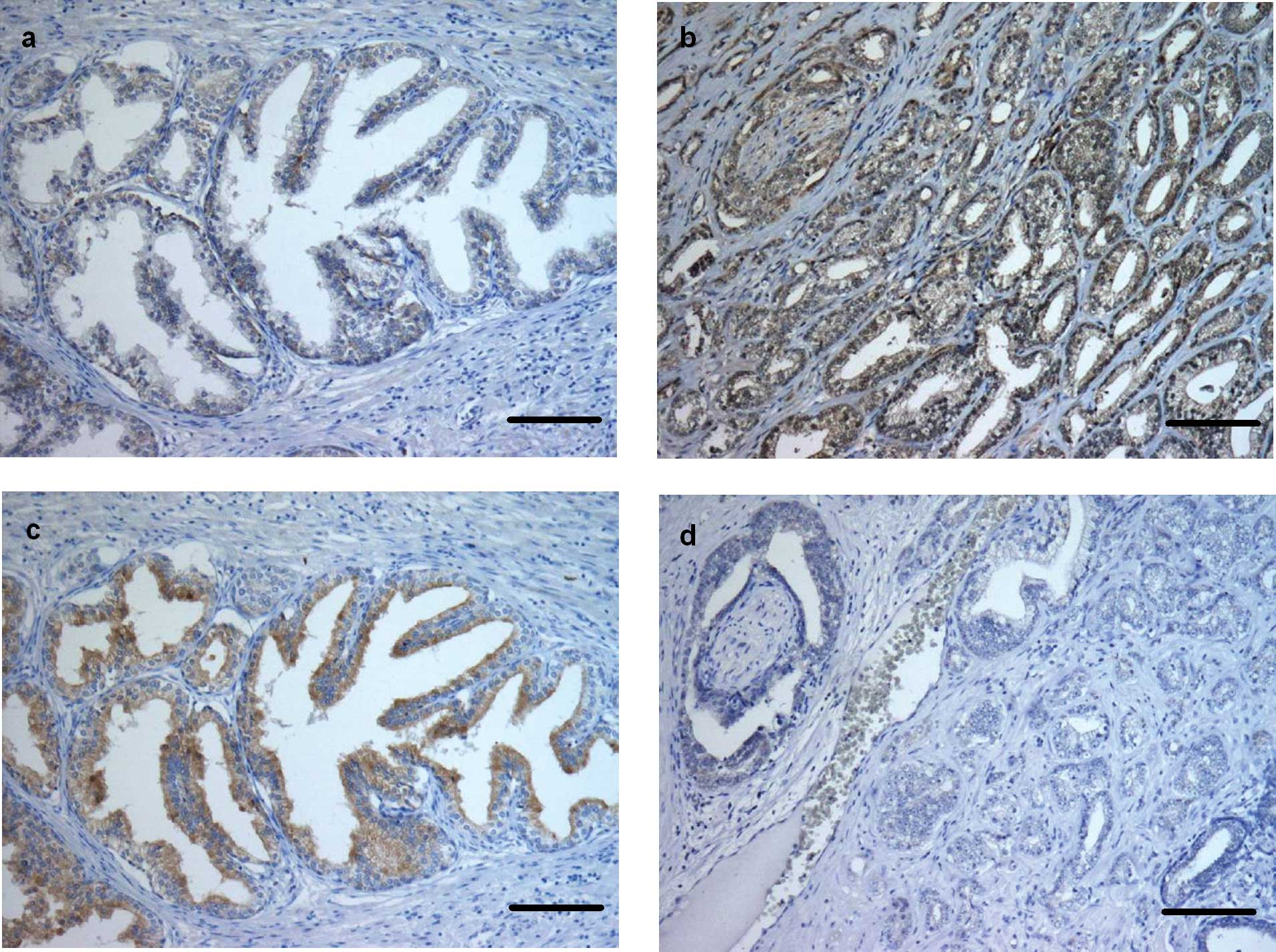
active caspase (+) (P<0.0001) (Figs. 1c and d, and 4b). Immunostaining for caspase-6 was
found in all basal and apical cells of normal prostate glands and
in all tumour cells (+++) (Figs.
2a and b, and 4c). Cleaved caspase-6 was strongly
expressed (+++) in apical cells of the normal prostate glands,
whereas the tumour cells showed moderate immunostaining (++)
(P<0.0001) (Figs. 2c and
d, and 4d). Immunohistochemical expression of
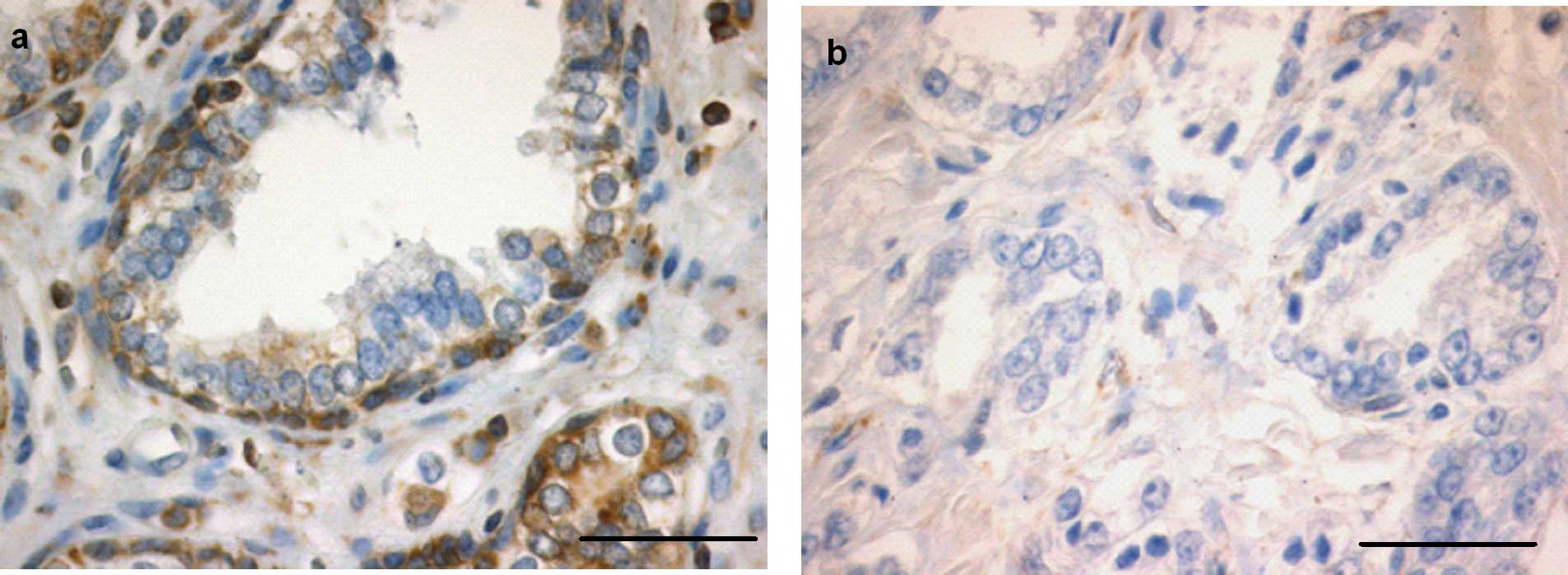
Bcl-2 was noted in nearly all basal cells of the non-neoplastic
prostate glands (+++). Tumour tissue, however, showed a weak immune
response for Bcl-2 (+) (P<0.0001) (Figs. 3a and b, and 4e). In the control sections no staining
was evident.
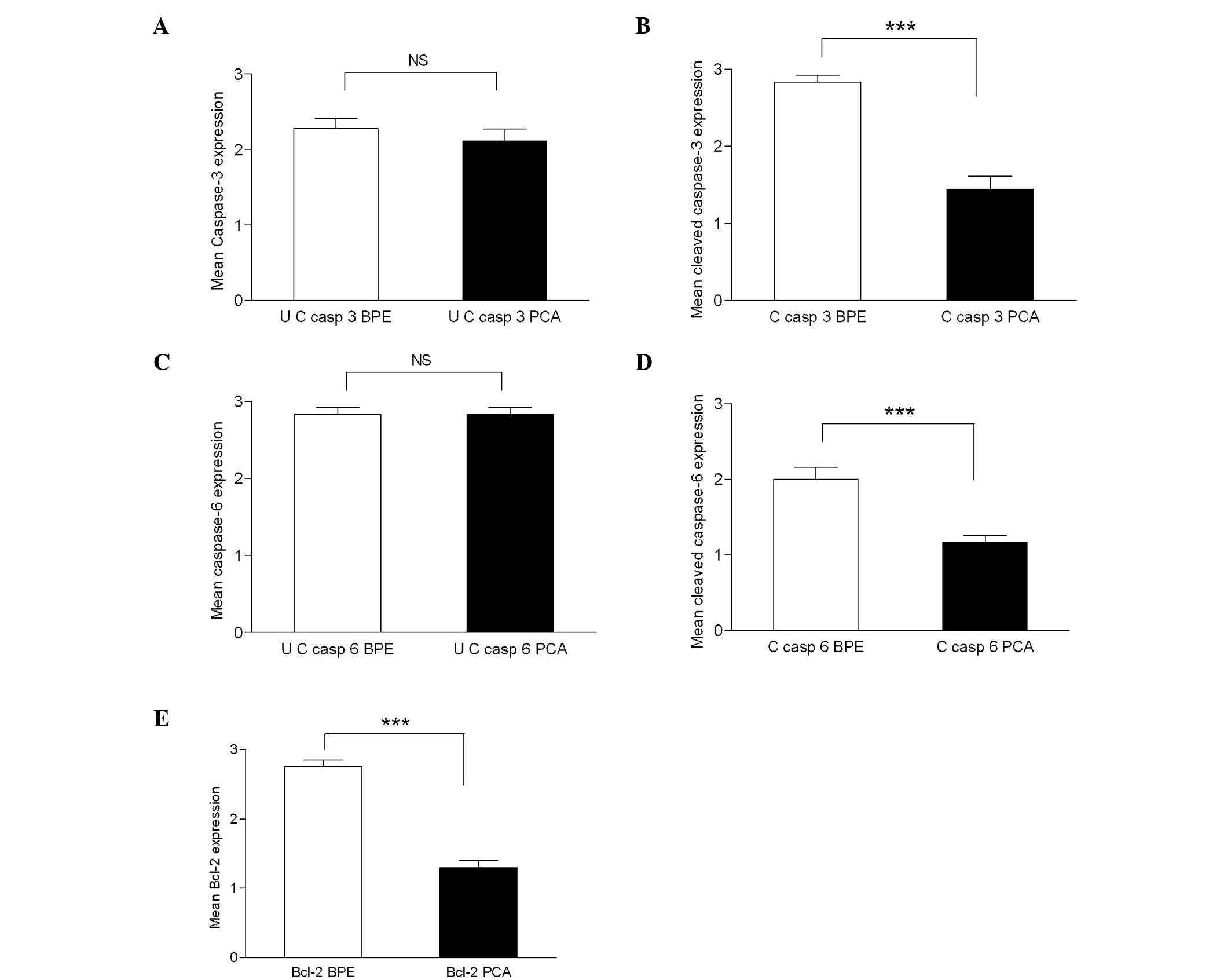 | Figure 4.Mean caspase-3 (a) and cleaved
caspase-3 (b) immunohistochemistry scores in normal/tumour samples.
Differential expression of caspase-3 (a) in PCa was not significant
(NS) compared to BPE, whereas cleaved caspase-3 expression (b) was
significant (P≤0.0001) in PCa (mean ± SEM, 1.444±0.166) in
comparison with adjacent BPE (mean ± SEM, 2.833±0.09). Mean
caspase-6 (c) and cleaved caspase-6 (d) immunohistochemistry scores
in normal/tumour samples. Differential expression of caspase-6 (c)
in PCa compared to BPE was not significant (NS), whereas cleaved
caspase-6 expression (d) was significant (P≤0.0001) in PCa (mean ±
SEM, 1.167±0.0903) in comparison with adjacent BPE (mean ± SEM,
2.00±0.1617). Mean values of Bcl-2 protein expression (e) in BPE
and PCA samples. A 1.45-fold decrease (P≤0.0001) in Bcl-2
expression was observed in PCa (mean ± SEM, 1.3±0.105) in
comparison with the BPE (mean ± SEM, 2.75±0.099). UC, uncleaved; C,
cleaved. |
 | Table II.Summary of patient data and expression
of caspases in normal and tumour tissue. |
Table II.
Summary of patient data and expression
of caspases in normal and tumour tissue.
| Specimen | A | Gl | G | pT | M | Caspase-3
| Caspase-6
| Caspase-1
| Caspase-9
| Bcl-2
|
|---|
| | | | | | Uncleaved
| Cleaved
| Uncleaved
| Cleaved
| | | | | | |
|---|
| | | | | | BPE | PCa | BPE | PCa | BPE | PCa | BPE | PCa | BPE PCa BPE | PCa | BPE | PCa |
|---|
| 1 | 62 | 3+4=7 | 3a | 2b | 0 | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 2 | 52 | 3+3=6 | 2a | 2b | 0 | 2 | 2 | 3 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 |
| 3 | 63 | 2+3=5 | 1b | 2b | 0 | 2 | 2 | 3 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 |
| 4 | 62 | 2+3=5 | 2a | 3a | 0 | 2 | 1 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 5 | 70 | 3+4=7 | 3a | 2c | 1 | 3 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 1 | 3 | 1 |
| 6 | 62 | 3+4=7 | 3a | 2c | 0 | 1 | 2 | 3 | 1 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 1 |
| 7 | 71 | 3+4=7 | 3a | 3c | 0 | 2 | 3 | 3 | 2 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 2 |
| 8 | 68 | 2+2=4 | 1b | 2c | 0 | 2 | 1 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 |
| 9 | 67 | 2+2=4 | 1b | 2a | 0 | 2 | 2 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 10 | 68 | 2+3=5 | 3a | 2c | 0 | 2 | 2 | 2 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 2 |
| 11 | 59 | 1+2=3 | 1b | 2b | 0 | 2 | 2 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 3 | 2 |
| 12 | 60 | 2+3=5 | 1b | 2b | 0 | 3 | 2 | 3 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 |
| 13 | 68 | 3+4=7 | 3a | 2c | 0 | 2 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 |
| 14 | 66 | 2+3=5 | 2b | 2a | 0 | 2 | 3 | 2 | 1 | 3 | 2 | 3 | 1 | 1 | 2 | 1 | 1 | 3 | 1 |
| 15 | 70 | 2+3=5 | 2b | 2b | 0 | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 1 |
| 16 | 65 | 2+3=5 | 2b | 2a | 0 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 1 | 2 | 2 | 1 | 1 | 3 | 2 |
| 17 | 66 | 3+4=7 | 3a | 3a | 0 | 3 | 2 | 3 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| 18 | 65 | 3+5=8 | 3a | 2b | 0 | 3 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 3 | 2 |
| 19 | 64 | 1+2=3 | 1b | 2a | 0 | * | * | * | * | * | * | * | * | 1 | 1 | 2 | 1 | 2 | 1 |
| 20 | 65 | 2+3=5 | 2a | 2b | 0 | * | * | * | * | * | * | * | * | 1 | 1 | 1 | 1 | 3 | 1 |
Discussion
The human prostate is composed of three anatomical
zones: the peripheral (PZ), central (CZ) and the transition (TZ)
zone (19). The PZ comprises 70%
of the prostate, and a majority of tumours originate from this zone
(20,21). Most of the benign hyperplastic
nodules called nodular hyperplasia, besides a few carcinomas,
originate from the TZ (22). The
CZ constitutes approximately 25% of the prostate and is less
frequently affected by prostate diseases (22,23).
In the present study, we evaluated the immunohistochemical
expression of caspases and Bcl-2 in the PZ of whole mount prostate
sections. Here, we demonstrated a decreased immunostaining for
active caspase-3 and -6, while expression of uncleaved caspase-3
and -6 appeared not to be altered in PCa compared to BPE. Previous
immunohistochemical studies revealed somewhat contradictory
results. For instance, immunohistochemistry showed the presence of
caspase-3 (inactivated form) in basal as well as secretory cells of
benign prostate hyperplasia (BPH). Moreover, increasing grades of
PCa showed a significant loss of caspase-3 expression, and it was
concluded that altered caspase-3 expression may represent an
additional mechanism of apoptotic resistance to androgen ablation
(24). On the other hand, it was
shown that expression of caspase-3 did not correlate with the
number of apoptotic bodies and Gleason score (25), while another study revealed that
the expression of caspase-3 was reduced in poorly differentiated
prostate tumours rather than well-differentiated PCa and BPE.
Therefore, a prognostic significance has been suggested in disease
progression (15). However, the
findings of Ananthanarayanan et al demonstrating decreased
expression of active caspase-3 in high grade prostate
intraepithelial neoplasia (PIN) and PCa (26) is in line with our findings. As in
our study, immunostaining for uncleaved caspase-3 was not altered,
but cleaved caspase-3 staining was significantly decreased in PCa.
This suggests an alteration of post-translational cleavage of
caspase-3 into active caspase-3. Therefore, modifications of
proteolytic cleavage of its precursor form could lead to lower
levels of active caspase-3. Consistently, this could represent a
mechanism of apoptosis suppression thus supporting cancer
development. As increased caspase-3 expression has been correlated
with increased apoptosis and high histologic grade in breast
carcinomas (27), cancer
progression appears not to be necessarily associated with the
down-regulation of caspase-3.
According to our current understanding, caspase-6 is
an effector caspase that is activated downstream of caspase-3
during apoptosis. Activation of procaspase-6 by caspase-3 results
in an active enzyme capable of cleaving an artificially introduced
lamin cleavage site. This suggests that caspase-3 is activated
prior to caspase-6 and may be responsible for the activation of
caspase-6 (28). However, these
results are in conflict with a previous study showing that
caspase-6 is capable of activating caspase-3 and that active
caspase-6 initiates activation of caspase-3 as shown in rodent
cerebellar granule cells (29).
Therefore, we focused our attention towards the investigation of
caspase-6 expression in PCa. Notably, immunohistochemistry revealed
no alteration in the expression of caspase-6, but a significant
reduction in immunoreactivity for cleaved caspase-6 in PCa compared
to BPE as noted for cleaved caspase-3. In recent studies, the
camptothesin resistant prostate cancer cell line DU145 showed
decreased expression of procaspase-6 indicating an important role
of caspase-6 in drug-induced apoptosis (30). Moreover, resveratrol-induced
apoptotic signalling led to caspase-6-mediated cleavage of lamin A
in colon carcinoma cells (31).
Immunohistochemical investigations for upstream
caspase-1 and -9 revealed a weak immunostaining in tumour cells as
well as in benign epithelial cells. In this context, it is worth
noting that the proenzyme form of caspase-1, but not its active
form, was previously detected in prostate cancer cells by Western
blot analysis (15).
Previous reports suggest overexpression of Bcl-2 in
PCa (32,33) and high grade PIN (34). This is in contrast to the results
of our study, as we detected a faint staining (compared to
non-neoplastic tissue) for the Bcl-2 protein in PCa of all of the
sections investigated. Notably, a recent study did not show any
significant alteration of Bcl-2 expression in pre-malignant tumours
(26). Overexpression of Bcl-2 is
linked to the restriction of cytochrome c release from
mitochondria which in turn activates caspase-9 to induce apoptosis
in PCa.
In conclusion, to the best of our knowledge, this is
the first study to report expression of active and inactive
caspases showing that caspases are expressed constitutively in
neoplastic and non-neoplastic prostate cells. We suggest that
activation of procaspases into active caspases could be
dysregulated in cancer tissue which in turn alters apoptosis.
Further investigations to elucidate altered pathways for caspase
activation must be pursued in order to discover novel strategies
for the treatment of cancer.
Acknowledgements
The authors thank Professor Gerhard
Lorenz (Department of Pathology, Ernst Moritz Arndt University of
Greifswald) for the pathological classification of prostate
sections. Ramesh Ummanni was supported by the Alfried-Krupp von
Bohlen und Halbach Stiftung, Graduiertenkolleg Tumorbiologie and
NGFN-Plus/NGFN-Transfer, Germany.
References
|
1.
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
View Article : Google Scholar
|
|
2.
|
Slee EA, Adrain C and Martin SJ: Serial
killers: ordering caspase activation events in apoptosis. Cell
Death Differ. 6:1067–1074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Woo M, Hakem R, Soengas MS, Duncan GS,
Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA,
Senaldi G, Howard T, Lowe SW and Mak TW: Essential contribution of
caspase 3/CPP32 to apoptosis and its associated nuclear changes.
Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Harvey NL and Kumar S: The role of
caspases in apoptosis. Adv Biochem Eng Biotechnol. 62:107–128.
1998.
|
|
5.
|
Stegh AH and Peter ME: Apoptosis and
caspases. Cardiol Clin. 19:13–29. 2001. View Article : Google Scholar
|
|
6.
|
Nunez G, Benedict MA, Hu Y and Inohara N:
Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Scaffidi C, Fulda S, Srinivasan A, Friesen
C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME: Two
CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Thornberry NA, Bull HG, Calaycay JR, et
al: A novel heterodimeric cysteine protease is required for
interleukin-1 beta processing in monocytes. Nature. 356:768–774.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Stennicke HR, Deveraux QL, Humke EW, Reed
JC, Dixit VM and Salvesen GS: Caspase-9 can be activated without
proteolytic processing. J Biol Chem. 274:8359–8362. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Slee EA, Harte MT, Kluck RM, Wolf BB,
Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri
ES, Green DR and Martin SJ: Ordering the cytochrome c-initiated
caspase cascade: hierarchical activation of caspases-2, -3, -6, -7,
-8 and -10 in a caspase-9-dependent manner. J Cell Biol.
144:281–292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Reed JC: Bcl-2 and the regulation of
programmed cell death. J Cell Biol. 124:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
15.
|
Winter RN, Kramer A, Borkowski A and
Kyprianou N: Loss of caspase-1 and caspase-3 protein expression in
human prostate cancer. Cancer Res. 61:1227–1232. 2001.PubMed/NCBI
|
|
16.
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974.
|
|
17.
|
Mellinger GT, Gleason D and Bailar J III:
The histology and prognosis of prostatic cancer. J Urol.
97:331–337. 1967.PubMed/NCBI
|
|
18.
|
Woenckhaus C, Giebel J, Failing K, Fenic
I, Dittberner T and Poetsch M: Expression of AP-2alpha, c-kit and
cleaved caspase-6 and -3 in naevi and malignant melanomas of the
skin. A possible role for caspases in melanoma progression? J
Pathol. 201:278–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
McNeal JE: Normal histology of the
prostate. Am J Surg Pathol. 12:619–633. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
McNeal JE: Regional morphology and
pathology of the prostate. Am J Clin Pathol. 49:347–357.
1968.PubMed/NCBI
|
|
21.
|
McNeal JE: Origin and development of
carcinoma in the prostate. Cancer. 23:24–34. 1969. View Article : Google Scholar
|
|
22.
|
McNeal JE: Origin and evolution of benign
prostatic enlargement. Invest Urol. 15:340–345. 1978.PubMed/NCBI
|
|
23.
|
Srodon M and Epstein JI: Central zone
histology of the prostate: a mimicker of high-grade prostatic
intraepithelial neoplasia. Hum Pathol. 33:518–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
O’Neill AJ, Boran SA, O’Keane C, Coffey
RN, Hegarty NJ, Hegarty P, Gaffney EF, Fitzpatrick JM and Watson
RW: Caspase 3 expression in benign prostatic hyperplasia and
prostate carcinoma. Prostate. 47:183–188. 2001.PubMed/NCBI
|
|
25.
|
Sohn JH, Kim DH, Choi NG, Park YE and Ro
JY: Caspase-3/CPP32 immunoreactivity and its correlation with
frequency of apoptotic bodies in human prostatic carcinomas and
benign nodular hyperplasias. Histopathology. 37:555–560. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ananthanarayanan V, Deaton RJ, Yang XJ,
Pins MR and Gann PH: Alteration of proliferation and apoptotic
markers in normal and premalignant tissue associated with prostate
cancer. BMC Cancer. 6:732006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Vakkala M, Paakko P and Soini Y:
Expression of caspases 3, 6 and 8 is increased in parallel with
apoptosis and histological aggressiveness of the breast lesion. Br
J Cancer. 81:592–599. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kang JJ, Schaber MD, Srinivasula SM,
Alnemri ES, Litwack G, Hall DJ and Bjornsti MA: Cascades of
mammalian caspase activation in the yeast Saccharomyces
cerevisiae. J Biol Chem. 274:3189–3198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Allsopp TE, McLuckie J, Kerr LE, Macleod
M, Sharkey J and Kelly JS: Caspase 6 activity initiates caspase 3
activation in cerebellar granule cell apoptosis. Cell Death Differ.
7:984–993. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Reinhold WC, Kouros-Mehr H, Kohn KW,
Maunakea AK, Lababidi S, Roschke A, Stover K, Alexander J, Pantazis
P, Miller L, Liu E, Kirsch IR, Urasaki Y, Pommier Y and Weinstein
JN: Apoptotic susceptibility of cancer cells selected for
camptothecin resistance: gene expression profiling, functional
analysis and molecular interaction mapping. Cancer Res.
63:1000–1011. 2003.
|
|
31.
|
Lee SC, Chan J, Clement MV and Pervaiz S:
Functional proteomics of resveratrol-induced colon cancer cell
apoptosis: caspase-6-mediated cleavage of lamin A is a major
signaling loop. Proteomics. 6:2386–2394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
McDonnell TJ, Troncoso P, Brisbay SM,
Logothetis C, Chung LW, Hsieh JT, Tu SM and Campbell ML: Expression
of the protooncogene bcl-2 in the prostate and its association with
emergence of androgen-independent prostate cancer. Cancer Res.
52:6940–6944. 1992.PubMed/NCBI
|
|
33.
|
Tu H, Jacobs SC, Borkowski A and Kyprianou
N: Incidence of apoptosis and cell proliferation in prostate
cancer: relationship with TGF-beta1 and bcl-2 expression. Int J
Cancer. 69:357–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Johnson MI, Robinson MC, Marsh C, Robson
CN, Neal DE and Hamdy FC: Expression of Bcl-2, Bax and p53 in
high-grade prostatic intraepithelial neoplasia and localized
prostate cancer: relationship with apoptosis and proliferation.
Prostate. 37:223–229. 1998. View Article : Google Scholar : PubMed/NCBI
|