Introduction
Ultrasound-mediated microbubble fragmentation
technology, which uses high-intensity ultrasound exposure to cause
‘sonoporation’ in cells and results in the uptake of genes, has
become a relatively safe and promising method of gene delivery in
recent years. Studies on ultrasound-targeted microbubble
destruction (UTMD)-mediated angiogenic gene delivery for the
treatment of ischemic heart disease have attracted much attention
(1,2). Fujii et al (3) demonstrated that repeated exposure to
UTMD promoted angiogenesis in the infarcted rat heart without
causing cardiac injury. Yuan et al (4) found that the direct intramyocardial
injection (IMI) of the hepatocyte growth factor (HGF) gene in
conjunction with microbubbles enhanced angiogenesis by
approximately 10.7-fold in dogs with myocardial infarction.
However, at present, this gene transfection technique has failed to
obtain satisfactory results in pre-clinical or clinical studies
when the gene was administrated intravenously, but not by direct
IMI (5–7). This low efficacy may be caused by
limitations of the technique or the wide distribution of
lipid-shelled microbubbles in the body (8,9).
As a result, the concentration and population of microbubbles in
the area of interest is not high enough to achieve biological
effects. Therefore, the enhancement of the microbubble population
or the density at the target site is essential in order to improve
the efficacy of UTMD via intravenous administration. In a previous
study, Browning et al (9)
found that the efficacy of ultrasound-mediated gene transfection
and the contrast agent, SonoVue, improved 3-fold by using larger
gauge needles to infuse more bubbles in rats, which indicated that
along with the increase in the number of microbubbles, the
biological effects increased as the bioeffects of cavitation were
thought to be the main mechanism of transfection (9). Their study focused on the total
number of microbubbles infused in vivo into the circulation
in animals. However, the suitable needle size may vary from large
to small animals, and may not vary that greatly between humans.
Thus, we hypothesized that the enhancement of the local microbubble
population at the site of interest rather than the greater number
of total microbubbles infused into the circulation would also
improve the efficacy of ultrasound-mediated gene transfection.
Currently, the targeted delivery technique, which
may enhance the microbubble population and density in the target
organ mainly involves 3 aspects: i) ultrasound-exposure mediated
microbubble destruction; ii) microbubbles loaded with a
tissue-specific ligand for the area of interest; iii) the
encapsulation of a gene or drug into the microbubbles and releasing
them by ultrasound triggering into the target tissue (10). In this study, we combined a
tissue-specific ligand with microbubbles in an aim to enhance the
local microbubble population in the infarcted myocardium, and
applied ultrasound irradiation for controlled gene release with
high efficacy.
It has been demonstrated that impaired endothelial
cells in the ischemic region overexpress intercellular adhesion
molecules (ICAMs), mainly ICAM-1 (11). Therefore, in this study, ICAM-1
was selected as a ligand to strengthen the targeting ability of
microbubbles in the infarcted myocardium. The therapeutic gene
introduced was angiopoietin-1 (Ang-1) gene, as its expression
product is a protein molecule which plays an important role in the
process of angiogenesis, and its effects are more long-term than
those of vascular endothelial growth factor (VEGF) (12). The Ang-1 gene also inhibits
endothelial cell apoptosis, promotes vessel matuarion, maintains
the stability of blood vessels and antagonizes the vascular
permeability caused by endothelial growth factors, ultimately
attenuating ventricular remodeling and cardiac dysfunction due to
the lack of myocardial cells (13,14). Based on these data, in this study,
we aimed to construct a microbubble loaded with anti-ICAM-1
monoclonal antibody as a Ang-1 gene carrier, and to determine the
ability of the targeted microbubbles to improve the efficacy of
therapeutic angiogenesis via ultrasound exposure in the infarcted
myocardium in comparison to common non-targeted microbubbles.
Materials and methods
Preparation and appraisal of
ICAM-1-targeted microbubbles
Our study was carried out at the Medicine and
Virology Laboratory of the College of Life Science in Wuhan
University (Wuhan, China). SonoVue microbubbles were diluted with
normal saline as per the manufacturer’s instructions (Bracco,
Milan, Italy). The mouse anti-human ICAM-1 antibody (jm-6140R;
Jingmei Biology Co., Shenzhen, China) marked by fluorescein
isothiocyanate (FITC; Sigma, St. Louis, MO, USA) was mixed with
phosphate-buffered saline (PBS) at a 1:50 volume ratio. Microbubble
suspension solution was mixed with the antibody at a 2:1 volume
ratio and incubated for 2 h at 4°C. Afterwards, the unbounded free
antibody was rinsed off with PBS solution and the upper foam was
re-suspended.
It was considered that the FITC-labeled ICAM-1
antibody had successfully incorporated into the SonoVue
microbubbles, as indicated by bright green fluorescence at the
fringe of the microbubble surface under a fluorescence microscope
(Olympus, Tokyo, Japan) during the experiment. The efficacy of the
microbubble and antibody combination was determined using a flow
cytometer (Beckman Coulter, Brea, CA, USA).
Determination of the targeting ability of
ICAM-1-targeted microbubbles for inflammatory endothelial
cells
Human vascular endothelial ECV304 cells (China
Center for Type Culture Collection, Wuhan, China) with or without
human interleukin-1β (IL-1β) stimulation were divided into 5 groups
as follows:
Group 1: IL-1β stimulation +
ICAM-1-targeted microbubbles (n=5)
The ECV304 cells were labeled with
3,3′-dioctadecyloxacarbocyanine perchlorate (DiO; Molecular Probes,
Sigma) and added to recombinant IL-1β solution (PeproTech, Rocky
Hill, NJ, USA). The final concentration of the IL-1β solution was
100 U/ml, as previously described (15). The ECV304 cells were stimulated
for 5 h in order to highly express ICAM-1. The ICAM-1-targeted
microbubbles were added to the IL-1β stimulated ECV304 cells (200
µl/well) and placed in 5% CO2 at 37°C for 30 min
of incubation.
Group 2: No stimulation + ICAM-1-targeted
microbubbles (n=5)
Normal ECV304 cells (not stimulated with IL-1β) were
added to 200 µl of the ICAM-1-targeted microbubbles and
incubated in 5% CO2 at 37°C for 30 min.
Group 3: IL-1β stimulation + common
microbubbles (n=5; negative control)
The IL-1β-stimulated ECV304 cells were added to 200
µl FITC-labeled microbubbles (without anti-ICAM-1 antibody)
and incubated in 5% CO2 at 37°C for 30 min.
Group 4: No stimulation + common
microbubbles (n=5)
Normal ECV304 cells were added to 200 µl
FITC-labeled microbubbles (without anti-ICAM-1 antibody) and
incubated in 5% CO2 at 37°C for 30 min.
Group 5: Blank control (n=5)
IL-1β-stimulated ECV304 cells without any reagent
and incubated in 5% CO2 at 37°C for 30 min.
The cells of each group were exposed to ultrasound
following incubation. The probe was placed under the 6-well plate
at a distance of 3–5 mm, the irradiation frequency ranged was 0.5–2
MHz with a continuous wave (UGT2007 ultrasound irradiation machine;
Ultrasonic Research Institute, Chongqing Medical School, Chongqing,
China). The intensity of ultrasound exposure was set at 1.5
W/cm2 and the time was 30 sec, as previously described
(16,17).
The determination of the adhesion of the
ICAM-1-targeted microbubbles to the ECV304 cells carried out by
measuring the fluorescence intensity under a fluorescence
microscope (IX51; Olympus). The targeting efficiency of the
microbubbles to the ECV304 cells was assessed using a flow
cytometer (Beckman Coulter). Following ultrasound irradiation, cell
viability was determined by 0.4% trypan blue staining (Sigma) and
the cell counting method.
Determination of the in vivo targeting
ability of ICAM-1-targeted microbubbles for the infarcted
myocardium
All experiments in this section were carried out in
accordance with the National Institutes of Health guide for the
care and use of laboratory animals (NIH Publications no. 8023,
revised in 1978). Approval from the Institutional Animal Care and
Use Committee of the Wuhan University Health Science Centre (Wuhan,
China) was also obtained to perform the experiments outlined
below.
Experimental groups
Healthy purebred New Zealand white rabbits (male or
female, weighing 2.5–3.5 kg) were provided by the Wuhan Institute
of Biology (Wuhan, China). In total, 15 rabbits were randomly
divided into 3 groups as follows: i) the acute myocardial
infarction (AMI) group, n=5: the rabbits with AMI received an
injection of 1 ml anti-ICAM-1-targeted microbubbles through the ear
vein and exposed to ultrasound irradiation; ii) the normal control
group (n=5): healthy (normal) rabbits received an injection of 1 ml
anti-ICAM-1-targeted microbubbles through the ear vein and exposed
to ultrasound irradiation; and iii) the blank control group (n=5):
rabbits with AMI did not receive a microbubble injection or were
exposed to ultrasound irradiation for the exclusion of
auto-fluorescence.
Establishment of model of AMI
After being anesthetized with 20% urethane (dose of
5 ml/kg; Sigma) by ear vein injection and an electrocardiogram
(ECG) monitor was connected, the heart was exposed from the left
sternal border while the rabbit was spontaneously breathing, and
the left circumflex branch of the coronary artery was ligated 5 mm
from the left atrial appendage. Following ligation, the contraction
of the regional myocardium was decreased and the infarcted
myocardium turned pale. In this study, we regarded the ST segment
elevation of the ECG (arched upward) of >2 mm as the sign of the
successful establishment of the model of AMI.
Microbubble transfer and ultrasound
exposure
The microbubbles were injected through the ear vein
of the rabbits. The iE33 ultrasound diagnostic platform (Philips
Medical Systems HSG, Andover, MA, USA) and an M3S transducer
(frequency 1–3 MHz) were used for ultrasound exposure. When the
transthoracic echocardiogram was obtained in short axis at the
mid-papillary muscle level, the ultrasound exposure commenced at
the time point of microbubble injection, and was completed when the
intra-myocardium microbubbles had completely vanished. The
mechanical index was set as 1.3 and the overall gain was adjusted
to 100% during the irradiation.
Immunofluorescence
The myocardial tissue of the infarcted region was
cut out and the sections were frozen immediately after microbubble
transfer, and the green fluorescence intensity on the cardiac
muscle vascular intima was observed under a fluorescence microscope
to determine the adherence of the targeted microbubbles to the
injured cardiac vascular intima. Hepatic and kidney tissue was also
taken from the rabbits in order to determine whether any green
fluorescence could be detected.
Ang-1 gene transfection into the ischemic
myocardium by UTMD using ICAM-1-targeted microbubbles
Experimental groups
In total, 30 rabbits with AMI were randomly divided
into 4 groups and were subjected to Ang-1 gene therapy by UTMD: i)
the ICAM-1-targeted microbubble group (TMB, n=8): the rabbits
received an injection of the mixture of microbubble carrying ICAM-1
antibody and Ang-1 gene suspension under ultrasound exposure; ii)
the non-TMB group (n=8): the rabbits only received an injection of
microbubbles carrying the Ang-1 gene under ultrasound exposure;
iii) the IMI group (n=8): the rabbits recevied a direct IMI of the
Ang-1 gene plasmid under ultrasound exposure; and iv) the control
group (n=6): the rabbits only received an injection of the Ang-1
gene plasmid intravenously under ultrasound exposure.
Gene transfection by UTMD
The Ang-1 gene plasmid was constructed by ligating
the Ang-1 gene into the pcDNA3.1 vector with a cytomegalovirus
promoter to drive Ang-1 expression. The SonoVue microbubble
suspension was prepared as as described above. The suspension was
mixed with 100 µg of the Ang-1 plasmid at room temperature
for 15 min, and was oscillated several times to ensure the
sufficient contact of the plasmids and microbubbles. The rabbits
were injected with 1 ml of the plasmid-microbubble solution (the
gene concentration was 100 µg/ml) through the ear vein, or
were directly injected with 100 µg of the plasmid at 5
points evenly distributed around the infarcted myocardium according
to their grouping. Subsequently, ultrasound irradiation was applied
for gene transfection.
The iE33 ultrasound diagnostic system and an M3S
transducer were used for ultrasound exposure. The ‘contrast’
procedure was selected and the second harmonic mode was switched
on. The probe emission frequency and the receiving frequency were
1.7 and 3.4 MHz, respectively, and the frame rate was 88 Hz.
ECG-triggering was performed for every 4–8 cardiac cycles and the
depth was set at 5 cm. Once the microbubble infusion commenced, the
ultrasound beam was continuously delivered from the chest wall
towards the heart of the rabbit. When the contrast agent was evenly
distributed in the myocardium, the ultrasound blasting function was
activated to explode the microbubbles. Ultrasound exposure
persisted for 5 min with a mechanical index of 1.3 and 100% overall
gain.
Echocardiography
A regular echocardiography examination was performed
in all the groups on day 2 after AMI and 2 weeks following gene
transfection. The regional wall motion was assessed, the left
ventricular end-diastolic dimension (LVEDD), left ventricular
ejection fraction (LVEF) and the infarct wall thickness of the left
ventricular anterior wall (LVAW) were measured. The ΔEF was defined
as [(LVEF post-treatment) - (LVEF pre-treatment)] and used to
assess the changes in left ventricular cardiac function.
Myocardial perfusion
Myocardial contrast echocardiography (MCE) was
performed on day 2 after AMI and 14 days following gene
transfection to assess the infarcted myocardial perfusion which was
described as uniform filling, partial filling and no filling
(perfusion defect), as previously described (18). The MCE parameters were the same as
those of the ‘contrast’ procedure mentioned above. The short axis
view at the mid-papillary muscle level was obtained to observe the
contrast agent filling and washout duration. All the acquired
images were transferred to the workstation for off-line analysis
frame by frame. The digital images were analyzed for the evaluation
of the myocardial blood volume by calculating the contrast agent
signal intensity ratio of the anterior wall (infarct area) to the
posterior wall (normal area). The MCE images were analyzed using
QLAB 6.0 software (Philips Medical Systems HSG).
Immunohistochemistry
Microvascular density (MVD; Factor VIII-positive
structures) observed under a light microscope (BX51; Olympus) at
x400 magnification was quantified immunohistochemically in the
tissue sections of the infarcted myocardium which were obtained 2
weeks following gene transfection according the criteria of Weidner
et al (19). Five visual
fields with the highest number of capillaries were calculated to
obtain the mean value. The left ventricle of the animal hearts was
removed and stained with 3% Evans blue. The blue-stained area
(non-ischemic) and the unstained area (ischemic) were separated and
both were weighed. The ratio of the infarct area was expressed as
the ratio of the weight of the infarcted myocardium/total
myocardial weight for evaluating the infarct size.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Semi-quantitative RT-PCR (Thermo Scientific,
Waltham, MA, USA) for the measuremnt of exogenous Ang-1 gene
expression in the myocardium in all the groups was performed 2
weeks following transfection to determine the gene transfection
efficiency. The Ang-1 primers were as follows:
5′-TGCCATTACCAGTCAGAGG-3′ (forward) and 5′-CAAGCATCAAACCACCATC-3′
(reverse); the PCR products were electrophoresed on a 1% agarose
gel and stained with ethidium bromide. The mRNA expression levels
of exogenous Ang-1 were normalized to the mRNA levels of
β-actin.
Western blot analysis
Western blot analysis was used to the determine the
Ang-1 protein expression level in the infarcted myocardium (which
was normalized to the protein levels of β-actin) 2 weeks following
gene transfection. Protein samples were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The
separated proteins were transferred onto polyvinylidene fluoride
membranes and incubated with goat anti-Ang-1 antibody (ab133425;
Abcam, Cambridge, UK) at 4°C overnight. The membranes were blocked
with 5% non-fat milk and incubated with horseradish
peroxidase-coupled mouse anti-goat IgG secondary antibodies
(ab6789; Abcam) for 1 h at room temperature. The membranes were
washed and exposed to X-ray film to detect the expression
bands.
Statistical analysis
Variables were normally distributed and presented as
the means ± standard deviation (SD). Differences between the data
before and after gene transfection were analyzed using the
Student’s t-tests. Comparisons among multiple stages were made
using one-way ANOVA. All statistical tests were two-sided, and a
p-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software version 19.0 (IBM SPSS, Chicago, IL, USA).
Results
Construction and appraisal of
ICAM-1-targeted microbubbles
As observed under an inverted microscope, the
microbubbles were unevenly distributed and were partially
aggregated in clusters (Fig. 1A).
Following repeated and gently pipetting, the clustered microbubbles
became dispersed again. Bright green fluorescence was detected at
the fringe of the microbubbles under a fluorescence microscope
(Fig. 1B), which demonstrated
that the FITC-labeled ICAM-1 antibody bound to the surface of the
microbubbles successfully. The flow cytometryshowed that the
percentage of microbubbles which expressed green fluorescence in
the mixed suspension was 52.3±5.3%.
Targeting ability of the
anti-ICAM-1-targeted microbubbles to inflammatory endothelial cells
in vitro
The fringe of the ECV304 cells expressed bright
green fluorescence in the IL-1β-stimulated group (Fig. 2A). However, only slight green
fluorescence was observed in the cells not stimulated with IL-1β;
this fluorescence intenstiy was much less than that of the
IL-1β-stimulated group (Fig. 2B).
In the blank control, no green fluorescence was observed which
excluded the influence of auto-fluorescence. In group 3, no green
fluorescence was observed which indicated that the common
microbubbles could not selectively adhere to the membrane of the
IL-1β-stimulated cells despite the high expression of ICAM-1. The
results indicated that the anti-ICAM-1-targeted microbubbles
aggregated and adhered to the ECV304 cells which highly expressed
ICAM-1. On the contrary, the anti-ICAM-1 targeted microbubbles did
not adhere and aggregate to the ECV304 cells which did not express
ICAM-1.
The combination efficiency of the ICAM-1-targeted
microbubbles and the IL-1β-stimulated ECV304 cells was 86.7±5.6%,
which was markedly higher than that of the microbubbles with the
cells not stimulated with IL-1β (2.3±0.9%, p<0.01). The cell
viability was 81.76±0.75, 83.04±1.05, 83.14±1.27, 82.54±1.32 and
93.06±0.94% in all 5 groups (groups 1–5, respectively). The
viability of the cells in the blank control was higher than that of
the cells in the other groups (p<0.01), while no significant
differences were observed among the other 4 groups (p>0.05).
Targeting ability of ICAM-1-targeted
microbubbles for the infarcted myocardium in vivo
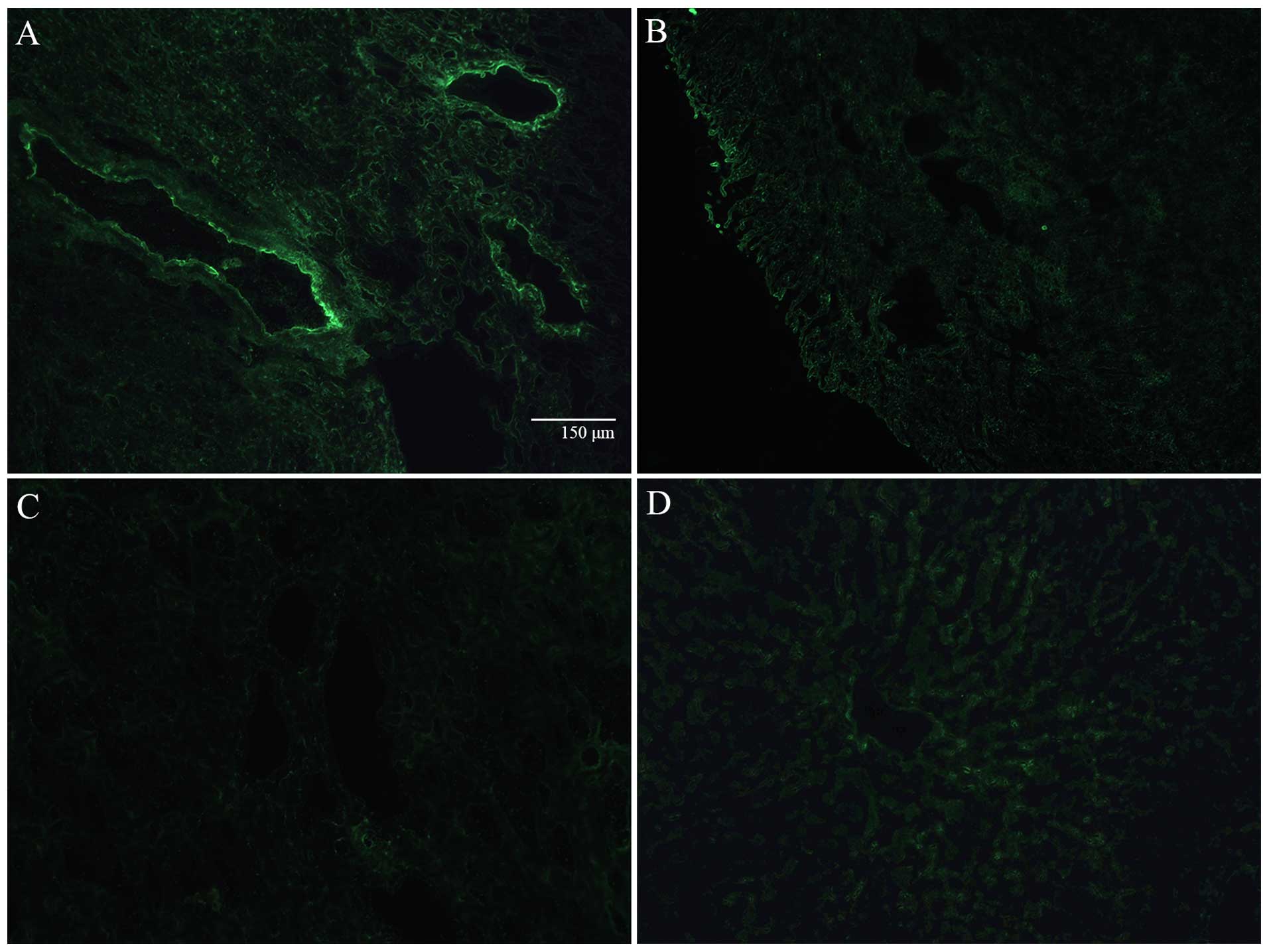
In the frozen sections, luminous green fluorescence
was observed in the injured myocardial vascular endothelium of the
infarcted myocardium in the experiment groups under a fluorescence
microscope, which indicated that a large number of ICAM-targeted
microbubbles was released and had adhered to the injured vascular
endothelium (Fig. 3A). Only
slight green fluorescence was observed in the normal myocardial
vascular endothelium in the control group, which indicated that few
ICAM-1-targeted microbubbles were released and had adhered at the
normal vascular endothelium (Fig.
3B). Green fluorescence was not observed in the blank control
group (Fig. 3C), which excluded
the influence of auto-fluorescence of the infarcted myocardium. No
green fluorescence was observed in the hepatic tissue (Fig. 3D).
Ang-1 gene transfection into the ischemic
myocardium by UTMD using ICAM-1-targeted microbubbles
After the model of AMI was successfully established,
the regional wall motion was significantly reduced and the LVEF was
decreased. At 2 weeks following gene transfection, compared to the
baseline levels, LVEDD was decreased, while the LVEF and the
infarct wall thickness were increased with varying degrees in all
the animals apart from the controls. Furthermore, the LVEDD, LVEF
and the infarct wall thickness in the TMB and IMI group were better
than those of non-TMB group (Table
I).
 | Table IEvaluation of echocardiography before
and after gene transfection (mean ± SD). |
Table I
Evaluation of echocardiography before
and after gene transfection (mean ± SD).
| Group | Control | Non-TMB | TMB | IMI |
|---|
| n | 6 | 8 | 8 | 8 |
| LVEF
(baseline) | 0.50±0.05 | 0.51±0.04 | 0.52±0.05 | 0.50±0.02 |
| LVEF (post) | 0.51±0.04 | 0.67±0.05a | 0.72±0.03a,b | 0.72±0.05a,b |
| ΔEF | 0.01±0.01 | 0.16±0.05a | 0.20±0.05a,b | 0.22±0.05a,b |
| LVEDD (baseline)
(mm) | 1.76±0.17 | 1.57±0.18 | 1.61±0.18 | 1.59±0.16 |
| LVEDD (post)
(mm) | 1.73±0.19 | 1.39±0.16a | 1.34±0.20a,b | 1.28±0.19a,b |
| Δdimension
(mm) | 0.03±0.03 | 0.18±0.10a | 0.26±0.06a,b | 0.31±0.71a,b |
| LVAW (baseline)
(mm) | 1.82±0.15 | 1.91±0.16 | 1.96±0.20 | 2.09±0.24 |
| LVAW (post)
(mm) | 1.82±0.08 | 2.09±0.12a | 2.40±0.16a,b | 2.43±0.20a,b |
| ΔLVAW (mm) | 0.00±0.09 | 0.18±0.07a | 0.44±0.16a,b | 0.34±0.09a,b |
| p-value for
LVEF | 0.199 | 0.000 | 0.000 | 0.000 |
| p-value for
LVEDD | 0.12 | 0.02 | 0.000 | 0.000 |
| p-value for
LVAW | 1.000 | 0.000 | 0.000 | 0.000 |
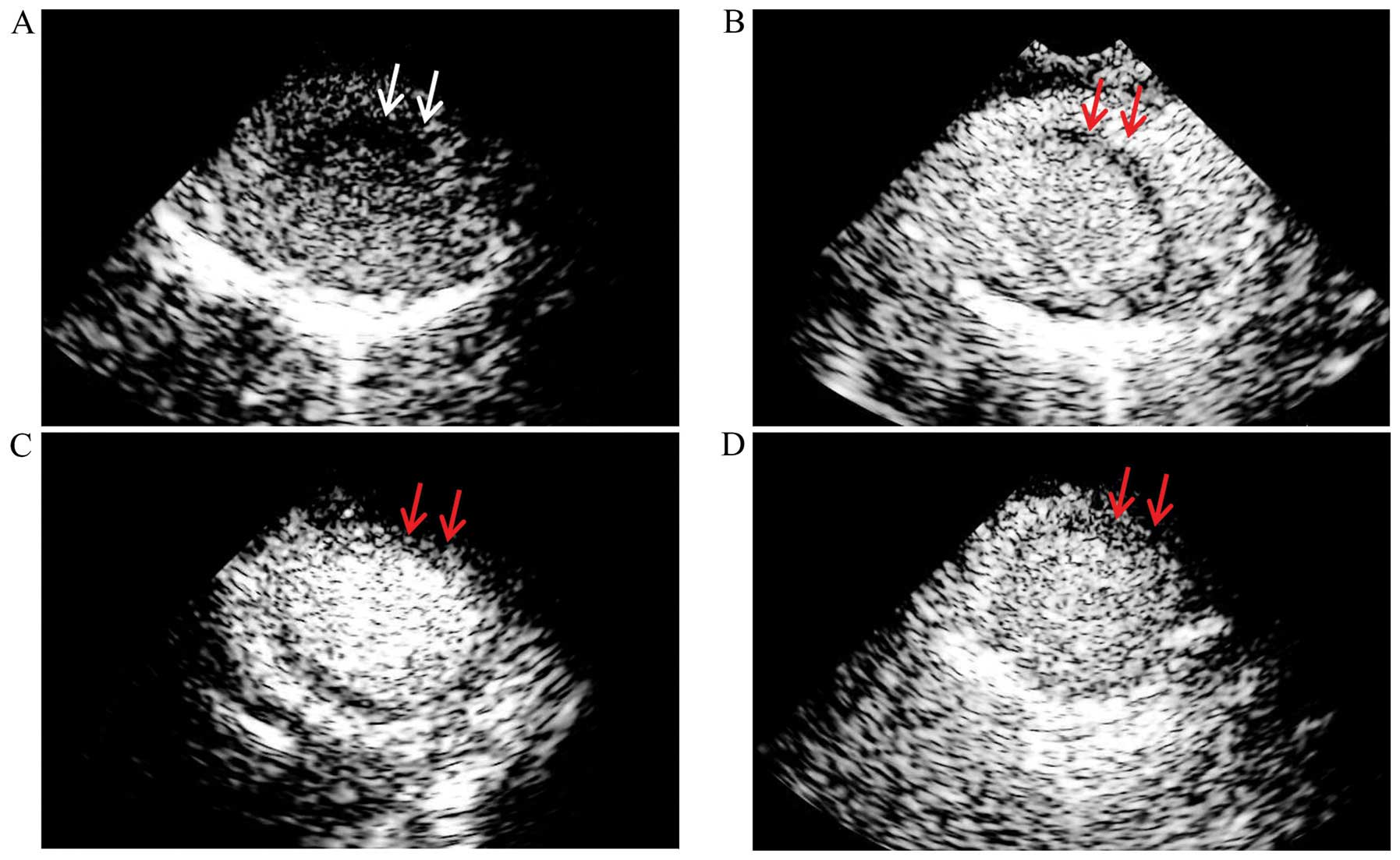
MCE indicated that the contrast agent was obviously
defective in the infarcted myocardium, while the filling of the
other regions was satisfactory in the rabbits with AMI. At 2 weeks
following transfection, the infarcted myocardium in the TMB and IMI
group was partially filled as shown by MCE, while the infarct area
of the animals in the non-TMB group was filled with less contrast
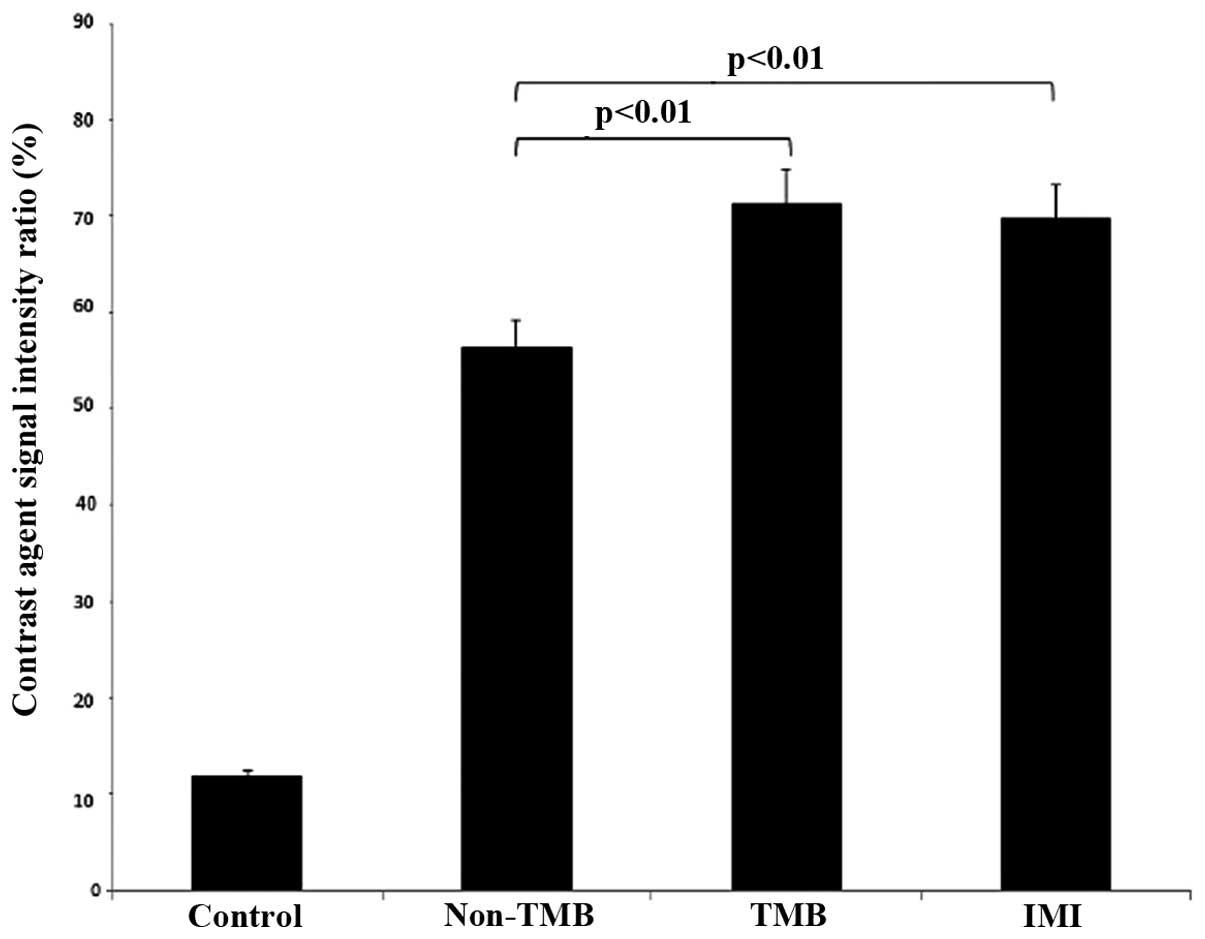
agent (Fig. 4). The contrast
agent signal intensity ratio of the anterior wall to the posterior
wall in the TMB and IMI group was 0.71±0.04 and 0.70±0.08,
respectively, which was significantly better than that of the
non-TMB group (0.56±0.08, p<0.01) and the control group
(0.12±0.02, p<0.01) (Fig.
5).
RT-PCR indicated that the relative expression of the
Ang-1 gene in the myocardium was 0.48±0.03, 0.81±0.06 and 0.82±0.03
in the non-TMB, TMB and IMI group, respectively. The expression
level in the non-TMB group was lower than that in the other 2
groups (p<0.01), and the expression level between the TMB and
IMI group did not differ significantly (p=0.72). No obvious mRNA
expression of Ang-1 was detected in the controls (Figs. 6 and 7).
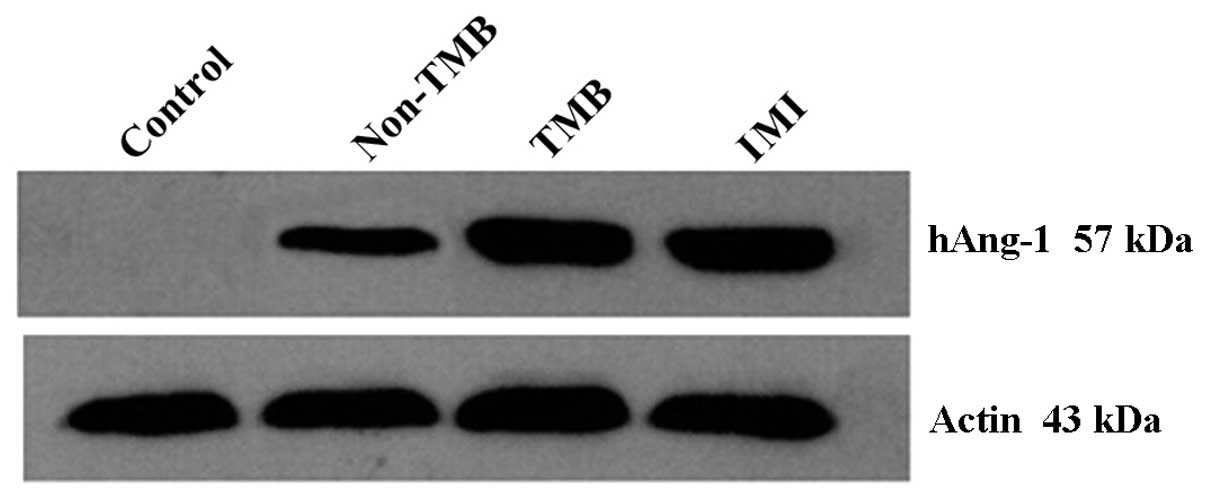
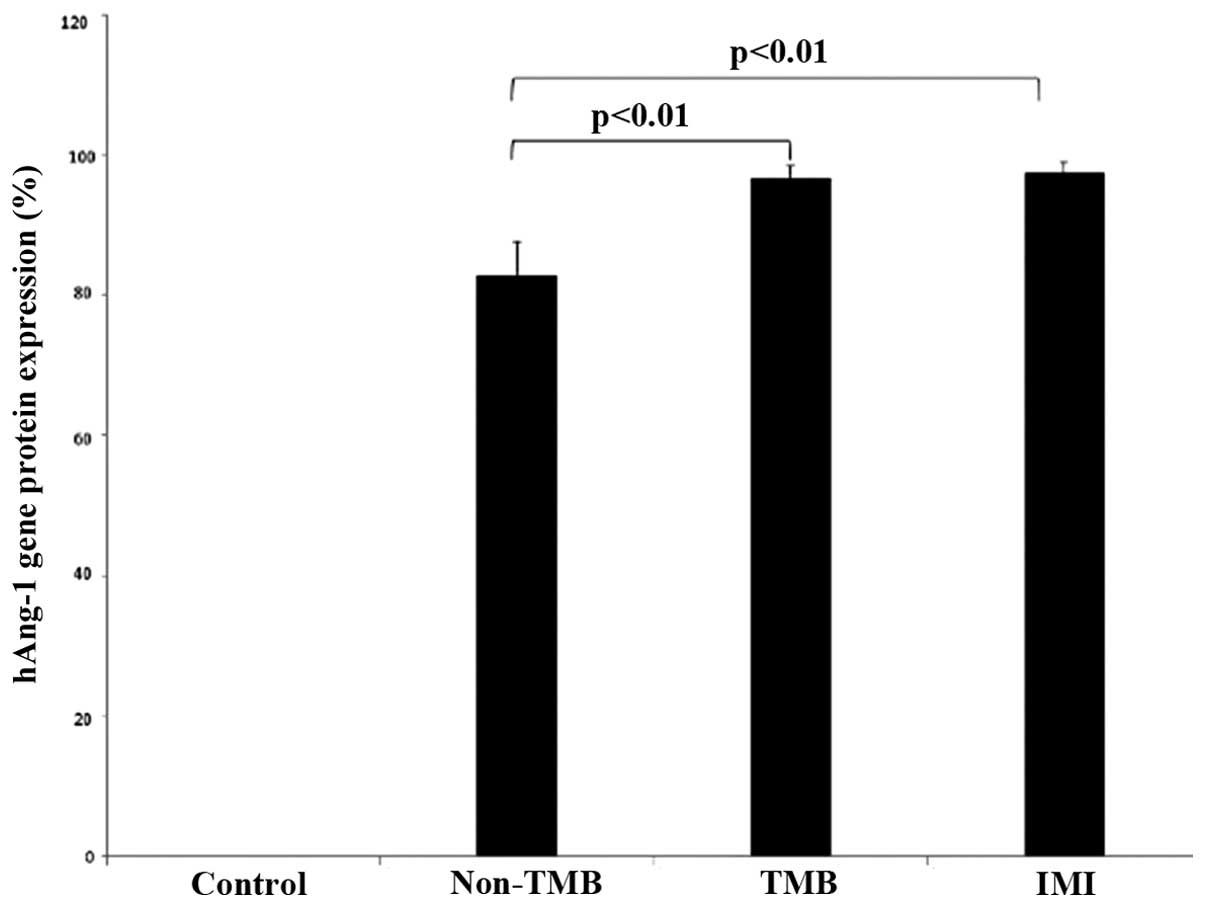
Ang-1 protein was detected in all the experimental
animals apart from the controls by western blot analysis. The
protein expression levels in the infarct area of the animals in the
non-TMB, TMB and IMI group were 0.83±0.05, 0.96±0.02 and 0.97±0.02,
respectively. The expression level in the TMB and IMI group was
significantly higher than that in the non-TMB group (p<0.01);
however, no statistically significant differences were observed in
the expression level between the first 2 groups (p=0.83). N obvious
protein expression was detected in the control group (Figs. 8 and 9).
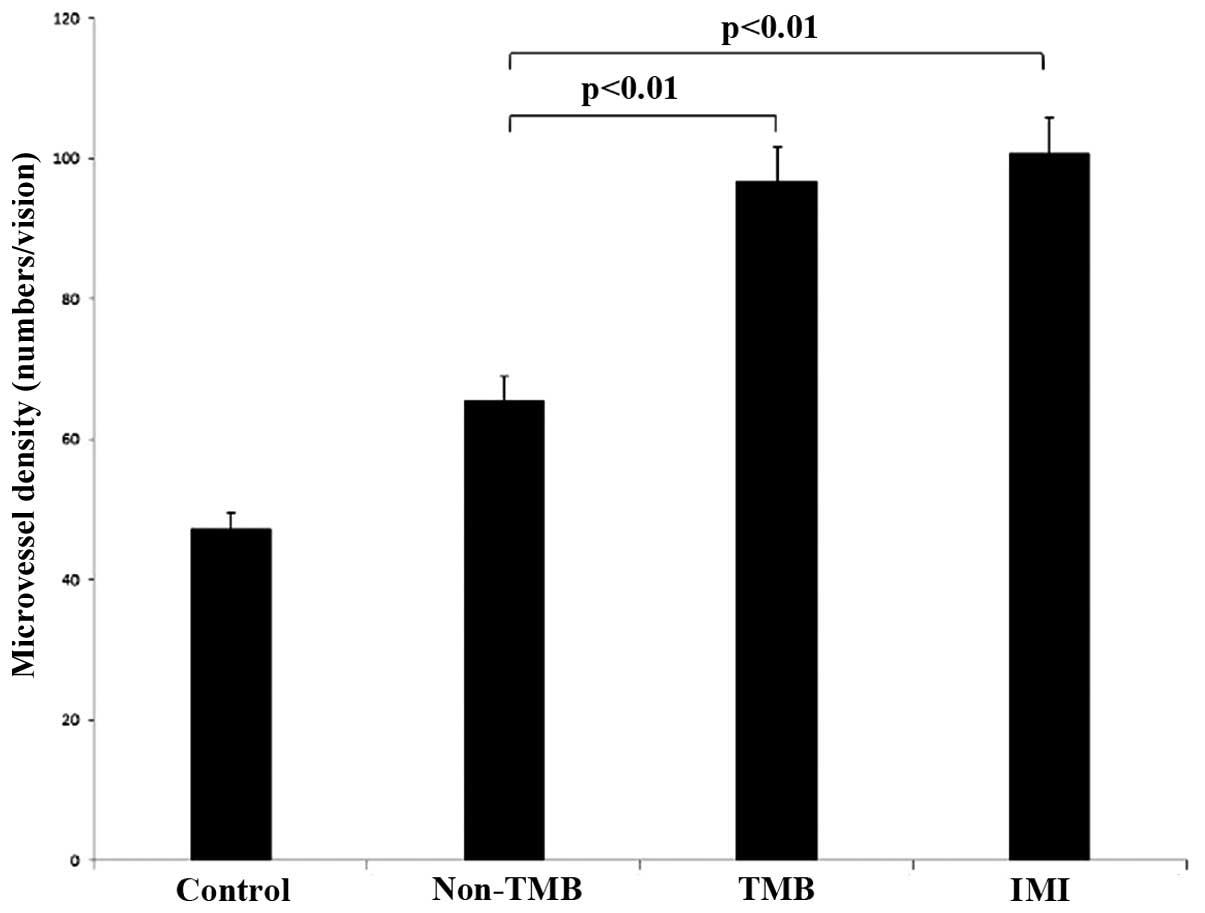
A greater number of factor VIII-positive structures
was observed in the infarct area following gene transfection, apart
from the control group. The MVD of the infarcted myocardium in the
control group at 2 weeks after the induction of AMI was 51.07±4.83,
which was lower than that of the other 3 groups (p<0.01). The
MVD of the TMB group was (96.7±2.1) which was higher than that of
the non-TMB group (65.6±4.4, p<0.01), but lower than that in the
IMI group (100.7±3.6, p=0.028) (Figs. 10 and 11).
Discussion
There is a high incidence and poor long-term outcome
of ischemic heart disease both in developing and developed
countries; thus, the treatment of refractory myocardial ischemia is
of particularly importance. In recent years, gene-based therapeutic
angiogenesis has been proven to be feasible in some pre-clinical
and clinical studies (20).
However, a good biosafety and high efficiency gene delivery system
is still required for future clinical application (21). At present, UTMD, as a novel gene
transfection technique, has shown its potential prosperity with
clinical diagnostic ultrasound exposure within a recommended safety
range. Ultrasound can produce and enhance bioeffects via acoustic
cavitation, while the microbubbles can reduce the threshold of
acoustic cavitation caused by ultrasound irradiation. Therefore,
the combined application of ultrasound with microbubbles may be an
effective and safe method for gene therapy. Although common
microbubbles can deliver a gene to the target tissue by ultrasound
exposure and achieve bioeffects, the transfer efficiency is not
satisfactory as low numbers of microbubbles attach to the organ of
interest, thus resulting in less gene uptake. A high gene
transfection efficiency is considered to be related to a greater
gene quantity and more microbubble destruction in the region of
interest. It is known that naked DNA administered intravenously is
easily degraded by DNase or captured by other cells before it
reaches the target organ; thus increasing the DNA quantity in the
region of interest is critical for improving the effects of gene
therapy. Direct IMI can be used to achieve a high plasmid dose in
the target tissue; however, this method of gene administration is
not suitable for repeated application in clinical treatment.
Therefore, in this study, we aimed to increase the quantity of the
microbubbles in the infarcted myocardium using a target microbubble
as a gene carrier, and as a consequence, greater microbubble
destruction by ultrasound, leading to more gene intake. We
progressively confirmed the feasibility and efficiency of the
transfection of the exogenous angiogenesis gene to the infarcted
myocardium by UTMD using microbubbles carrying anti-ICAM-1
antibody. First, we successfully constructed a
‘microbubble-anti-ICAM-1 monoclonal antibody’ complex by
electrostatic attraction as a Ang-1 gene carrier and then found
that this microbubble had better adhesion to inflammatory
endothelial cells in vitro and the infarcted myocardium
in vivo as compared with the non-targeted microbubbles.
Moreover, we proved that the ICAM-1-targeted microbubbles are a
more effective and feasible gene carrier for myocardial infarction
gene therapy than common microbubbles, as their use increases the
chance of the release of more bubbles to the area of interest and
exerts better bioeffects, as determined by the standard of the
effects of the direct IMI which is recognized as the most efficient
method for exogenous gene transfection.
Previous studies have demonstrated a site
orientation technique by connecting specific ligands to
microbubbles, which gives the microbubbles the ability to recognize
the lesion area of interest (22–25). Leong-Poi et al (26) prepared αV integrin-targeted
microbubbles by connecting a monoclonal antibody to αV integrin on
the surface. Ferrante et al (27) reported a dual-targeted
phospholipid microbubble, which contained fluorocarbon gas
internally and adhesion molecule P-selectin and vascular cell
adhesion molecule-1 on the surface, and proved that microbubbles
may be used for the detection of atherosclerotic plaque. It is
known that ICAM is widely distributed on the surface of many cells,
such as endothelial cells, and its expression level is relatively
low under normal conditions. The expression of ICAM-1 is effected
by various factors, such as IL-1, tumor necrosis factor (TNF),
ischemia and hypoxia (28,29).
The enhancement of ICAM-1 expression is a symbol of endothelial
cell injury and leukocyte activation (30). Once ischemia occurs, multiple
inflammatory factors induce the overexpression of ICAM-1 in
microvascular endothelial cells. Furthermore, in injured
endothelial cells, some inflammatory binding sites, such as the
ICAM-1 spot are observed (31).
Thus, in theory, targeted microbubbles carrying ICAM-1 antibodies
would directionally transport more exogenous gene to the ischemic
myocardium by selective combination with the injured endothelial
cells expressing ICAM-1. To verify this point, we examined the
adhesion ability of the ICAM-1-targeted microbubbles to
IL-1β-stimulated ECV304 cells in vitro and the ischemic
myocardium in rabbits with AMI in vivo. Our results revealed
the feasibility of utilizing ICAM-1 antibody as a tissue-specific
ligand for inflammatory cells, and compared with the non-targeted
microbubbles, although in a complex internal environment, the
targeted microbubbles remained stable, and still showed good
adhesion to the ischemic myocardium.
A limitation of this study was the complex blood
components and the sheer stress caused by blood flow in vivo
which may weaken the intermolecular electrostatic adsorption
capacity of the antibody and microbubbles, or the microbubbles
themselves (32,33), which may reduce the targeting
ability of ICAM-1-targeted microbubbles as compared to the level
in vitro. Another limitation was the time window for the
assessment of the bioeffects on angiogenesis. For better detecting
the expression of the transfected gene, the time window was
selected as 2 weeks following transfection, as previously described
(34), although it was relatively
short for ventricular remodeling. Therefore, the improvement of the
regional function of the infarcted myocardium or heart function
caused by therapeutic angiogenesis may have been more evident if
the observation time was longer in our study.
In conclusion, our data demonstrate that SonoVue
microbubbles carrying anti-ICAM-1 antibody provide a
tissue-specific targeting strategy which leads to the release of a
greatery quantity of microbubbles and higher gene levels into the
infarcted myocardium. UTMD using ICAM-1-targeted microbubbles may
significantly enhance the angiogenic effects of the Ang-1 gene than
common microbubbles, which may become an outlet for the clinical
application of gene therapy in the future.
Acknowledgments
This study was supported by the Chinese National
Science Foundation Committee Grant (no. 81471674) and the Wuhan
University Independent Interdisciplinary Foundation Program (no.
2042014kf 0277).
Abbreviations:
|
UTMD
|
ultrasound-targeted microbubble
destruction
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
References
|
1
|
Phillips LC, Klibanov AL, Wamhoff BR and
Hossack JA: Targeted gene transfection from microbubbles into
vascular smooth muscle cells using focused, ultrasound-mediated
delivery. Ultrasound Med Biol. 36:1470–1480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou J, Wang Y, Xiong Y, Wang H, Feng Y
and Chen J: Delivery of TFPI-2 using ultrasound with a microbubble
agent (SonoVue) inhibits intimal hyperplasia after balloon injury
in a rabbit carotid artery model. Ultrasound Med Biol.
36:1876–1883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujii H, Li SH, Wu J, Miyagi Y, Yau TM,
Rakowski H, Egashira K, Guo J, Weisel RD and Li RK: Repeated and
targeted transfer of angiogenic plasmids into the infarcted rat
heart via ultrasound targeted microbubble destruction enhances
cardiac repair. Eur Heart J. 32:2075–2084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan QY, Huang J, Chu BC, Li XS and Si LY:
A visible, targeted high-efficiency gene delivery and transfection
strategy. BMC Biotechnol. 11:562011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henry TD, Annex BH, McKendall GR, Azrin
MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman
DS, et al VIVA Investigators: The VIVA trial: Vascular endothelial
growth factor in Ischemia for Vascular Angiogenesis. Circulation.
107:1359–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kornowski R, Fuchs S, Leon MB and Epstein
SE: Delivery strategies to achieve therapeutic myocardial
angiogenesis. Circulation. 101:454–458. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kusumanto YH, Mulder NH, Dam WA, Losen M,
De Baets MH, Meijer C and Hospers GA: Improvement of in vivo
transfer of plasmid DNA in muscle: Comparison of electroporation
versus ultrasound. Drug Deliv. 14:273–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki R, Takizawa T, Negishi Y, Hagisawa
K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T and Maruyama K:
Gene delivery by combination of novel liposomal bubbles with
perfluoropropane and ultrasound. J Control Release. 117:130–136.
2007. View Article : Google Scholar
|
|
9
|
Browning RJ, Mulvana H, Tang M, Hajnal JV,
Wells DJ and Eckersley RJ: Influence of needle gauge on in vivo
ultrasound and microbubble-mediated gene transfection. Ultrasound
Med Biol. 37:1531–1537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laing ST and McPherson DD: Cardiovascular
therapeutic uses of targeted ultrasound contrast agents. Cardiovasc
Res. 83:626–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar
|
|
12
|
Siddiqui AJ, Blomberg P, Wärdell E,
Hellgren I, Eskandarpour M, Islam KB and Sylvén C: Combination of
angiopoietin-1 and vascular endothelial growth factor gene therapy
enhances arteriogenesis in the ischemic myocardium. Biochem Biophys
Res Commun. 310:1002–1009. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onda T, Honmou O, Harada K, Houkin K,
Hamada H and Kocsis JD: Therapeutic benefits by human mesenchymal
stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral
ischemia. J Cereb Blood Flow Metab. 28:329–340. 2008. View Article : Google Scholar :
|
|
14
|
Chen JX and Stinnett A: Ang-1 gene therapy
inhibits hypoxia-inducible factor-1alpha
(HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha
expression, and normalizes immature vasculature in db/db mice.
Diabetes. 57:3335–3343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weller GE, Lu E, Csikari MM, Klibanov AL,
Fischer D, Wagner WR and Villanueva FS: Ultrasound imaging of acute
cardiac transplant rejection with microbubbles targeted to
intercellular adhesion molecule-1. Circulation. 108:218–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Zhou Q, Wang YH, Wang X and Guo
RQ: Effects of ultrasound-mediated SonoVue microbubbles destruction
on the integrity and expression of hAng-1 gene. Chin J
Ultrasonography. 18:1080–1084. 2009.
|
|
17
|
Rahim A, Taylor SL, Bush NL, ter Haar GR,
Bamber JC and Porter CD: Physical parameters affecting
ultrasound/microbubble-mediated gene delivery efficiency in vitro.
Ultrasound Med Biol. 32:1269–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dijkmans PA, Senior R, Becher H, Porter
TR, Wei K, Visser CA and Kamp O: Myocardial contrast
echocardiography evolving as a clinically feasible technique for
accurate, rapid, and safe assessment of myocardial perfusion: The
evidence so far. J Am Coll Cardiol. 48:2168–2177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka M, Taketomi K and Yonemitsu Y:
Therapeutic angiogenesis: recent and future prospects of gene
therapy in peripheral artery disease. Curr Gene Ther. 14:300–308.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Villanueva FS: Ultrasound mediated
destruction of DNA-loaded microbubbles for enhancement of
cell-based therapies: New promise amidst a confluence of
uncertainties? JACC Cardiovasc Imaging. 2:880–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaufmann BA, Sanders JM, Davis C, Xie A,
Aldred P, Sarembock IJ and Lindner JR: Molecular imaging of
inflammation in atherosclerosis with targeted ultrasound detection
of vascular cell adhesion molecule-1. Circulation. 116:276–284.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klibanov AL: Ultrasound molecular imaging
with targeted microbubble contrast agents. J Nucl Cardiol.
14:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schumann PA, Christiansen JP, Quigley RM,
McCreery TP, Sweitzer RH, Unger EC, Lindner JR and Matsunaga TO:
Targeted-microbubble binding selectively to GPIIb IIIa receptors of
platelet thrombi. Invest Radiol. 37:587–593. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai W and Chen X: Multimodality molecular
imaging of tumor angiogenesis. J Nucl Med. 49(Suppl 2): 113S–128S.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leong-Poi H, Christiansen J, Klibanov AL,
Kaul S and Lindner JR: Noninvasive assessment of angiogenesis by
ultrasound and microbubbles targeted to alpha(v)-integrins.
Circulation. 107:455–460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrante EA, Pickard JE, Rychak J,
Klibanov A and Ley K: Dual targeting improves microbubble contrast
agent adhesion to VCAM-1 and P-selectin under flow. J Control
Release. 140:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Almenar-Queralt A, Duperray A, Miles LA,
Felez J and Altieri DC: Apical topography and modulation of ICAM-1
expression on activated endothelium. Am J Pathol. 147:1278–1288.
1995.PubMed/NCBI
|
|
29
|
Benson V, McMahon AC and Lowe HC: ICAM-1
in acute myocardial infarction: A potential therapeutic target.
Curr Mol Med. 7:219–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lawson C and Wolf S: ICAM-1 signaling in
endothelial cells. Pharmacol Rep. 61:22–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilhelmi MH, Leyh RG, Wilhelmi M and
Haverich A: Upregulation of endothelial adhesion molecules in
hearts with congestive and ischemic cardiomyopathy:
Immunohistochemical evaluation of inflammatory endothelial cell
activation. Eur J Cardiothorac Surg. 27:122–127. 2005. View Article : Google Scholar
|
|
32
|
Kaufmann BA and Lindner JR: Molecular
imaging with targeted contrast ultrasound. Curr Opin Biotechnol.
18:11–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weller GE, Villanueva FS, Klibanov AL and
Wagner WR: Modulating targeted adhesion of an ultrasound contrast
agent to dysfunctional endothelium. Ann Biomed Eng. 30:1012–1019.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaspar BK, Roth DM, Lai NC, Drumm JD,
Erickson DA, McKirnan MD and Hammond HK: Myocardial gene transfer
and long-term expression following intracoronary delivery of
adeno-associated virus. J Gene Med. 7:316–324. 2005. View Article : Google Scholar
|