Introduction
Bone marrow-derived mesenchymal stem cells (BMSCs)
can be easily isolated and expanded from bone marrow aspirates.
BMSCs are promising sources for regenerative medicine as they are
harvested directly from patients (1). BMSCs are immunomodulatory,
suppressing mixed lymphocyte reactions and attenuating
alloresponses (2). Moreover, they
are multipotent progenitor cells that have the capacity to
differentiate into various types of cells, such as bone, cartilage,
muscle, endothelial, vascular smooth muscle and other connective
tissues (3). Due to their broad
capacity for differentiation, BMSCs have been tested in multiple
diseases. including diseases of the nervous system (4), skeletal (5) and renal system (6), and have been extensively used in
myocardial disease (7,8). A previous study reported that BMSCs
can produce a variety of cytokines, including vascular endothelial
growth factor, basic fibroblast growth factor, interleukin-1,
platelet-derived growth factor and transforming growth factor,
which contribute to the functional improvement of infarcted hearts
by inhibiting the apoptosis of cardiomyocytes and inducing
therapeutic angiogenesis (9). The
potential of therapy using BMSCs to differentiate into viable
cardiomyocytes and regenerate vascularization is, therefore, an
attractive prospect, with the aim of reversing ventricular
remodeling, preventing heart failure and alleviating the need for
heart transplantation. Pre-clinical studies found that the
implantation of BMSCs to the infarct zone in the heart improved the
wall thickness of the left ventricle (LV), and promoted
neo-vascularization in a rat model (7). In addition, implantation
significantly increased LV function, cardiac blood flow and
vascular density in a pig model (10). However, only a small fraction of
transplanted cells engraft and survive in the injured heart, which
limits the efficacy of cell transplantation (11). A clinical study demonstrated that
BMSC intracoronary transplantation in patients with anterior acute
myocardial infarction did not result in an increase in ejection
fraction, although slight improvements in myocardial perfusion were
noted in the BMSC group (12).
Advanced imaging technologies are essential for the pre-clinical
evaluation of novel cell-based therapeutics, as they permit
longitudinal tracking and monitoring of cellular grafts and donor
cell survival, which provides a more detailed understanding of the
mechanisms involved in stem cell transplantation in ischemic heart
disease.
To date, the majority of studies on stem cell
viability have relied on ex vivo analysis, such as
histological staining for green fluorescent protein or
β-galactosidase. Another approach is to label cells with iron
particles and track cell viability by magnetic resonance imaging
(MRI) (13). MRI, however, is
unable to distinguish viable from non-viable cells, as iron
particles may be retained by living, dead, or scavenger cells
(14). Another promising approach
is based on the transfer of a sodium iodide symporter (NIS)
reporter gene construct into stem cells using a viral vector
(15), which permits the
detection of viable transplanted cells by positron emission
tomography (PET) or single photon emission computed tomography
(SPECT) following iodine-125 (125I) or
99mTc99g (Tc, technetium; 99m indicates that
technetium is at its excited stage; 99g indicates the atomic weight
of technetium) radiotracer administration (16). A number of studies have
successfully introduced the ectopic expression of NIS for
non-invasive imaging analyses (17,18).
In the current study, we employed a lentiviral
vector to induce the expression of the NIS reporter gene and
enhanced green fluorescence protein (EGFP) in BMSCs. The potential
of NIS as an imaging reporter gene for the uptake and accumulation
of 125I and 99mTc99g in
vitro and in vivo was investigated using a rat model of
ischemia.
Materials and methods
Animals
Sprague-Dawley rats were obtained from Slaccas
Experimental Animal Corp. (Shanghai, China). Animal studies were
approved by the local Ethics Committee (Shanghai Jiaotong
University School of Medicine) and performed according to ethical
principles of animal experimentation. All animals were anesthetized
with pentobarbital (100 mg/kg, 1 dose intraperitoneally) prior to
sacrifice. The efficacy of the anesthesia was monitored by pinching
the hind paw. When sufficiently sedated, the rats were euthanized
by cervical dislocation.
BMSC isolation and culture
conditions
Four-week-old male Sprague-Dawley rats (weighing
80±5 g) were used for BMSC isolation. The BMSCs were harvested,
propagated and characterized as previously described (19). Briefly, both ends of the femur
were cut off at the epiphysis, and the BMSCs were flushed out from
the femurs and tibias with Dulbecco’s modified Eagle’s medium
(DMEM; Gibco-BRL, NY, USA) containing 23 mM NaHCO3
(Gibco Biocult, Paisley, UK) and supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco Biocult) and
antibiotics (50 μg/ml streptomycin sulfate and 100 U/ml
penicillin). The cells were cultured in DMEM at 37°C in a
humidified 5% CO2 incubator.
Virus production and cell culture
For the generation of transgenic BMSC lines, the
lentiviral vector, Lv-EF1α-NIS-IRES-EGFP, was constructed.
Lv-EF1α-OCT4-IRES-EGFP was kindly provided by the Institute of
Molecular Biology, Chinese Academy of Sciences, Shanghai, China;
pcDNA3.1-NIS was obtained from our own library, as previously
described (20). The NIS gene was
amplified from pcDNA3.1-NIS by PCR using the following primers:
forward, 5′-GCGC GGATCCCGGGTATCGATGGAGGCCGTG-3′ and reverse,
5′-CGCGTCTAGATCAGAGGTTTGTAGGTAGTGAGC-3′. The product was digested
with XbaI and BamHI, and cloned into the XbaI
and BamHI sites of Lv-EF1α-OCT4-IRES-EGFP generating a
functional vector featuring NIS under the control of the human
elongation factor-1α (EF1α) promoter, while the octamer-binding
transcription factor 4 (OCT4) transgene of Lv-EF1α-OCT4-IRES-EGFP
was replaced with NIS.
The HEK293T cell line (Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) was cultured in RPMI-1640
medium (Gibco-BRL) supplemented with 10% FBS and 1%
penicillin/streptomycin.
Viral particles were generated by co-transfection of
the HEK293T cells with Lv-EF1α-NIS-IRES-EGFP and the 3 packaging
plasmids, pRsv-REV, pMDIg-pRRE and pMD2G (Biovector Science
Laboratory, Beijing, China). The viral particles were harvested by
collecting the cell culture medium at 48 h post-transfection. The
supernatants were filtered through 0.45-μm filters, centrifuged at
10,000 × g for 15 min and the resulting pellet was resuspended in
100 μl culture medium.
Gene transduction and cell viability
assay
The Lv-EF1α-NIS-IRES-EGFP virus at various
multiplicities of infection (MOI) from 10 to 1,200 was used to
infect the BMSCs and the infection efficiency was detected by
fluorescence microscopy of the expression of green fluorescent
protein.
Following gene transduction, cell viability and
proliferation were determined using the cell counting kit-8 (CCK-8)
assay (Beyotime Institute of Biotechnology, Shanghai, China).
Transduced or non-transduced BMSCs were plated into 96-well plates
(2×103 cells/well), and incubated for 12, 24, 36, 48 or
72 h. The blank group contained medium without cells. CCK-8 reagent
(10 μl) was added to the wells, and the cells were incubated for 1
h. Absorbance was measured using a Multiskan MK3 Microplate Reader
(Thermo Fisher Scientific, Hudson, NH, USA) at 450 nm. The
absorbance was calculated as Atest-Ablank,
where Atest represents the measured absorbance of each
experimental group, and Ablank represents absorbance of
each blank group. The mean ± standard deviation (SD) of
quadruplicate replicates from at least 3 independent experiments
are presented.
Phenotypic expression and differentiation
of BMSCs
To verify the phenotype of the isolated BMSCs, the
cells were examined for the expression of various surface markers
(CD105, CD29, CD90, CD14, CD34 and CD45) characteristic of BMSCs by
flow cytometry (Beckman Coulter, Miami, FL, USA). Following 3
passages, the cells were used for in vitro and in
vivo experiments. The BMSCs were plated on 6-well plates at a
density of 105 cells per well and cultured in DMEM at
37°C in a humidified 5% CO2 incubator. Adipogenic
differentiation was induced by treating 50% confluent cultures
twice weekly for 2 weeks with 10 nM dexamethasone and 5 μg/ml
insulin, as previously described (21). Osteocyte differentiation was
induced by treating 50% confluent cultures twice weekly for 4 weeks
with 10 nM dexamethasone, 50 μg/ml ascorbic acid and 10 mM
β-glycerol phosphate, as previously described (22). The cells were fixed for 20 min in
10% buffered formalin. Lipid droplets in the adipocytes were
stained with Oil Red O (0.5% in isopropropanol stock diluted 3:2 in
H2O) for 10 min, and bone matrix was stained for 20 min
in 2% alizarin red.
Quantitative reverse transcription PCR
(RT-qPCR) and western blot analysis
To examine the expression levels of NIS in the
Lv-EF1α-NIS-IRES-EGFP-transfected BMSCs, RT-qPCR was performed on
days 1, 4, 7, 14 and 21 following viral infection, and western blot
analysis was performed on day 7. Total RNA was extracted using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was
synthesized using the Superscript RT kit (Invitrogen). RT-qPCR was
performed using SYBR® Premix Ex Taq™ II (Takara Bio,
Inc., Shiga, Japan) according to the manufacturer’s instructions.
The primers used for amplification were as follows: NIS forward,
5-GTACATTGTAGCCACGAT GCTGTA-3′ and reverse, 5′-CCGTGTAGAAGGTGCAGAT
AATTC-3′; GAPDH (internal control) forward, 5′-GTCAAG
CTCATTTCCTGGTATGAC-3′ and reverse, 5′-CTCTCTC TTCCTCTTGTGCTCTTG-3′
at 95°C for 30 sec followed by 40 cycles of 5 sec at 95°C and 30
sec at 60°C and one cycles of 95°C for 15 sec, 60°C for 1 min, 95°C
for 15 sec. According to the manufacture’s instructions, the NIS
expression levels were normalized to those of the GAPDH endogenous
reference gene as follows: F value = 2−ΔΔCt, as
previously described (23).
Total protein was harvested from the cultured cells
on day 7 following viral infection. The cells were incubated in
lysis buffer (SDS lysis buffer), 1% phenylmethanesulfonyl fluoride
(PMSF) on ice, centrifuged at 10,000 × g, and the protein
concentration of the supernatants was measured using the BCA
Protein Assay kit (all from Beyotime Institute of Biotechnology).
Equal quantities of protein were subjected to western blot analysis
using a polyclonal goat anti-NIS antibody (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and an anti-goat IgG-HRP
secondary antibody (1:5,000; MultiSciences Biotech Co., Ltd.,
Shanghai, China). Relative protein levels were normalized against
GAPDH (1:1,000; Beyotime Institute of Biotechnology). All
experiments were performed in triplicate.
Analysis of 125I uptake and
efflux
125I uptake and efflux were measured in
triplicate as previously described (24). On day 1, transduced or control
BMSCs were plated in 24-well plates (2×105 cells/well).
On day 2, 500 μl of Hank’s Balanced Salt Solution (HBSS) containing
3.7 kBq 125I and 10 μmol/l sodium iodide (NaI) were
added. The cells in the control group were treated with 10 μmol/l
NaI, whereas the cells in the test group were treated with 50 μM
sodium perchlorate (NaClO4). The cells were incubated at
37°C for 5–120 min, washed twice with ice-cold HBSS, and lysed
using 0.5 mol/l sodium hydroxide (NaOH). The radioactivity [counts
per minute, (CPM)] of the cell lysates was measured using an
automatic gamma counter (Shanghai Hesuo Rihuan Photoelectric
Instrument Co., Ltd., Shanghai, China).
In order to measure the efflux, the cells were
incubated with 3.7 kBq Na125I and 10 μM NaI in 500 μl of
HBSS at 37°C for 60 min, washed twice with HBSS, and incubated in
500 μl of HBSS containing 10 μM NaI (without radioactive
Na125I). The buffer was replaced every 5 min for up to
40 min and the level of radioactivity of the solutions was
determined. Following the removal of the last sample, the cells
were lysed using 0.5 M NaOH. Total radioactivity at the initiation
of the measurement of the efflux was calculated by adding the final
cell radioactivity to the total medium radioactivity.
Animal model of myocardial
infarction
The experimental animals used in this study were
male Sprague-Dawley rats, weighing 200–220 g. The rats were
intraperitoneally anesthetized with pentobarbital (35 mg/100 g). A
midline anterior cervical skin incision was made, and the trachea
was exposed by sharp dissection. The trachea was intubated with an
angiocatheter and ventilated to a rodent ventilator with room air.
A 1.5 cm vertical left parasternal skin incision was made, the
chest cavity was entered through the fourth interspace, and the
pericardium was vertically opened. The left anterior descending
(LAD) coronary artery was ligated with a 6-0 polypropylene suture.
Ventricle blanching indicated the successful occlusion of the
vessel.
Implantation of BMSCs
Adult male Sprague-Dawley rats were randomly divided
into 2 groups. Immediately following the ligation of the LAD, the
experimental group received 5×106 BMSCs transfected with
Lv-EF1α-NIS-IRES-EGFP; the control group received 5×106
BMSCs.
Micro-SPECT/computed tomography (CT)
imaging
One week following BMSC transplantation, the rats
were intravenously injected with 74 MBq of
99mTc99g. Anesthesia was induced and
maintained by isoflurane inhalation, and the rats were placed in a
spread-prone position and scanned using a small-animal micro-SPECT
scanner (NanoSPECT/CT® PLUS; Bioscan, Washington, DC,
USA) 60 min after the injection of 99mTc99g.
CT images were acquired (CTDI = 6.1 cGy) before whole-body
NanoSPECT images (10 s/frame for systematic scans) were obtained,
without moving the rats. The images were processed and
reconstructed using Nuclear v1.02 software and HiSPECT 1.4.2
software (both from Bioscan) for image acquisition.
Histological analysis
After imaging, some animals from the
Lv-EF1α-NIS-IRES-EGFP group were sacrificed by cervical
dislocation. The hearts were harvested and immersed in 4%
paraformaldehyde for 24 h. The fixed hearts were sliced into 2
sections according to the injection sites. Heart sections
containing the injection sites were then embedded in paraffin and
cut into 2–3 μm sections. Hematoxylin and eosin (H&E) staining
was performed and unstained sections on positively charged slides
were used for immunohistochemical staining using primary polyclonal
rabbit anti-NIS antibody (1:50; Proteintech, Chicago, IL, USA).
Statistical analysis
Data were analyzed using GaphPad Prism software
(version 5.0; GraphPad Software, Inc., San Diego, CA, USA); the
mean ± SD values are presented. Statistical analyses were performed
using two-tailed Student’s t-tests. For all analyses, a value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
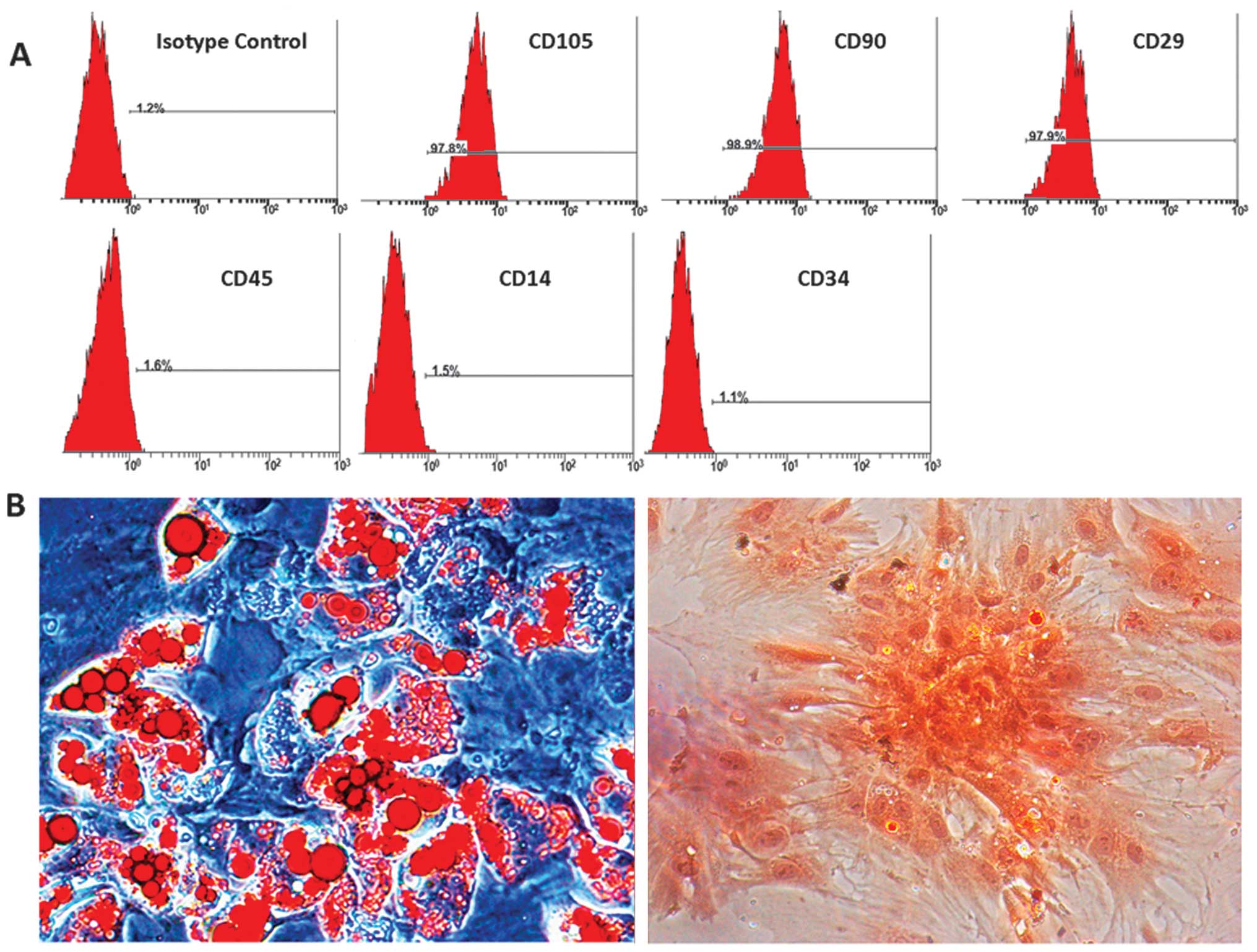
Immunophenotyping of BMSCs
The BMSCs were analyzed by flow cytometry and were
found to be positive for the cell surface antigens, CD105, CD29 and
CD90, and negative for CD14, CD34 and CD45 (Fig. 1A). The differentiation assay
confirmed that the isolated BMSCs differentiated into adipocytes
and osteoblasts. Lipid droplets in adipocytes were stained red with
Oil Red O, and bone matrix was stained in orange red with alizarin
red (Fig. 1B).
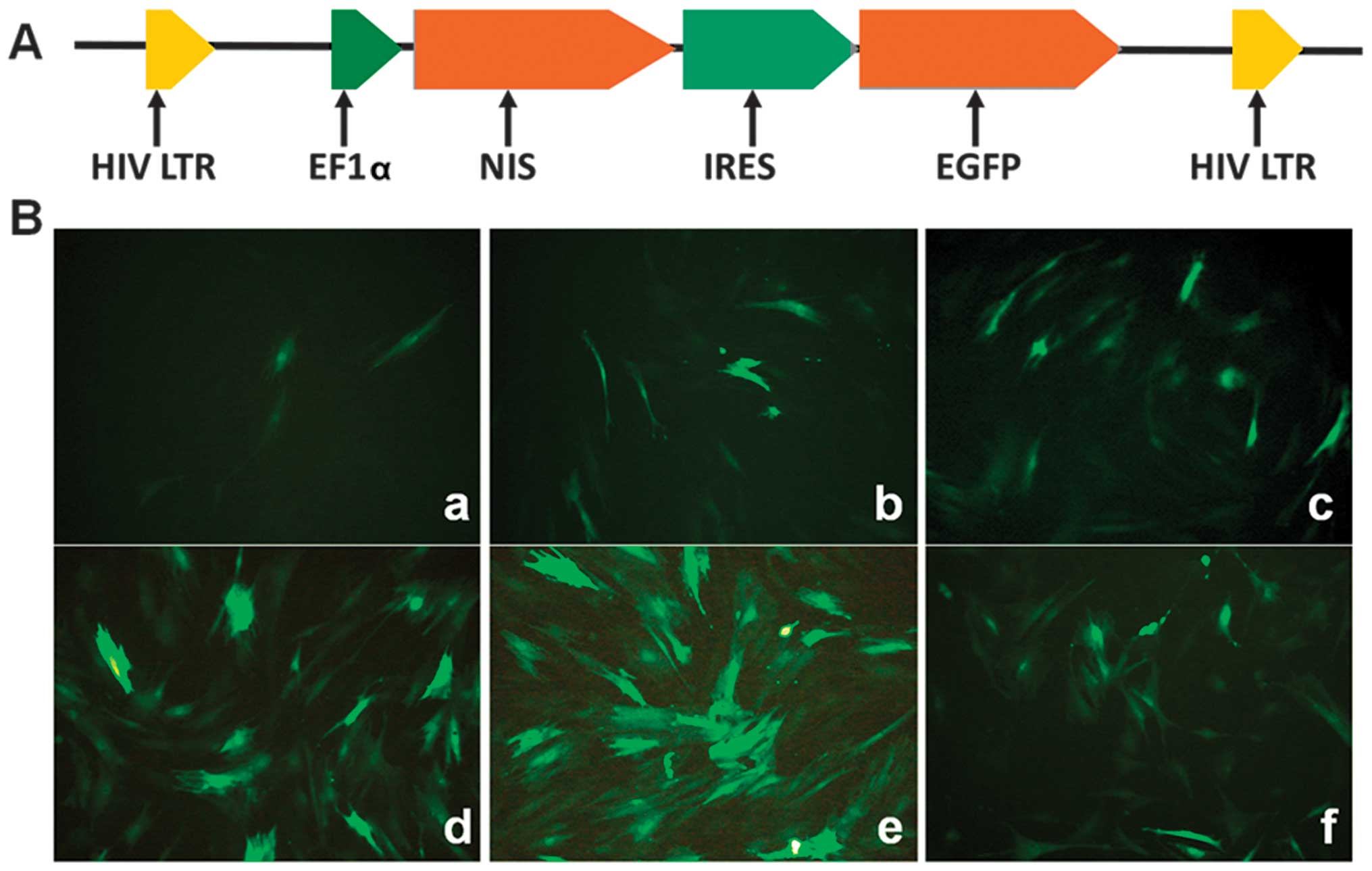
Infection with the lentiviral vector and
determination of the optimal MOI
As illustrated in Fig.
2, the BMSCs were distributed uniformly at 48 h following
infection with Lv-EF1α-NIS-IRES-EGFP at MOIs of 10, 50, 100, 400,
600 and 1,200. The majority of the cells (>90%) expressed EGFP
at an MOI of 600.
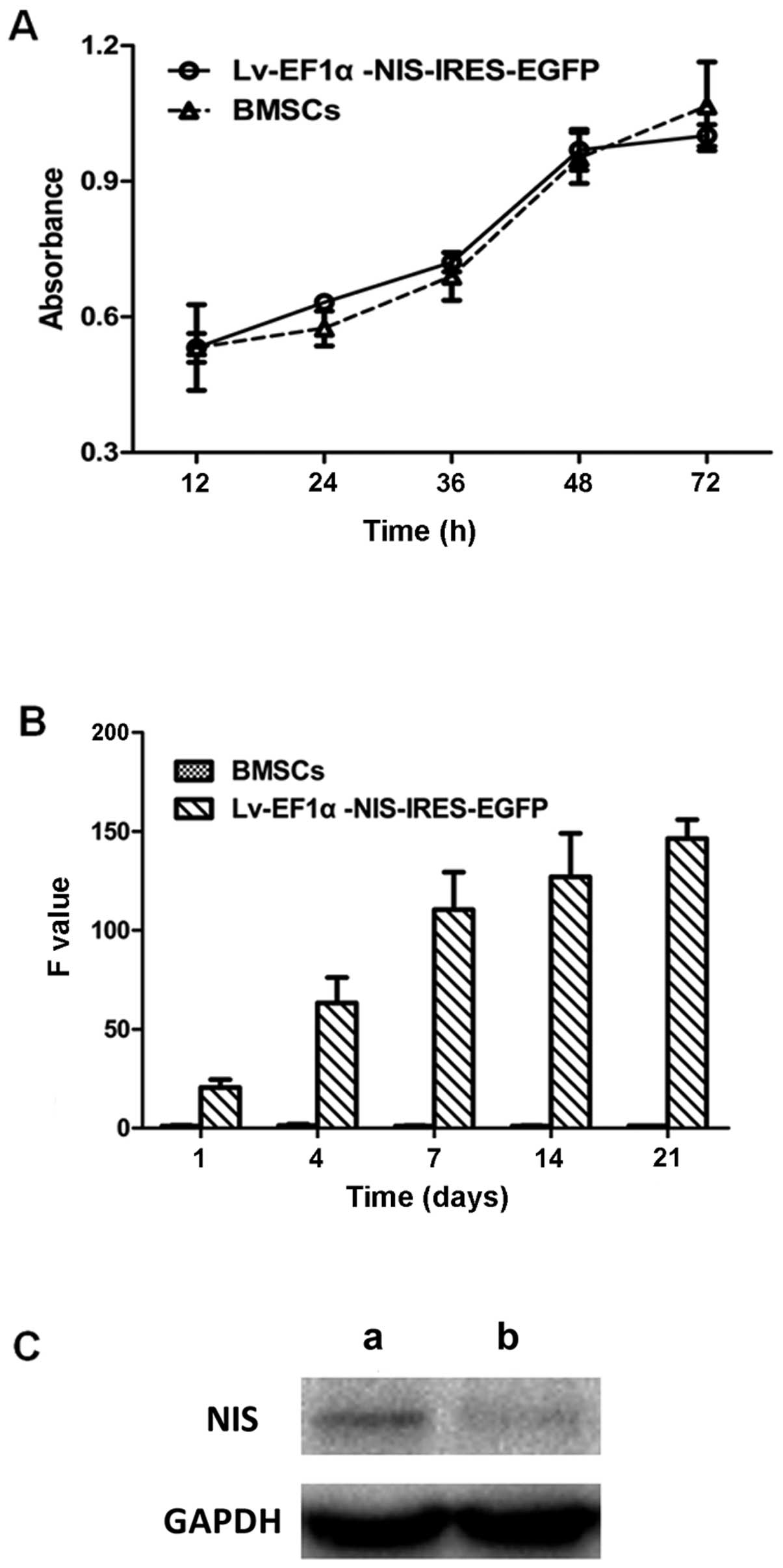
CCK-8 assay, RT-qPCR and western blot
analysis
There was no significant difference in cell
viability and proliferation between the BMSCs infected with
Lv-EF1α-NIS-IRES-EGFP and the BMSC control group (Fig. 3A). To examine the expression
levels of NIS in the BMSCs infected with Lv-EF1α-NIS-IRES-EGFP,
RT-qPCR was performed on days 1, 4, 7, 14 and 21 following viral
infection and western blot analysis was performed on day 7. NIS
mRNA and protein expression was clearly detected in the
Lv-EF1α-NIS-IRES-EGFP-treated BMSCs compared with the control group
(Fig. 3B and C). The results
revealed that the expression of NIS increased from day 4 to 7, and
remained at a consistently high level from day 7 to 21.
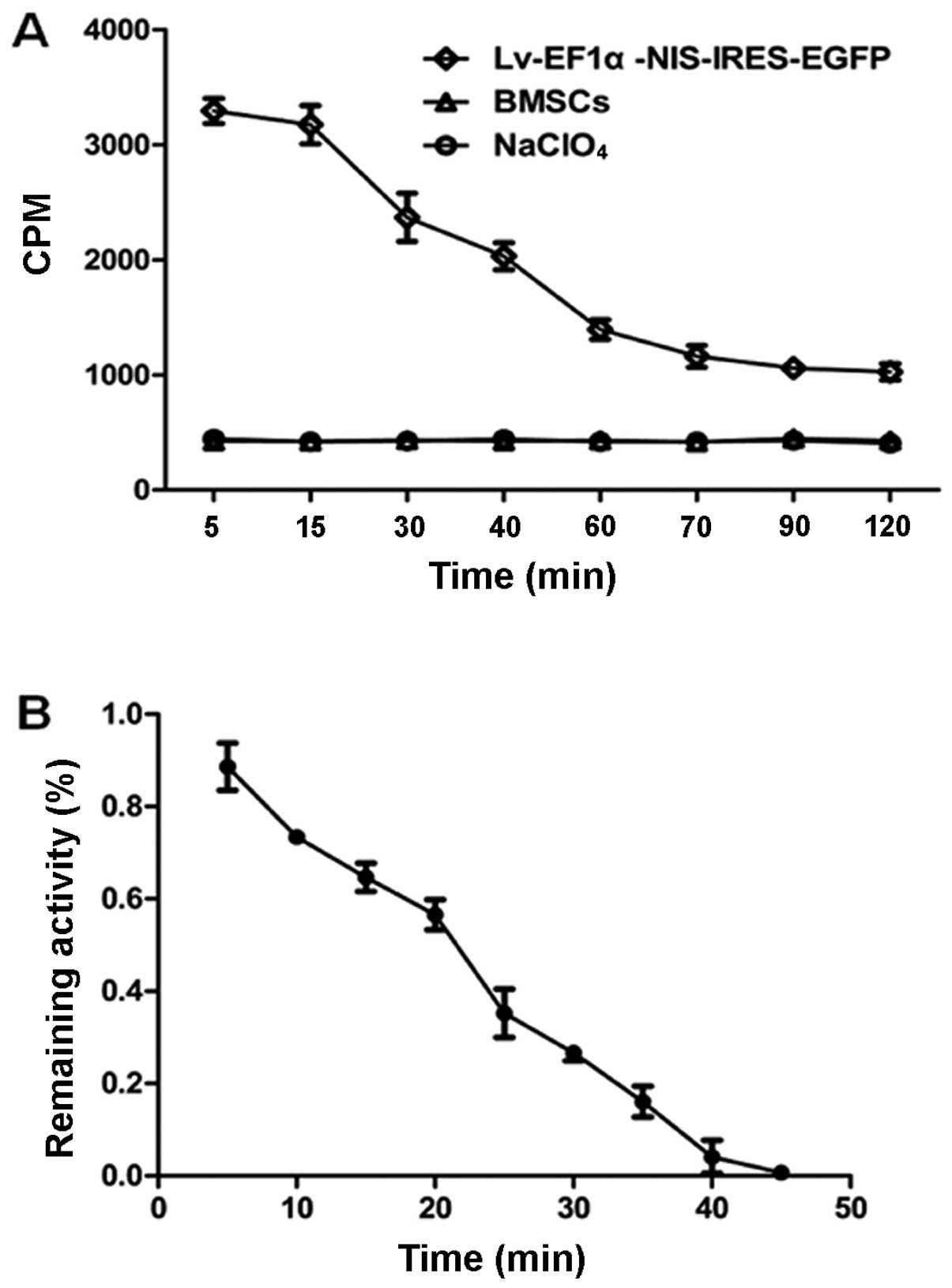
125I uptake and efflux
125I uptake by the
Lv-EF1α-NIS-IRES-EGFP-infected cells varied depending on the
incubation time, and peaked at approximately 3,300 CPM at 5 min.
This was 8-fold higher than the level of 125I uptake by
the control BMSCs at the same time point. After 5 min,
125I uptake decreased with time. 125I uptake
by the Lv-EF1α-NIS-IRES-EGFP-infected cells was completely blocked
with NaClO4. There was no functional 125I
uptake observed in the control BMSC group (Fig. 4A). 125I was rapidly
effluxed from the Lv-EF1α-NIS-IRES-EGFP-infected BMSCs, with a half
life (t1/2) of approximately 25 min (Fig. 4B).
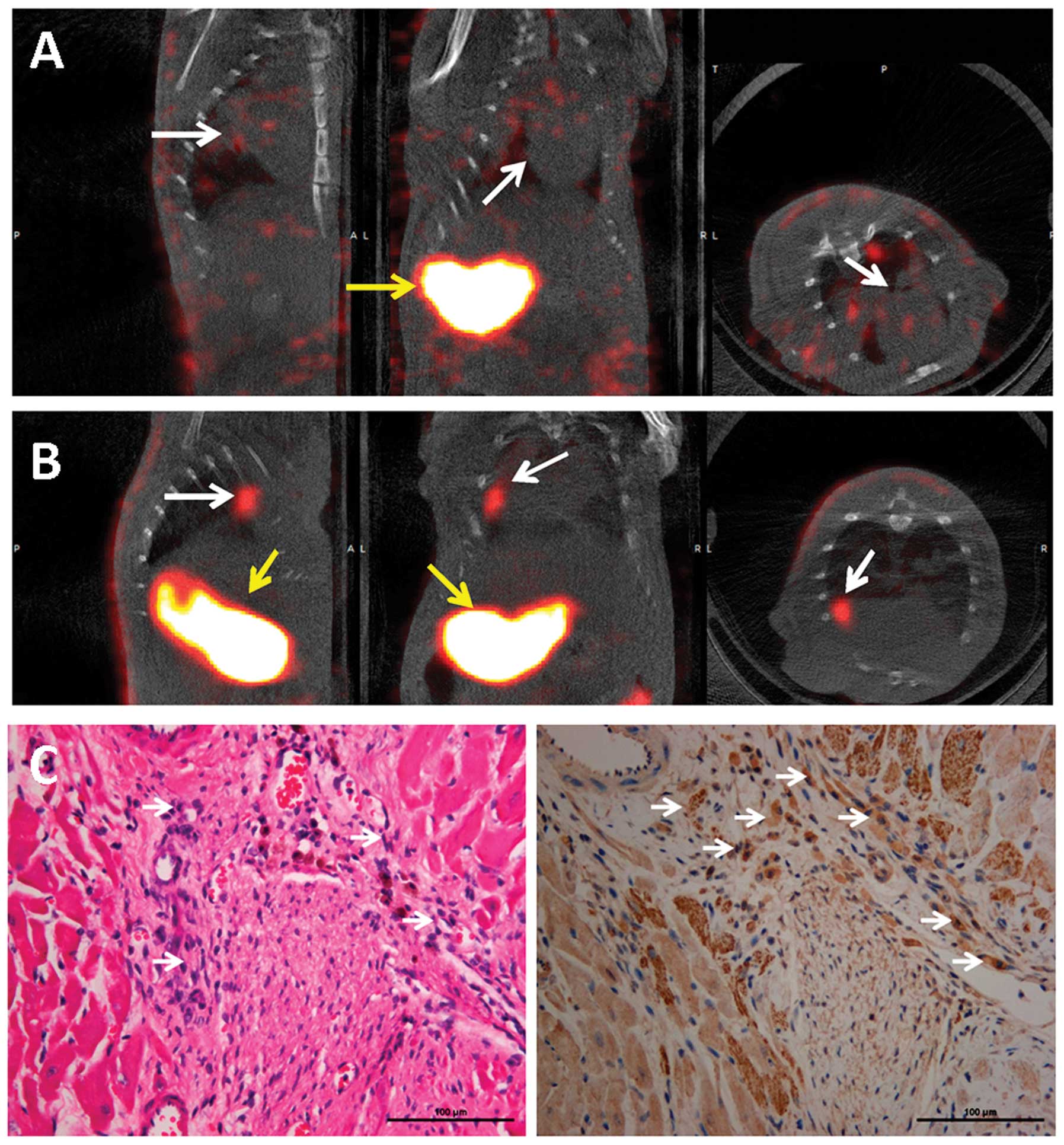
Micro-SPECT/CT imaging and
immunostaining
One week following cell transplantation, the rats
were intravenously injected with 74 MBq of
99mTc99g. 99mTc99g
uptake was not detectable in the heart, although significant uptake
was observed in the stomach of the rats in the BMSC control group
(Fig. 5A). By contrast,
significant uptake was observed in the transplanted zone of the
hearts of rats transplanted with Lv-EF1α-NIS-IRES-EGFP-infected
BMSCs and in the stomach 45 min following
99mTc99g injection (Fig. 5B). H&E staining identified the
transplanted cells in the infarct zone of the rat hearts (Fig. 5C, left panel). The BMSCs
transfected with Lv-EF1α-NIS-IRES-EGFP were positive for NIS
expression (Fig. 5C, right
panel).
Discussion
Despite rapid progress in the medical treatment of
cardiometabolic disease, ischemic heart disease remains the leading
cause of mortality in the developed world (25). Pre-clinical and clinical studies
have demonstrated that BMSC transplantation into the infarcted
myocardium can augment cardiac function and attenuate ventricular
remodeling (7,10,12,26). However, 99% of BMSCs do not
survive within 3–4 days following transplantation into the ischemic
heart (27). In an attempt to
prolong survival in vivo, genetically engineered BMSCs have
been suggested as an effective strategy to improve the survival
rate and therapeutic efficacy of BMSCs by inducing the expression
of proteins, such as CXC chemokine receptor 4 (28), angiogenin (29), vascular endothelial growth factor
(30), heme oxygenase-1 (31,32), and hypoxia-inducible factor-1α
(8). The tracking and monitoring
of transplanted cells relies on ex vivo analyses, such as
histologic staining for green fluorescent protein or
β-galactosidase or cellular labeling with Dil. These techniques
require a large number of animals to be sacrificed and cannot be
applied in clinical research. Advanced imaging technologies and
non-invasive techniques are therefore be required. MRI has been
used to track cell viability after labelling with iron particles
(13). This technique, however,
is unable to distinguish viable from non-viable cells, as iron
particles may be retained by living, dead, or scavenger cells
(14). NIS is a transmembrane
carrier that selectively transports iodine (I), technetium (Tc),
rhenium (Re) and their isotopes, 123I, 125I,
131I, 99mTc99g and
188Re (16,17,32), that can be detected by SPECT or
PET, and offers several advantages for in vivo reporter gene
imaging (33).
In this study, we evaluated the ability of the NIS
reporter gene to monitor transplanted BMSCs in the ischemic
myocardium of living rats. The technique involved inducing the
expression of the NIS and EGFP genes, which was achieved with high
efficiency at an MOI of 600 and without adverse effects following
infection with a lentiviral vector driven by a single promoter,
EF1α. The Lv-EF1α-NIS-IRES-EGFP lentiviral particles were
successfully packaged and efficiently infected the BMSCs. The
expression of NIS was confirmed by RT-qPCR and western blot
analysis. To address concerns regarding the biosafety of the
lentivirus, we assessed the effects of exogenous NIS expression on
the viability and proliferation of BMSCs in vitro. However,
no significant differences in cell viability or proliferation were
measured in the control or treated BMSCs. The absorption and
accumulation of 125I was successfully observed in the
BMSCs transfected with the lentivirus in vitro and was
specifically inhibited by NaClO4. One week following
BMSC transplantation, the BMSCs were successfully monitored by
99mTc99g-SPECT.
A number of viruses have been used for gene
transfer, and each has advantages and disadvantages. Herpes simplex
virus has a broad infectivity, but low titers and short term
episomal expression. Adenoviruses can be obtained with high titers
and can infect non-dividing cells, but have life-threatening
immunogenicity and short-term episomal expression. Adeno-associated
viruses also have broad infectivity similar to the herpes simplex
virus, infect non-dividing cells and are non-cytopathic; however,
short-term expression with limited integration limits their broad
use in gene transfer. Fortunately, lentiviruses can be obtained
with high viral titers that can permanently infect non-dividing
cells, although safety concerns exist due to their HIV derivation
(34). To address this issue, the
current packaging cell line requires transient transfection with
three distinct plasmids, all containing gene sequences required for
an active infectious virus. This system ensures that the
possibility of three recombination events occurring in one cell to
produce an actively infectious product is extremely unlikely
(35). In our study, we used
lentivirus for transfecting BMSCs to establish cell lines
expressing the NIS and the EGFP genes which is useful for further
study on BMSC viability and migration following transplantation
into the infarcted myocardium.
NIS expression is limited to only a few tissues,
such as the thyroid gland, salivary glands, stomach, lactating
mammary glands, small intestine and rectum (36). A limitation of this imaging
technology is the physiological expression of NIS in these tissues.
If the signals from these organs are strong, they may cover up weak
signals from adjacent organs of cell transplantation. In our study,
significant radioactive uptake was observed in the transplanted
Lv-EF1α-NIS-IRES-EGFP-treated BMSCs in the heart, stomach, urinary
bladder, intestine and only a slight uptake in the thyroid in the
Lv-EF1α-NIS-IRES-EGFP group at 45 min following
99mTc99g injection.
99mTc99g uptake of other tissues did not
affect the signals from the transplanted BMSCs.
With rapid advances in genetically engineered stem
cell-based therapy for myocardial infarction, non-invasive in
vivo imaging may play a critical role in future studies. Our
strategy of using lentivirus as a gene delivery vector in a
radionuclide-based reporter gene imaging system may provide a
valuable method for further studies on BMSC transplantation therapy
for myocardial infarction.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (NSFC; no. 81271610), the
Shanghai Outstanding Academic Leaders Project (11XD1403700), the
Discipline Leaders Climbing Project of Ruijin Hospital and the
Medical Engineering (Science) Cross micro-PET Special Foundation of
Shanghai Jiaotong University (no. YG08PETZD01), national leading
clinical discipline project.
References
|
1
|
Hess DC and Borlongan CV: Stem cells and
neurological diseases. Cell Prolif. 41(Suppl 1): 94–114. 2008.
View Article : Google Scholar
|
|
2
|
Jitschin R, Mougiakakos D, Von Bahr L, et
al: Alterations in the cellular immune compartment of patients
treated with third-party mesenchymal stromal cells following
allogeneic hematopoietic stem cell transplantation. Stem Cells.
31:1715–1725. 2013. View Article : Google Scholar
|
|
3
|
Wang CH, Cherng WJ, Yang NI, et al:
Late-outgrowth endothelial cells attenuate intimal hyperplasia
contributed by mesenchymal stem cells after vascular injury.
Arterioscler Thromb Vasc Biol. 28:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shichinohe H, Kuroda S, Maruichi K, et al:
Bone marrow stromal cells and bone marrow-derived mononuclear
cells: which are suitable as cell source of transplantation for
mice infarct brain? Neuropathology. 30:113–122. 2010.PubMed/NCBI
|
|
5
|
Zou D, Zhang Z, Ye D, et al: Repair of
critical-sized rat calvarial defects using genetically engineered
bone marrow-derived mesenchymal stem cells overexpressing
hypoxia-inducible factor-1α. Stem Cells. 29:1380–1390.
2011.PubMed/NCBI
|
|
6
|
Liu N, Patzak A and Zhang J:
CXCR4-overexpressing bone marrow-derived mesenchymal stem cells
improve repair of acute kidney injury. Am J Physiol Renal Physiol.
305:F1064–F1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chi NH, Yang MC, Chung TW, Chen JY, Chou
NK and Wang SS: Cardiac repair achieved by bone marrow mesenchymal
stem cells/silk fibroin/hyaluronic acid patches in a rat of
myocardial infarction model. Biomaterials. 33:5541–5551. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang B, Qian J, Ma J, et al: Myocardial
transfection of hypoxia-inducible factor-1alpha and
co-transplantation of mesenchymal stem cells enhance cardiac repair
in rats with experimental myocardial infarction. Stem Cell Res
Ther. 5:222014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi M, Li TS, Suzuki R, et al:
Cytokines produced by bone marrow cells can contribute to
functional improvement of the infarcted heart by protecting
cardiomyocytes from ischemic injury. Am J Physiol Heart Circ
Physiol. 291:H886–H893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Liu XC, Zhang GW, et al: A new
transmyocardial degradable stent combined with growth factor,
heparin, and stem cells in acute myocardial infarction. Cardiovasc
Res. 84:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barbash IM, Chouraqui P, Baron J, et al:
Systemic delivery of bone marrow-derived mesenchymal stem cells to
the infarcted myocardium: feasibility, cell migration, and body
distribution. Circulation. 108:863–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grajek S, Popiel M, Gil L, et al:
Influence of bone marrow stem cells on left ventricle perfusion and
ejection fraction in patients with acute myocardial infarction of
anterior wall: randomized clinical trial: Impact of bone marrow
stem cell intracoronary infusion on improvement of
microcirculation. Eur Heart J. 31:691–702. 2010.
|
|
13
|
Hoehn M, Küstermann E, Blunk J, et al:
Monitoring of implanted stem cell migration in vivo: a highly
resolved in vivo magnetic resonance imaging investigation of
experimental stroke in rat. Proc Natl Acad Sci USA. 99:16267–16272.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bulte JW and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Templin C, Zweigerdt R, Schwanke K, et al:
Transplantation and tracking of human-induced pluripotent stem
cells in a pig model of myocardial infarction: assessment of cell
survival, engraftment, and distribution by hybrid single photon
emission computed tomography/computed tomography of sodium iodide
symporter transgene expression. Circulation. 126:430–439. 2012.
|
|
16
|
Dwyer RM, Schatz SM, Bergert ER, et al: A
preclinical large animal model of adenovirus-mediated expression of
the sodium-iodide symporter for radioiodide imaging and therapy of
locally recurrent prostate cancer. Mol Ther. 12:835–841. 2005.
View Article : Google Scholar
|
|
17
|
Pan Y, Liu S, Wu H, Lv J, Xu X and Zhang
Y: Baculovirus as an ideal radionuclide reporter gene vector: a new
strategy for monitoring the fate of human stem cells in vivo. PLoS
One. 8:e613052013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grünwald GK, Vetter A, Klutz K, et al:
Systemic image-guided liver cancer radiovirotherapy using
dendrimer-coated adenovirus encoding the sodium iodide symporter as
theranostic gene. J Nucl Med. 54:1450–1457. 2013.
|
|
19
|
Li W, Ma N, Ong LL, et al: Bcl-2
engineered MSCs inhibited apoptosis and improved heart function.
Stem Cells. 25:2118–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo R, Zhang R, Pan Y, et al: Feasibility
of a novel positive feedback effect of 131I-promoted Bac-Egr1-hNIS
expression in malignant glioma through baculovirus: a comparative
study with Bac-CMV-hNIS. Nucl Med Commun. 32:402–409. 2011.
View Article : Google Scholar
|
|
21
|
da Meirelles LS and Nardi NB: Murine
marrow-derived mesenchymal stem cell: isolation, in vitro
expansion, and characterization. Br J Haematol. 123:702–711.
2003.
|
|
22
|
Rombouts WJ and Ploemacher RE: Primary
murine MSC show highly efficient homing to the bone marrow but lose
homing ability following culture. Leukemia. 17:160–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss SJ, Philp NJ and Grollman EF: Iodide
transport in a continuous line of cultured cells from rat thyroid.
Endocrinology. 114:1090–1098. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Libby P and Ridker PM: Novel inflammatory
markers of coronary risk: theory versus practice. Circulation.
100:1148–1150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang SN, Sun AJ, Ge JB, et al:
Intracoronary autologous bone marrow stem cells transfer for
patients with acute myocardial infarction: a meta-analysis of
randomised controlled trials. Int J Cardiol. 136:178–185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reinecke H and Murry CE: Cell grafting for
cardiac repair. Methods Mol Biol. 219:97–112. 2003.PubMed/NCBI
|
|
28
|
Cheng Z, Ou L, Zhou X, et al: Targeted
migration of mesenchymal stem cells modified with CXCR4 gene to
infarcted myocardium improves cardiac performance. Mol Ther.
16:571–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XH, Bai CG, Xu ZY, et al: Therapeutic
potential of angiogenin modified mesenchymal stem cells: angiogenin
improves mesenchymal stem cells survival under hypoxia and enhances
vasculogenesis in myocardial infarction. Microvasc Res. 76:23–30.
2008. View Article : Google Scholar
|
|
30
|
Kim SH, Moon HH, Kim HA, Hwang KC, Lee M
and Choi D: Hypoxia-inducible vascular endothelial growth
factor-engineered mesenchymal stem cells prevent myocardial
ischemic injury. Mol Ther. 19:741–750. 2011. View Article : Google Scholar
|
|
31
|
Zeng B, Lin G, Ren X, Zhang Y and Chen H:
Over-expression of HO-1 on mesenchymal stem cells promotes
angiogenesis and improves myocardial function in infarcted
myocardium. J Biomed Sci. 17:802010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo R, Ma Y, Zhang R, et al: Rhenium-188
labeled recombinant human plasminogen kringle5 (rhk5) and
preliminary biodistribution. Evaluation in mice bearing A549
tumours. Nuklearmedizin. 50:234–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Acton PD and Zhou R: Imaging reporter
genes for cell tracking with PET and SPECT. Q J Nucl Med Mol
Imaging. 49:349–360. 2005.PubMed/NCBI
|
|
34
|
Selkirk SM: Gene therapy in clinical
medicine. Postgrad Med J. 80:560–570. 2004. View Article : Google Scholar
|
|
35
|
Yee JK and Zaia JA: Prospects for gene
therapy using HIV-based vectors. Somat Cell Mol Genet. 26:159–174.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nicola JP, Basquin C, Portulano C,
Reyna-Neyra A, Paroder M and Carrasco N: The Na+/I- symporter
mediates active iodide uptake in the intestine. Am J Physiol Cell
Physiol. 296:C654–C662. 2009. View Article : Google Scholar : PubMed/NCBI
|