Introduction
Colorectal cancer (CRC) is one of the most common
malignances and the third lethal cancer with an estimated incidence
of one million new cases and a mortality rate of >600,000
annually worldwide (1,2). Recent progress in surgical operation
combined with chemotherapy for CRC has been beneficial in the early
stages of CRC. However, effective treatment for patients with
advanced CRC remains unsatisfactory. Metastasis is a critical
factor resulting in difficulty in curing cancer. Therefore, studies
should be conducted on the therapeutic targets for CRC
metastasis.
Angiopoietin is important in angiogenesis and the
maintenance of hematopoietic stem cells (3,4). A
family of proteins structurally similar to angiopoietin was
identified and designated ‘angiopoietin-like proteins’ (ANGPTLs)
(5). It has been well established
that ANGPTL2 functions as a chronic inflammatory mediator in
obesity (6), atherosclerotic
disease (7) and rheumatoid
arthritis (RA) (8). Findings of
recent studies have shown that ANGPTL2 is commonly upregulated in
various types of cancer and plays an oncogenic role in inflammatory
carcinogenesis, tumor invasion and metastasis (9–11).
Notably, a reduced ANGPTL2 expression induced by methylation
surrounding the ANGPTL2 CpG-island and an anti-oncogene role in a
stage-dependent manner were observed in ovarian cancer (12). These findings suggested that the
specific role of ANGPTL2 in cancer may differ depending on cancer
type or stage. Thus, the expression and role of ANGPTL2 in CRC
remain unclear.
microRNAs (miRs) have been shown to regulate the
expression of a variety of genes pivotal for tumor development and
highlight a novel mechanism participating in CRC pathogenesis
(13,14). A recent study demonstrated that
miR-25 functions in various types of cancer including CRC (15). ANGPTL2 may be a direct target gene
of miR-25 as predicted by bioinformatical analysis. However,
whether the targeted relationship is established in CRC as well as
the consequent functional influence remains unclear.
In this study, we determined the expression levels
of ANGPTL2 in CRC tissues and cells. The roles of ANGPTL2 and
miR-25 in the migration and invasion of CRC SW620 and HCT-116 cells
were investigated using transwell assays and scratch wound assays.
The results showed that ANGPTL2 upregulation induced by miR-25
downregulation contributes to the malignant progression of CRC.
Accordingly, we suggest that ANGPTL2 and miR-25 may serve as novel
diagnostic or therapeutic targets for CRC metastasis.
Materials and methods
Cell culture
The protocols for this study were approved by the
Ethics Committee of Central South University. CRC HT-29, SW480,
SW620, and HCT-116 cell lines as well as human colonic epithelial
cells (HCEpic) were obtained from the China Center for Type Culture
Collection, Wuhan, China. Cells were cultured in DMEM supplemented
with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100
μg/ml streptomycin sulfate at 37°C in a humidified incubator
containing 5% CO2. The ANGPTL2 siRNA, pre-miR-25,
pre-miR-con, anti-miR-25 or anti-miR-con (Fulengen Co., Ltd.,
Guangzhou, China) were transfected into SW620 and HCT-116 cells
using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions.
Quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Life Technologies) following the manufacturer’s
instructions. The relative expression of miR-25 was determined
using RT-qPCR using a mirVana™ qRT-PCR microRNA Detection kit (Life
Technologies) according to the manufacturer’s instructions.
Specific primer sets for miR-25 and U6 (used as an internal
reference) were obtained from Life Technologies. ANGPTL2 mRNA
expression was detected RT-qPCR using the standard SYBR-Green
RT-PCR kit (Takara Bio Inc., Otsu, Japan) as per the manufacturer’s
instructions. The specific primer pairs used were: ANGPTL2 sense,
5′-GCCACCAAGTGTCAGCCTCA-3′ and antisense,
5′-TGGACAGTACCAAACATCCAACATC-3′; β-actin as an internal sense,
5′-AGGGGCCGGACTCGTCATACT-3′ and antisense,
5′-GGCGGCACCACCATGTACCCT-3′. The relative expression of ANGPTL2
mRNA or miR-25 was quantified using GraphPad Prism 4.0 software
(GraphPad Software, La Jolla, CA, USA) and the 2−ΔΔCt
method (16).
Immunohistochemical analysis
A human colon adenocarcinoma tissue microarray
(Auragene Bioscience Co., Changsha, China), containing 50 cases
(including 9 cases of T2, 16 cases of T3, and 25 cases of T4) of
colon adenocarcinoma tissues as well as their matched adjacent
normal colon tissues, duplicate scores per case, was processed for
immunohistochemical analysis using anti-ANGPTL2 antibody
(Millipore, Billerica, MA, USA) as described previously (17). The mean optical density value (D)
and area (A) of brown particles in three visual fields of each
section were calculated by the Leica Q550 image analysis system
(Leica Co., Solms, Germany). The expression levels of ANGPTL2 in
tissues were evaluated using the formula: integral density = D ×
A.
Cell invasion assay
The cell invasive ability was estimated using a Cell
Invasion Assay kit (Chemicon International, Inc., Temecula, CA,
USA) according to the manufacturer’s instruction as described
previously (18). Briefly, the
cells were placed in the upper compartment of the chambers, and
RPMI-1640 containing 10% FBS was added in the lower chambers. After
24 h of incubation at 37°C, the cells on the upper face of the
membrane were scraped using a cotton swab and cells on the lower
face were fixed, stained and observed under a microscope (AE31;
Motic Group Co., Ltd., Xiamen, China). The dye on the membrane was
dissolved with 10% acetic acid and dispensed into 96-well plates
(150 μl/well). The optical density at 570 nm (OD570) of each well
was subsequently measured with an ELISA reader (ELX-800 type;
BioTek, Winooski, VT, USA).
Cell migration assay
Cell migratory abilitiy was estimated using a wound
healing assay as described previously (19). In brief, cells transfected with
miRs or its inhibitor were cultured to confluence. Wounds of ~1 mm
width were created with a plastic scriber, and cells were washed
and incubated in a serum-free medium. After wounding for 24 h, the
cells were incubated in a medium including 10% FBS. Cultures at 0,
24 and 48 h were fixed and observed under a microscope.
Western blotting
Total protein was extracted and the protein
concentration was measured by the Bradford DC protein assay
(Bio-Rad, Hercules, CA, USA). Proteins were then separated in 10%
SDS-PAGE and blotted onto a polyvinylidene difluoride (PVDF)
membrane. The PVDF membrane was treated with TBST containing 50 g/l
skimmed milk at room temperature for 4 h, followed by incubation
with the primary antibodies anti-ANGPTL2 and anti-β-actin (both
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
respectively, at 37°C for 1 h. The membranes were rinsed and
incubated for 1 h with the correspondent peroxidase-conjugated
secondary antibodies. Chemiluminent detection was performed using
an ECL kit (Pierce Chemical Co., Rockford, IL, USA).
Dual luciferase reporter assay
HCT-116 cells were co-transfected with the reporter
constructs ANGPTL2–3′ UTR-psi-CHECK2 (containing the 3′-UTR of
ANGPTL2 including the miRNA-25 binding sites) or Mut-ANGPTL2–3′
UTR-psi-CHECK2 (containing the corresponding mutated sequence of 3′
UTR of ANGPTL2), and miR-25 mimics or negative control (Life
Technologies) using Lipofectamine 2000. Luciferase activity was
determined after 48 h using the Dual-Glo substrate system (Promega,
Madison, WI, USA) and LD400 Luminometer (Beckman Coulter, Brea, CA,
USA). Data were presented as the ratio of experimental (Renilla)
luciferase to control (Firefly) luciferase.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Differences between the two groups were determined using a
Student’s t-test. Analyses were performed using SPSS 16.0 software.
P<0.05 was considered statistically significant.
Result
Expression of ANGPTL2 and miR-25 in CRC
tissues and cells
To investigate the expression of ANGPTL2 protein in
CRC tissues, a CRC tissue microarray was used. Fig. 1A and B showed that ANGPTL2 was
highly expressed in CRC tissues compared with normal tissues, and
gradually increased with the metastatic progression of CRC. The
levels of ANGPTL2 protein and mRNA expression in CRC HT-29, SW480,
SW620, HCT-116 cells and HCEpic were determined using RT-qPCR. In
contrast to HCEpic, the ANGPTL2 expression levels were elevated in
the CRC cells (Fig. 1C and D).
Notably, the RT-qPCR results showed that the miR-25 expression
levels in the four CRC cell lines were significantly decreased
compared with the control (Fig.
1E). These results suggested that ANGPTL2 likely plays an
important role in the malignant progression of CRC. Additionally,
the coexistence of ANGPTL2 upregulation and miR-25 downregulation
in CRC cells suggests a mutual potential regulatory
correlation.
Roles of ANGPTL2 in CRC cells
To investigate the functions of ANGPTL2 in CRC,
SW620 and HCT-116 cells were transfected with ANGPTL2 siRNA.
Fig. 2A and B shows that the
ANGPTL2 mRNA and protein expression of the two cell lines was
significantly decreased, indicating that ANGPTL2 was downregulated
in SW620 and HCT-116 cells. Knockdown of ANGPTL2 reduced cell
colony formation, and the invasive and migratory abilities of SW620
and HCT-116 cells (Figs. 2C–E and
3). Inhibition of ANGPTL2
expression suppressed the invasion and migration of CRC cells. This
result suggested that ANGPTL2 plays a promotional role in CRC
metastatic progression.
Roles of miR-25 in CRC cells
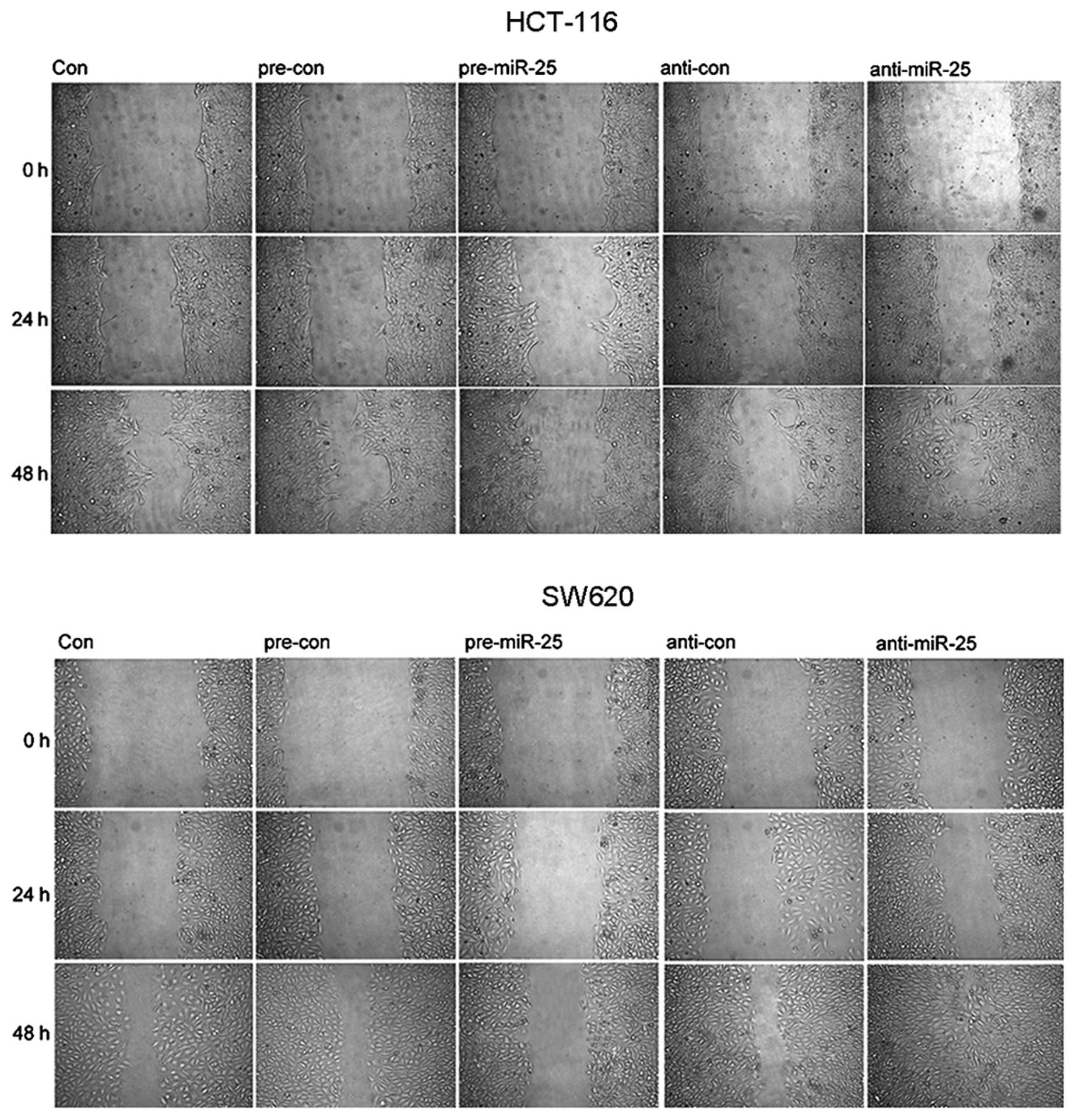
To verify the biological roles of miR-25 in CRC,
SW620 and HCT-116 cells were transfected with pre-miR-25 or miR-25
inhibitor. Fig. 4A shows that the
induction of pre-miR-25 significantly increased miR-25 expression
in SW620 and HCT-116 cells. Induction of miR-25 inhibitor
significantly decreased its expression. It was then confirmed that
the overexpression of miR-25 reduced colony formation, and the
invasive and migratory abilities of SW620 and HCT-116 cells.
However, inhibition of miR-25 increased proliferation, invasion and
migration of the two cell lines (Figs. 4B–D and 5). This result suggested that miR-25
functions as an anti-oncogene in CRC.
Regulation of ANGPTL2 expression by
miR-25
To examine the effects of miR-25 on ANGPTL2
expression, the mRNA and protein levels of ANGPTL2 in SW620 and
HCT-116 cells transfected with pre-miR-25 or miR-25 inhibitor were
determined using RT-qPCR and western blotting. The results showed
that the overexpression of miR-25 significantly decreased ANGPTL2
expression in SW620 and HCT-116 cells. By contrast, the
downregulation of miR-25 significantly increased ANGPTL2 expression
in the two cell lines (Fig.
6A–C). This result demonstrated that miR-25 negatively affected
ANGPTL2 expression in CRC cells.
To investigate whether ANGPTL2 is a direct target of
miR-25, the ANGPTL2 3′-UTR fragment containing the miR-25 binding
site and mutated targeting sequence were subcloned into psi-CHECK2
dual luciferase reporter vectors. Ectopic expression of miR-25
significantly reduced the luciferase activity in HCT-116 cells
transfected with the ANGPTL2–3′ UTR-psi-CHECK2 reporter vector
(Fig. 6D). The luciferase
activity levels in HCT-116 transfected with Mut-ANGPTL2–3′
UTR-psi-CHECK2 reporter vector plus miR-25 were not significantly
different from those of the control. It was confirmed that miR-25
directly targets ANGPTL2. The above results suggested that ANGPTL2
upregulation partly induced by miR-25 downregulation promotes the
malignant progression of CRC.
Discussion
It has been well-established that the initiation and
progression of cancers involve the deregulation of various genes,
such as oncogene upregulation, anti-oncogene downregulation or loss
(20–23). ANGPTL2 expression is reported to
be upregulated in lymph node metastasis-positive lung cancer
tissues compared with lymph node metastasis-negative cases
(11). A high ANGPTL2 expression
in lung cancer suggests a poor prognosis in terms of disease-free
survival (10). Results of the
present study show that ANGPTL2 expression was higher in CRC
tissues than in matched adjacent normal colon tissue, and gradually
increased with metastatic progression. The levels of ANGPTL2
protein and mRNA expression were also elevated in CRC HT-29, SW480,
SW620 and HCT-116 cells as compared to HCEpic. These findings
suggest that ANGPTL2 is involved in the malignant progression of
CRC. We further showed that the knockdown of ANGPTL2 reduced colony
formation, and the invasive and migratory abilities of SW620 and
HCT-116 cells, suggesting that ANGPTL2 acts as an oncogene in CRC
and its upregulation may promote its metastatic progression.
ANGPTL2 upregulation can enhance distant metastasis
of cancer possibly through increased tumor angiogenesis depending
on Rac activation, and tumor cell epithelial-to-mesenchymal
transitions (EMT) (9), and can
increase in vitro motility and invasion of cancer cells in
an autocrine/paracrine manner (10). It has been demonstrated that
ANGPTL2 upregulation is associated with increased transcription
factors NFATc, ATF2, and c-Jun expression, which form a complex and
bind to the ANGPTL2 promoter region (10). However, increasing evidence
suggests that the deregulation of tumor-associated genes is partly
due to the abnormal expression of its regulatory miRs (24–26). In this study, we examined the
expression of miR-25 in CRC cells, which is a theoretic regulatory
miR of ANGPTL2 predicted by algorithms available. Our data show
that the miR-25 expression levels in the four CRC cell lines were
significantly decreased compared to the control, which is
consistent with findings obtained by Li et al (15) who found that miR-25 was
downregulated in human CRC tissues. Overexpression of miR-25
inhibited colony formation, and the invasive and migratory
abilities of SW620 and HCT-116 cells, while the inhibition of
miR-25 promoted the invasion and migration of the two cell lines,
suggesting that miR-25 contributes to the development of CRC,
possibly by targeting ANGPTL2.
An inverse correlation between the expression of
miR-25 and the ANGPTL2 mRNA and protein expression levels in CRC
cells was identified, suggesting a critical role of miR-25 in the
regulation of ANGPTL2 expression by targeting its 3′-UTR. Thus,
ANGPTL2 upregulation in CRC is partly due to a decreased miR-25
expression. The regulatory mechanisms of the tumor-associated gene
are complicated, involving methylation (27,28), mutation (29), transcription factors (30) and miRs (31). In CRC, an increased ANGPTL2
expression is also possibly associated with NFATc, ATF2, and c-Jun
as well as in lung cancer (10),
or the other potential miRs which may target ANGPTL2. A gene can be
regulated by multiple miRs and a miR may also target multiple genes
(32,33). Recently, it has been reported that
miR-25 is capable of targeting Smad7 leading to inhibition of the
proliferation and migratory ability of CRC cells (15), and target the polycomb protein
enhancer of zeste 2 (EZH2) resulting in inhibition of proliferation
and colony formation of anaplastic thyroid carcinoma cells by
inducing G2/M-phase cell-cycle arrest (34). In addition, molecules involved in
the regulation of proliferation, apoptosis, cell cycle, migration,
invasion, and other biological process, are also targets of miR-25
in various types of cell, such as the TNF-related apoptosis
inducing ligand (TRAIL) death receptor-4 (35), Bim (36), Scratch2 (37), Wwp2, Fbxw7 (38) and MITF (39). Whether these genes regulated by
miR-25 play a role in CRC should be investigated.
In summary, the ANGPTL2 is upregulated in CRC and
gradually increases with its metastatic progression. The
upregulation of ANGPTL2 may be partly induced by miR-25
downregulation and miR-25 targeting ANGPTL2 contributes to the
metastatic progression of CRC. Our results provide evidence
supporting miR-25 and ANGPTL2 as diagnostic or therapeutic tools
for CRC.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Suda T, Arai F and Shimmura S: Regulation
of stem cells in the niche. Cornea. 24:S12–S17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabata M, Kadomatsu T, Fukuhara S, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tazume H, Miyata K, Tian Z, et al:
Macrophage-derived angiopoietin-like protein 2 accelerates
development of abdominal aortic aneurysm. Arterioscler Thromb Vasc
Biol. 32:1400–1409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada T, Tsukano H, Endo M, et al:
Synoviocyte-derived angiopoietin-like protein 2 contributes to
synovial chronic inflammation in rheumatoid arthritis. Am J Pathol.
176:2309–2319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoi J, Endo M, Kadomatsu T, et al:
Angiopoietin-like protein 2 is an important facilitator of
inflammatory carcinogenesis and metastasis. Cancer Res.
71:7502–7512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endo M, Nakano M, Kadomatsu T, et al:
Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical
driver of metastasis. Cancer Res. 72:1784–1794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki H, Suzuki A, Shitara M, et al:
Angiopoietin-like protein ANGPTL2 gene expression is
correlated with lymph node metastasis in lung cancer. Oncol Lett.
4:1325–1328. 2012.
|
|
12
|
Kikuchi R, Tsuda H, Kozaki K, et al:
Frequent inactivation of a putative tumor suppressor,
angiopoietin-like protein 2, in ovarian cancer. Cancer Res.
68:5067–5075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schetter AJ and Harris CC: Alterations of
microRNAs contribute to colon carcinogenesis. Semin Oncol.
38:734–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu WK, Law PT, Lee CW, et al: MicroRNA in
colorectal cancer: from benchtop to bedside. Carcinogenesis.
32:247–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Zou C, Zou C, et al: MicroRNA-25
functions as a potential tumor suppressor in colon cancer by
targeting Smad7. Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006.PubMed/NCBI
|
|
17
|
Jiang X, Yue J, Lu H, et al: Inhibition of
filamin-A reduces cancer metastatic potential. Int J Biol Sci.
9:67–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aspenström P, Fransson A and Saras J: Rho
GTPases have diverse effects on the organization of the actin
filament system. Biochem J. 377:327–337. 2004.PubMed/NCBI
|
|
19
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lai AZ, Durrant M, Zuo D, Ratcliffe CD and
Park M: Met kinase-dependent loss of the E3 ligase Cbl in gastric
cancer. J Biol Chem. 287:8048–8059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baldwin A, Huh KW and Munger K: Human
papillomavirus E7 oncoprotein dysregulates steroid receptor
coactivator 1 localization and function. J Virol. 80:6669–6677.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill R, Calvopina JH, Kim C, et al: PTEN
loss accelerates KrasG12D-induced pancreatic cancer development.
Cancer Res. 70:7114–7124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woods NT, Yamaguchi H, Lee FY, Bhalla KN
and Wang HG: Anoikis, initiated by Mcl-1 degradation and Bim
induction, is deregulated during oncogenesis. Cancer Res.
67:10744–10752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadera BE, Li L, Toste PA, et al:
MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated
fibroblasts promotes metastasis. PLoS One. 8:e719782013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahlhaus M, Schult C, Lange S, Freund M
and Junghanss C: MicroRNA 181a influences the expression of HMGB1
and CD4 in acute Leukemias. Anticancer Res. 33:445–452.
2013.PubMed/NCBI
|
|
26
|
Sandhu R, Rivenbark AG and Coleman WB:
Loss of post-transcriptional regulation of DNMT3b by
microRNAs: A possible molecular mechanism for the hypermethylation
defect observed in a subset of breast cancer cell lines. Int J
Oncol. 41:721–732. 2012.PubMed/NCBI
|
|
27
|
Fang JY, Lu J, Chen YX and Yang L: Effects
of DNA methylation on expression of tumor suppressor genes and
proto-oncogene in human colon cancer cell lines. World J
Gastroenterol. 9:1976–1980. 2003.PubMed/NCBI
|
|
28
|
Pakneshan P, Szyf M and Rabbani SA:
Methylation and inhibition of expression of uPA by the RAS
oncogene: divergence of growth control and invasion in breast
cancer cells. Carcinogenesis. 26:557–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chien CH and Chow SN: Point mutation of
the ras oncogene in human ovarian cancer. DNA Cell Biol.
12:623–627. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beger M, Butz K, Denk C, Williams T, Hurst
HC and Hoppe-Seyler F: Expression pattern of AP-2 transcription
factors in cervical cancer cells and analysis of their influence on
human papillomavirus oncogene transcription. J Mol Med (Berl).
79:314–320. 2001. View Article : Google Scholar
|
|
31
|
Patel JB, Appaiah HN, Burnett RM, et al:
Control of EVI-1 oncogene expression in metastatic breast cancer
cells through microRNA miR-22. Oncogene. 30:1290–1301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009.PubMed/NCBI
|
|
33
|
Lukiw WJ and Alexandrov PN: Regulation of
complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease
(AD) brain. Mol Neurobiol. 46:11–19. 2012.PubMed/NCBI
|
|
34
|
Esposito F, Tornincasa M, Pallante P, et
al: Down-regulation of the miR-25 and miR-30d contributes to the
development of anaplastic thyroid carcinoma targeting the polycomb
protein EZH2. J Clin Endocrinol Metab. 97:E710–E718. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Razumilava N, Bronk SF, Smoot RL, et al:
miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death
receptor-4 and promotes apoptosis resistance in cholangiocarcinoma.
Hepatology. 55:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: miR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.PubMed/NCBI
|
|
37
|
Rodríguez-Aznar E, Barrallo-Gimeno A and
Nieto MA: Scratch2 prevents cell cycle re-entry by repressing
miR-25 in postmitotic primary neurons. J Neurosci. 33:5095–5105.
2013.PubMed/NCBI
|
|
38
|
Lu D, Davis MP, Abreu-Goodger C, et al:
miR-25 regulates Wwp2 and Fbxw7 and promotes reprogramming of mouse
fibroblast cells to iPSCs. PLoS One. 7:e409382012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Z, He J, Jia X, et al: MicroRNA-25
functions in regulation of pigmentation by targeting the
transcription factor MITF in alpaca (Lama pacos) skin
melanocytes. Domest Anim Endocrinol. 38:200–209. 2010. View Article : Google Scholar : PubMed/NCBI
|