Introduction
Over the years, the average global lifespan has
increased due to economic development and advancements in medicine,
and there is great interest in skin health and anti-aging
technologies in order to delay the aging process and maintain youth
(1). Studies on anti-wrinkle
agents have been conducted with the aim of maintaining skin health
(2). As a result, various
cosmetics and foods which assist in delaying the aging process have
been developed (3). Aging
involves structural, functional and biochemical changes that occur
throughout cells and bodily tissues; the amount of hormones
secreted from of all human organs, including the skin, decreases
over time (4). Skin aging is
associated with environmental and genetic factors, and is divided
into extrinsic aging caused by various environmental factors, such
as UV rays, polluted air and gravity (5); by contrast, endogenous aging (which
is associated with genetic factors) can also lead to skin wrinkling
(6,7). Wrinkles are a typical symptom of
skin aging caused by the loss of elasticity, which is caused by a
reduction in the amount of collagen associated with the elasticity
of the skin dermal tissue. Collagen (approximately 90% of which is
found in dermal cells) has a close association with skin
elasticity, as it protects the skin against external stimuli and
provides it with tension and strength (3). Procollagen type 1 and the
extracellular matrix are decomposed and the expression of elastin
(related to skin elasticity) is inhibited by matrix
metalloproteinases (MMPs) induced by the transcription factor,
activating protein-1 (AP-1) and MMP-1 and -8, which are known to
degrade collagen. Tissue inhibitors of metalloproteinase-1 (TIMP-1)
exert the opposite effect by inhibiting the activity of MMPs
(8,9). In addition, elastase derived from
fibroblasts plays an important role in the three-dimensional
distortion of skin elasticity fibers, and increased elastase
activity contributes to the acceleration of wrinkle formation by
reducing the number of elastic fibers in the skin (10). UV rays and the production of
reactive oxygen species cause skin aging and damage cells by
attacking the plasma membrane, resulting in loss of function. This
creates a harsh environment and causes skin wrinkles by reducing
the glossy appearance of skin (11,12). Of the factors involved in cell
proliferation, phosphatidylinositol 3-kinase (PI3K) is an important
protein that performs regulatory functions in a number of signaling
pathways and consists of two subunits (p110 and p85) and increases
the activity of Akt, a serine/threonine kinase (13). Serine 473 autophosphorylation
promotes the proliferation and differentiation of cells by
activating sub-molecules and transcription factors (14).
The Akt/PI3K signaling pathway is a signal
transduction pathway that promotes cell growth and survival in
response to extracellular signals. In order to maintain the
elasticity of skin cells, it is important to increase procollagen
type 1 c-peptide (PIP) and elastin activity by increasing TIMP-1
expression and inhibiting that of MMP, and it is also necessary to
activate the PI3K/Akt pathway to directly induce cell growth. In
this study, we confirmed the anti-wrinkle effects of tuna heart
extract, which were mediated through the inhibition of the
expression of MMPs and the increase in PI3K/Akt levels. By-products
of global seafood processing amount to 75% of the total catch, and
these by-products are disposed of together with a considerable
amount of useable ingredients. Omega-3 fatty acid separated through
protein hydrolysate, and concentrates of its by-products, has
previously been used in the food and pharmaceutical industries
(15–17). Studies on immune enhancement and
on unsaturated fats in tuna by-products have previously been
performed (18–20). In this study, we examined the
anti-wrinkle effects of a physiologically active substance isolated
from tuna heart extract on skin by measuring the expression levels
MMPs in skin fibroblasts and determining whether they are directly
involved in the anti-wrinkle effects.
Materials and methods
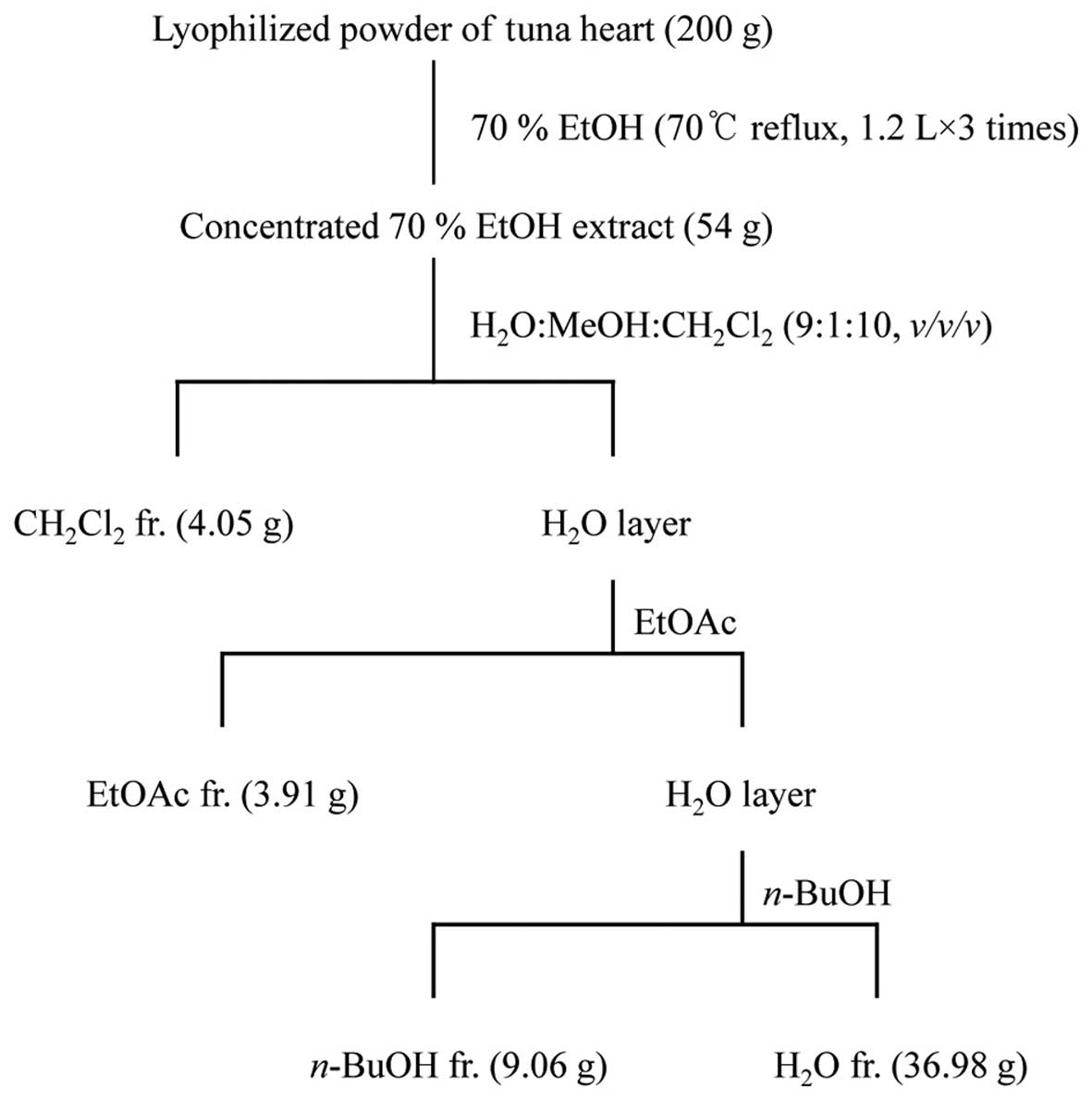
Extraction and fractionation of tuna
heart
Lyophilized powder (200 g) of tuna heart was
refluxed with 70% ethanol (EtOH; 1.2 liters for 3 times) for 3 h,
and each filtrate was concentrated to dryness in vacuo at
40°C to obtain the 70% EtOH extract (54 g). The 70% EtOH extract
was then suspended in distilled water (H2O) and
partitioned in sequence with dichloromethane
(CH2Cl2), ethyl acetate (EtOAc),
n-butanol (n-BuOH) and H2O to yield 4
fractions. The respective yields of the
CH2Cl2 (4.05 g), EtOAc (3.91 g),
n-BuOH (9.06 g) and H2O (36.98 g) fractions were
2.03, 1.96, 4.53 and 18.49%, respectively (Fig. 1). The
CH2Cl2, EtOAc and n-BuOH fraction had
no effect on the viability of Hs27 cells (data not shown). In this
study, we confirm the effects of the H2O fraction on the
viability of Hs27 cells. Thus, we used various concentrations of
the H2O fraction (25, 50 and 100 µg/ml).
Cell culture
Hs27 human skin fibroblasts (American Type Culture
Collection, Manassas, VA, USA) were maintained at 37°C in a
humidified atmosphere with 5% CO2. The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 100
U/ml penicillin/100 mg/ml streptomycin. The Hs27 cells were
cultured to ~60–80% confluence in a 100-mm diameter plate. The
medium was replaced daily.
Cell proliferation assays
Hs27 cell proliferation was measured using a
CellTiter 96® aqueous non-radioactive cell proliferation
assay (Promega, Madison, WI, USA), which is based on the cleavage
of
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium
(MTS) into a formazan product which is soluble in tissue culture
medium. The cells were seeded onto 96-well plates at
2×104 cells/well, and the medium was replaced with
serum-free medium (SFM) following culture for 24 h. After a further
24 h, the medium was replaced with SFM containing tuna heart
(TH)-H2O fraction (25, 50 and 100 µg/ml),
followed by incubation for 24 h. For the assay, MTS solution was
added to the cells in each well and was allowed to react for 30 min
at 37°C. The absorbance at 490 nm of the solution in each well was
measured using a microplate reader (Benchmark microplate reader;
Bio-Rad Laboratories, Hercules, CA, USA).
Determination of elastase activity
To determine the activity of elastase and its
effects on elastic fibers, the decomposition of elastase was
measured. Elastic fibers are bundles of elastin found in the
extracellular matrix of connective tissue and produced by skin
fibroblasts (21). Elastic fiber
includes elastin. The upregulation of elastin is important in order
to prevent skin cell aging (22).
The cells were seeded into 6-well plates at 2×104
cells/well and the medium was replaced with SFM containing the
TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h.
The cells were collected in 100 mM Tris-HCl buffer (pH 7.6) with
0.1% Triton X-100 buffer. The supernatant was then transferred to
the 96-well plate. Subsequently, 2 mM
N-Succinyl-Ala-Ala-Ala-p-nitroanilide was added to each well
(Sigma-Aldrich, St. Louis, MO, USA), adjusted to a 100-µl
volume using elastase enzyme solution, and incubated at 37°C for 30
min. The absorbance at 410 nm was measured using a microplate
reader (Benchmark microplate reader; Bio-Rad Laboratories).
β-galactosidase staining
β-galactosidase staining was performed using the
Takara V2600 kit (Takara Bio Inc., Tokyo, Japan). The cells were
seeded into 6-well plates at 2×104 cells/well and the
medium was replaced with SFM containing the TH-H2O
fraction (25, 50 and 100 µg/ml) for 24 h. Cell fixative
working solution (0.5 ml) was added to each well and incubated at
room temperature for 5 min, after which the working solution was
removed. Each well was then washed twice with 1 ml
phosphate-buffered saline (PBS), and 0.5 ml of cell staining
working solution was added. Cell morphology and staining were
observed using a light microscope (ECLIPSE TS100-F; Nikon, Tokyo,
Japan).
PIP EIA assay
PIP EIA assay was performed using the Takara MK101
kit (Takara Bio Inc.). The cells were seeded into 6-well plates at
2×104 cells/well and the medium was replaced with SFM
containing the TH-H2O fraction (25, 50 and 100
µg/ml) for 24 h. The cell supernatant was then collected by
centrifugation (12,000 rpm, 4°C, 10 min). Antibody-POD conjugate
solution (100 µl) and 20 µl of cell supernatant were
added to each well, followed by incubation at 37°C for 3 h. Each
well was washed 4 times with 400 µl PBS, after which 100
µl of substrate solution (TMBZ,
3,3′,5,5′-tetramethylbenzidine) was added, followed by incubation
at room temperature for 15 min. Finally, 100 µl of stop
solution (1 N H2SO4) were added and the
absorbance at 450 nm was measured using a microplate reader
(Benchmark microplate reader; Bio-Rad Laboratories).
Western blot analysis
The Hs27 cells in 100-mm diameter plates were
cultured to 60% confluence and incubated in SFM containing the
TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h.
The collected cells were washed with PBS, after which extraction
lysis buffer (20 mM Tris-base, pH 8, 150 mM NaCl, 100 µM
sodium vanadate, 100 µM ammonium molybdate, 10% glycerol,
0.1% Nonidet P-40, 0.1% SDS, 1 mM glycerophosphate, 1 µg/ml
aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin A and
1 mM PMSF) was added. The proteins were separated on 7–15% SDS-PAGE
gels and transferred onto polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked at room
temperature with 1% bovine serum albumin in TBS-T (10 mM Tris-HCl,
pH 7.5, 150 mM NaCl, 0.1% Tween-20) and then incubated with the
following primary antibodies: anti-MMP-1 (sc-21731, anti-mouse,
1:1,000), anti-MMP-8 (sc-8848, anti-goat, 1:1,000), anti-elastin
(sc-166543, anti-mouse, 1:1,000), anti-col1A (sc-59772, anti-mouse,
1:1,000), anti-PI3K (sc-7248, anti-goat, 1:1,000), anti-Akt
(sc-8312, anti-rabbit, 1:1,000), anti-TIMP1 (sc-5538, anti-rabbit,
1:1,000), and anti-GAPDH (sc-25778, anti-rabbit, 1:1,000) (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The secondary
antibody was a peroxidase-conjugated goat (sc-2741), mouse
(sc-2032), or rabbit (sc-2031) antibody (1:10,000; Santa Cruz
Biotechnology, Inc.). The signal was developed using SuperSignal
West Pico Stable Peroxide Solution and the SuperSignal West Pico
Luminol/Enhancer solution (Thermo Fisher Scientific, Rockford, IL,
USA) before exposure to Kodak X-ray film.
Reverse transcription-polymerase chain
reaction (RT-PCR) for analysis of mRNA expression
The Hs27 cells in 100-mm diameter plates were
cultured to 60% confluence and incubated in SFM containing the
TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h.
The cells were then treated with TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), after which the extracted RNA was used as a
template for cDNA synthesis using an oligo(dT) primer (Intron Co.,
Gyeonggi, Korea). The synthe sized cDNA was mixed with 2X TOPsimple
DyeMIX-nTaq (Enzynomics Inc., Daejeon, Korea) and primers (MMP-1
forward, AAAGGG AATAAGTACTGGGC and reverse, AATTCCAGGAAAG TCATGTG;
MMP-8 forward, GCTCATTTTGATGCCGAAG and reverse,
AGTAGTTGCTGGTTTCCCT; col1A forward, CTCCAACGAGATCGAGATC and
reverse, GTTACAGGAA GCAGACAGG; TIMP-1 forward, TGGGGACACCAG
AAGTCAAC and reverse, TTTTCAGAGCCTTGGAGGAG; PI3K forward,
AGGAGCGGTACAGCAAAGAA and reverse, GCCGAACACCTTTTTGAGTC; Akt
forward, CAACTTCT CTGTGGCGCAGTG and reverse, GACAGGTGGAAGAA
CAGCTCG; GAPDH forward, AAGGGCTCATGACCAC AGTC and reverse,
GGATGCAGGGATGTTCT) in 0.1% diethylpyrocarbonate (DEPC)-treated
water for PCR. Using a 1% agarose gel, the PCR products were
separated and stained with Red Safe nucleic acid staining solution
(Intron Co.).
Statistical analysis
The results are expressed as the means ± SDs. SPSS
software (v. 10.0; SPSS, Inc., Chicago, IL, USA) was used.
Comparisons were made using ANOVA and Duncan's multiple range test.
A p-value <0.05 was deemed to indicate a statistically
significant difference.
Results
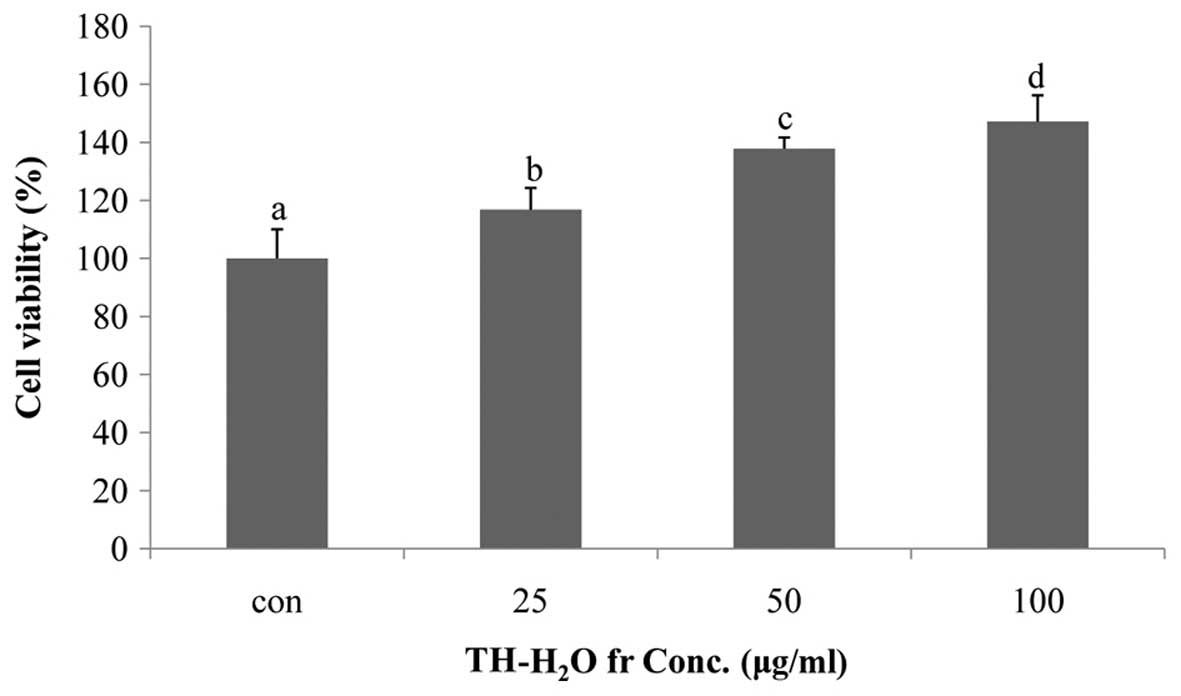
Effects of TH-H2O fraction on
Hs27 cell proliferation
The effect of the TH-H2O fraction on Hs27
cell proliferation was examined by MTS assay. The Hs27 cells
treated with the TH-H2O fraction at 25, 50 and 100
µg/ml exhibited a dose-dependent increase in proliferation,
with a maximum increase of 47% compared to the controls (untreated
cells) being observed in the cells treated with 100 µg/ml of
the TH-H2O fraction. In other words, treatment of Hs27
skin fibroblasts with the TH-H2O fraction did not result
in toxicity and may have affected differentiation and reproduction
(Fig. 2).
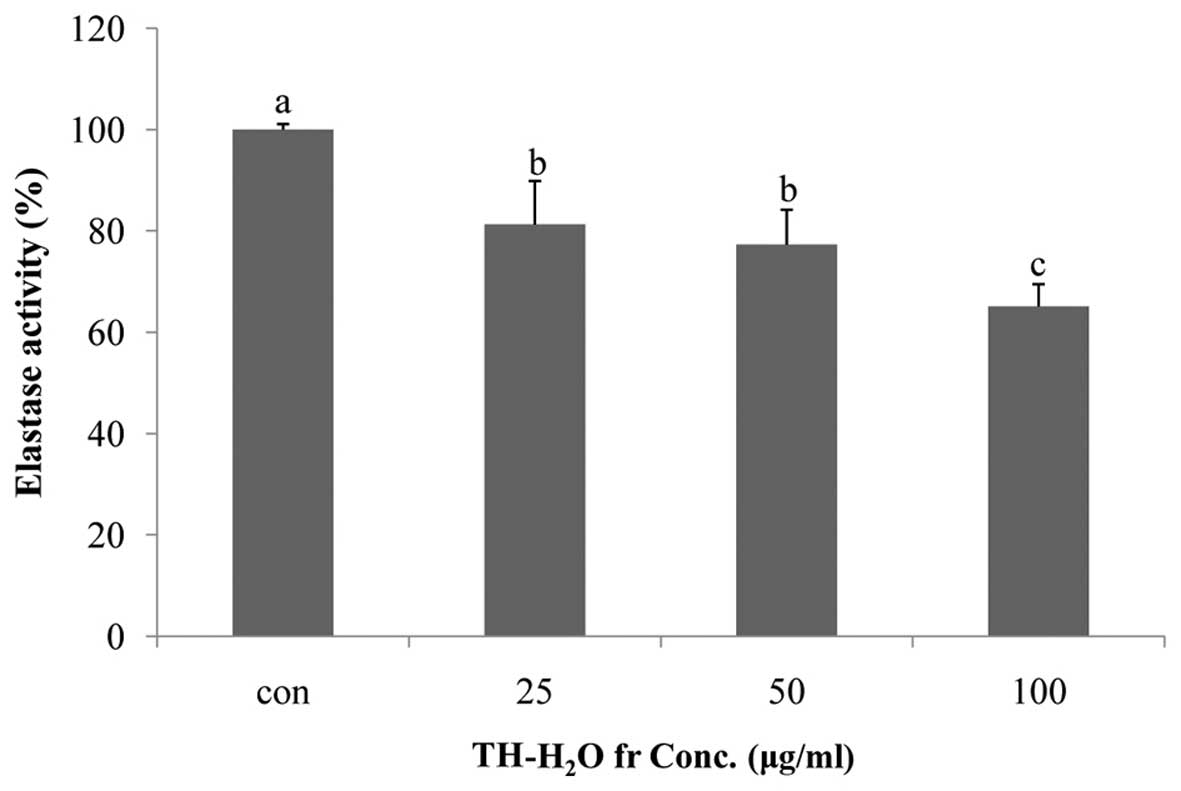
Inhibitory effects of the
TH-H2O fraction on elastase activity
Elastin is a substrate protein that accounts for 4%
of the dermis and plays a role in maintaining skin elasticity
(23). Elastin is degraded by
elastase in neutrophil granulocytes; elastase is a non-specific
hydrolytic enzyme capable of degrading the matrix protein collagen.
Elastase degrades matrix proteins in the network structure of the
basal cells of the skin and causes wrinkles and/or destroys tissue
(1). In addition, as previously
demonsrated in a study on skin wrinkles, increased elastase
activity accelerates wrinkle formation by reducing the amount of
elastic fibers in the skin (10).
In this study, we noted that treatment of the Hs27 cells with the
TH-H2O fraction inhibited elastase activity. The level
of elastase was reduced in a dose-dependent manner, with a maximum
decrease of 35% compared to the controls (untreated cells) being
observed in the cells treated with 100 µg/ml of the
TH-H2O fraction (Fig.
3).
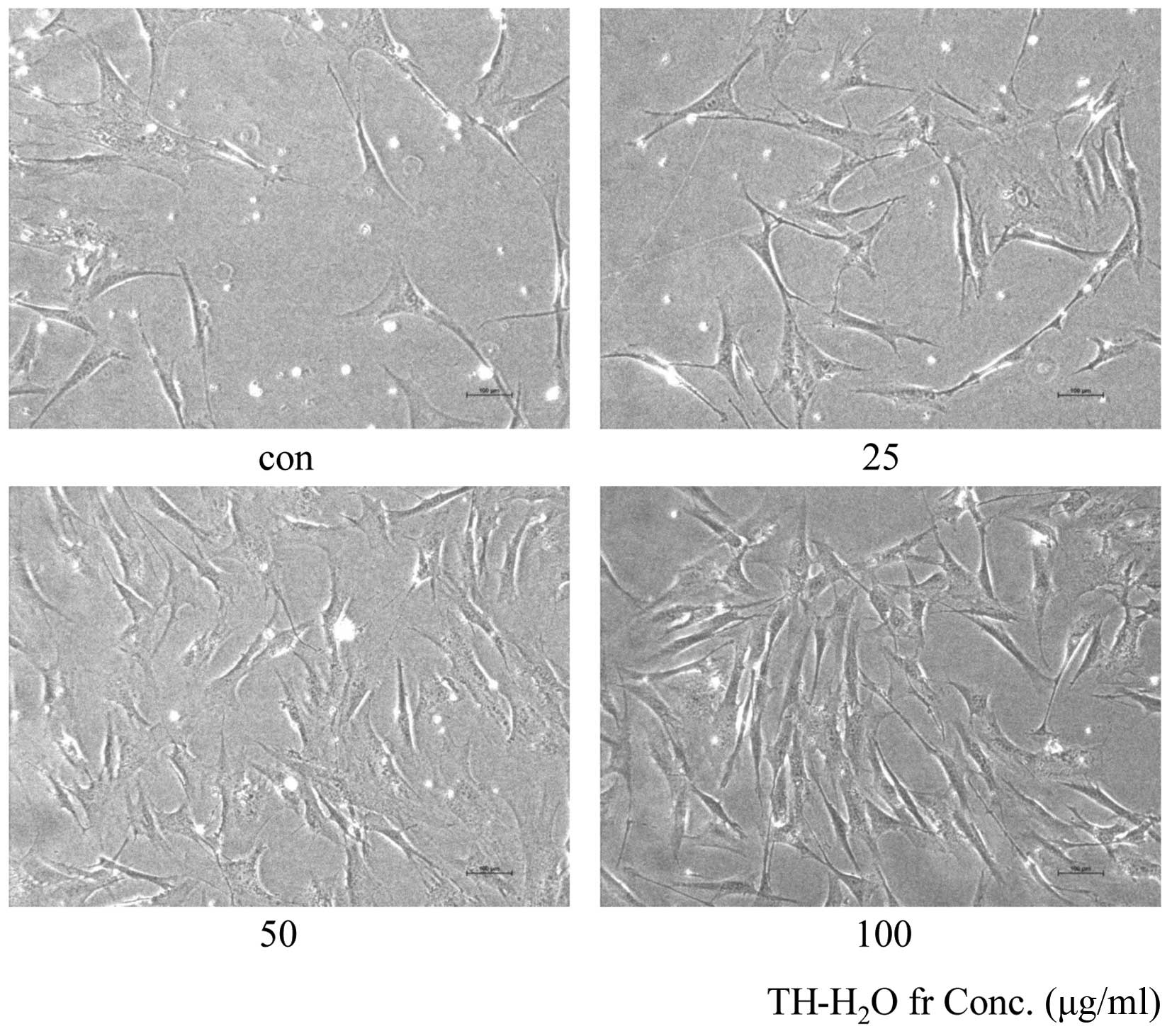
Morphological changes and β-galactosidase
staining
The aging of skin fibroblasts is evidenced by blue
color upon β-galactosidase staining. The decomposition of X-gal by
β-galactosidase in transfected cells leads to the generation of the
blue color. Treatment with the TH-H2O fraction at 25, 50
and 100 µg/ml resulted in a dose-dependent increase in the
number of Hs27 cells and in no β-galactosidase staining (Fig. 4). This suggests that cell aging
did not occur and that the cells were proliferating.
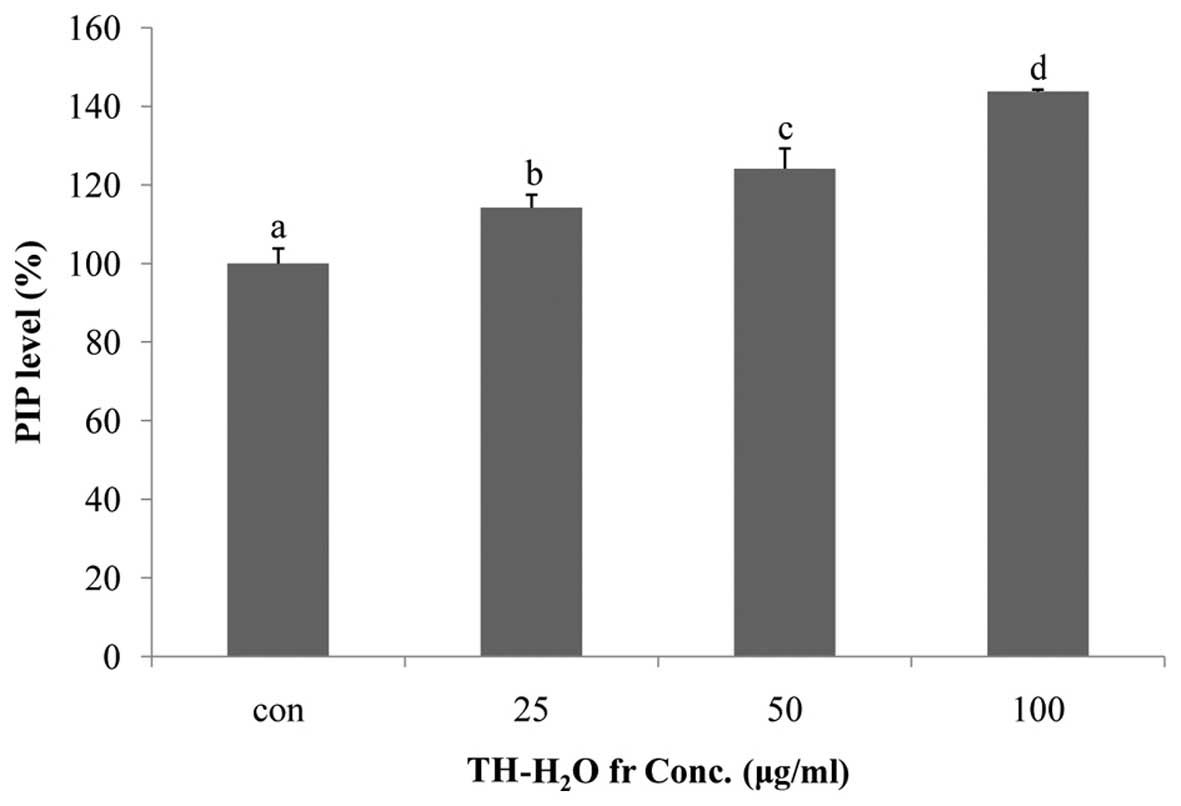
Effects of the TH-H2O fraction
on PIP levels
Collagen is the main structural protein in
extracellular space in connective tissues in mammals. Collagen is
present at high concentrations in the skin, bones, ligaments,
cartilage and teeth, and is a particularlyimportant component of
the extracellular matrix in fibroblasts (24). In addition, collagen is associated
with the mechanical robustness of skin, resistance of the skin
connective tissue, tissue bonding, support of cell adhesion,
differentiated function and the induction of cell division
(23). Collagen is synthesized
from the precursor, procollagen, which has a propeptide sequence at
the amino and carboxyl termini (25). The propeptide plays a role in the
folding of procollagen, and upon polymerization it is separated
from collagen molecules. This suggests that increased PIP
activation is associated with cell proliferation and anti-wrinkle
effects, as it promotes collagen synthesis (26). In this study, we measured the PIP
levels using the Takara MK101 kit. We noted a dose-dependent
increase in PIP levels, with a maximum increase of 43% compared to
the controls being observed in the cells treted with 100
µg/ml of the TH-H2O fraction (Fig. 5).
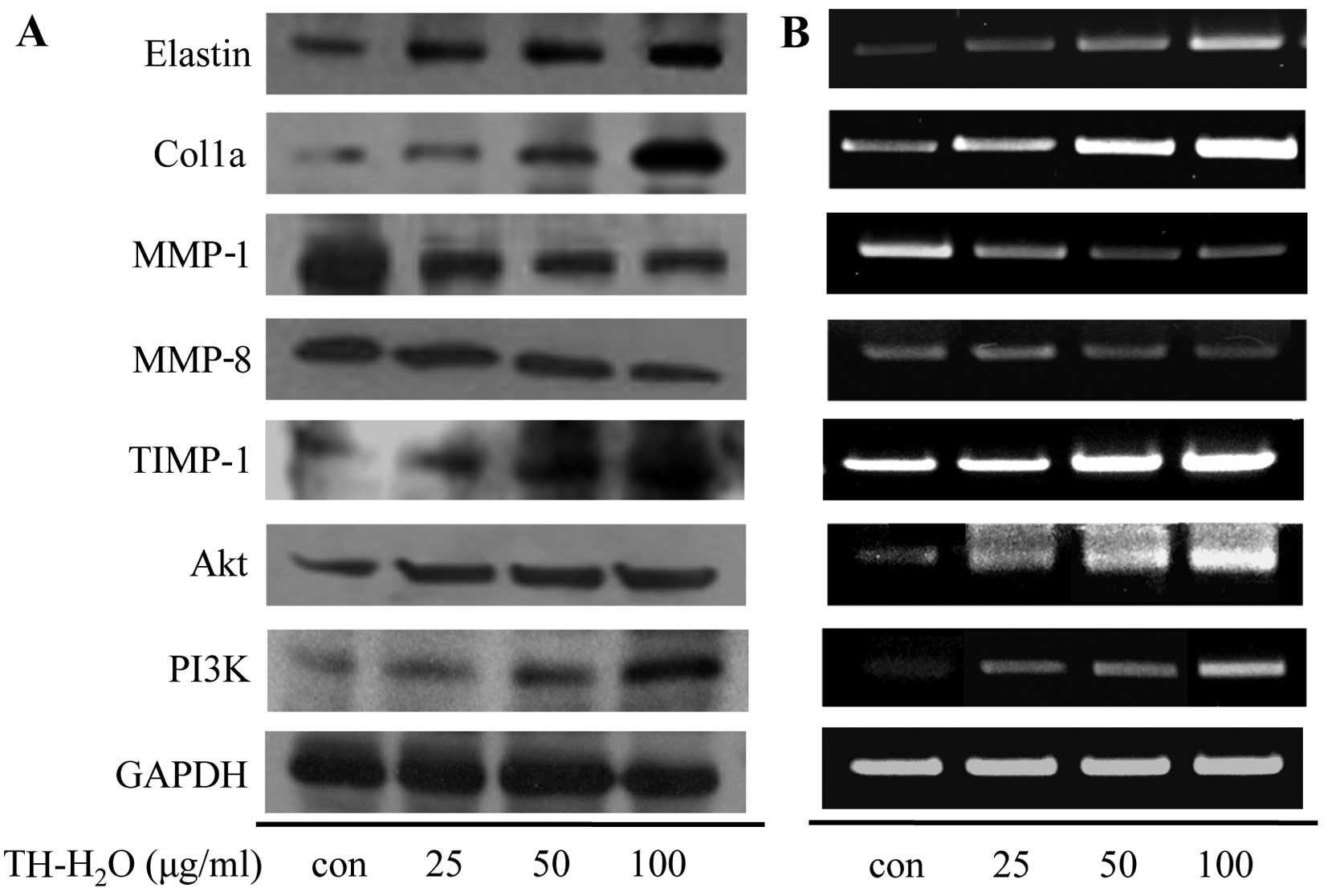
Effect of the TH-H2O fraction
on the mRNA expression levels of PI3K/Akt and MMP in Hs27
cells
MMP-1 and -8 are enzymes that degrade collagen and
inhibit collagen synthesis by specifically decomposing procollagen.
In addition, they degrade the extracellular matrix (27) and inhibit the expression of
elasticity-related genes [elastin and collagen, type I, alpha 1
(Col1a)]. The MMPs are inhibited by specific endogenous TIMPs, and
TIMP-1 regulates MMP activity and protects against collagen and
elastin degradation (28). Thus,
upregulation of TIMP-1 plays an important anti-wrinkle role. One of
the genes involved in cell growth, PI3K, is involved in the lipid
components of phophoinisitide and components of the cell membrane,
and functions as a lipid kinase that phosphorylates and activates
Akt by generating a secondary transfer (29,30). We confirmed increased cell
proliferation and PIP levels. Thus, we measured the expression
levels of MMP-encoding genes and PI3K/Akt by western blot analysis
and RT-PCR. The MMP-1 and -8 levels decreased upon the activation
of TIMP-1 in the cells treated with the TH-H2O fraction,
and the Col1a and elastin levels increased. In addition, the
TH-H2O fraction upregulated the PI3K/Akt levels in a
dose-dependent manner (Fig.
6).
Discussion
Interest in a healthy lifestyle has increased
worldwide and living standards have also increased; thus it is
important to also improve the treatments associated with the
bioactive effect of various marine resources (seaweed and fish), in
order to further protect against various diseases (31). Interest in marine resources and
fishery byproducts to replace depleted land resources has thus
increased. Tuna is mainly used in canning, and there is a growing
demand for tuna to be used in simple and highly functional foods.
However, the processing of marine products, such as tuna generates
30–35% byproducts of total materials (32). Recently, certain studies have been
conducted in order to take advantage of fishery by-products since
they contain a variety of functional proteins (20). Typically, tuna is a high-protein
food which exerts anti-atherosclerotic and anticancer effects, and
is known to protect against high cholesterol levels in the blood
(18). Thus, the present study
was conducted on tuna waste, and previous studies reported on the
anti-obesity effects of boiled tuna extract on 3T3-L1 cells
(18,19) and the anti-atopic effect of an
ethanol extract of tuna heart (20). However, bioactivity studies using
tuna heart are not common. In this study we confirmed the
anti-wrinkle effect of the TH-H2O fraction on Hs27 human
fibroblasts. First, we evaluated cell proliferation following
treatment with 25, 50 and 100 µg/ml of the TH-H2O
fraction by MTS assay. As shown in Fig. 2, we noted a dose-dependent
increase in cell proliferation, with a maximum increase of 47%
compared to the controls in the cells treated with 100 µg/ml
of the TH-H2O fraction. Elastase activity induces skin
wrinkling by destroying the network structure of the basal cells of
the skin through the degradation of matrix proteins (1).
The upregulation of elastase activity leads to
tissue destruction and loss of elasticity (33,34). The data in Fig. 3 show a dose-dependent decrease in
elastase activity upon treatment with the TH-H2O
fraction, with a maximum inhibition of 35% compared to the controls
in the cells treated with 100 µg/ml of the TH-H2O
fraction. In addition, we noted an increase in the number of cells,
but not in blue β-galactosidase staining. This suggests that the
TH-H2O fraction is not induced in aging cells (Fig. 4). To explore the mechanism
underlying this induction of cell proliferation, we examined the
PIP levels by PIP EIA assay. Collagen is present at the highest
concentration in the dermal layer of the skin, accounting for
70–80% of the total dry weight, and plays a role in supporting the
skin (35). However, stress
caused by genetic factors and the environment induces wrinkles by
increasing collagenase activity and reactive oxygen species
(36,37). In other words, collagen is an
important factor for anti-wrinkle effects and cell growth. The
level of collagen biosynthesis can be determined based on the
biosynthetic effects by measuring the amount of propeptide
(26). As shown in Fig. 5, we noted a dose-dependent
increase in PIP levels upon treatment with the TH-H2O
fraction, with a maximum increase of 43% compared to the controls
being observed in the cells treated with 100 µg/ml of the
TH-H2O fraction. This indicates increased collagen
synthesis due to elevated PIP levels. MMP-1 and -8 can degrade
collagen, and TIMP-1 exerts the opposite effect by inhibiting MMP
activity. In addition, tissue-specific inhibitors of TIMP-1 are
known to maintain the biological equilibrium by inactivating MMPs
(38). In the present study we
examined the changes in protein and mRNA expression by western blot
analysis and RT-PCR. The MMP-1 and -8 levels were decreased by the
upregulation of TIMP-1. This suggests that collagen biosynthesis is
promoted by decreased MMP expression. In addition, the expression
levels of elastin and Col1a were increased by the inhibition of
elastase. Moreover, treatment with the TH-H2O fraction
induced the upregulation of PI3K/Akt (Fig. 6). TH-H2O induced the
upregulation of TIMP-1 and inhibited elastase expression. Thus, the
increased expression of the genes encoding elastin, Col1a and
PI3K/Akt is a factor involved in skin elasticity, anti-wrinkling
and cell growth. In conclusion, the TH-H2O fraction
significantly decreased MMP levels and upregulated the expression
of genes associated with cell growth. Therefore, we posit that tuna
heart extract modulates cell growth and induces an anti-wrinkle
effect.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education (2014R1A6A3A01058410).
This study was a part of the project entitled 'Functional materials
and foods using fisheries by-products', funded by the Ministry of
Oceans and Fisheries, Korea (20130279).
References
|
1
|
Kim SH, Yong HJ, Shin C and Ko SG:
Research of traditional herbal medicines for anti-aging, inhibition
effect of wrinkle and whitening effect in the skin. Korean J Ori
Physiol Pathol. 22:691–698. 2008.In Korean.
|
|
2
|
Gancevicine R, Liakou AI, Theodoridis A,
Makrantonaki E and Zouboulis CC: Skin anti-aging strategies.
Dermato-Endocrinol. 4:308–319. 2012. View Article : Google Scholar
|
|
3
|
Park KJ, Park SH and Kim JK: Anti-wrinkle
activity of Acanthopanax senticosus extract in ultraviolet B
(UBV)-induced photoaging. J Korean Soc Food Sci Nutr. 39:42–46.
2010.In Korean. View Article : Google Scholar
|
|
4
|
Gilchrest BA: Skin aging and photoaging.
Dermatol Nurs. 2:79–82. 1990.PubMed/NCBI
|
|
5
|
Ha TY: Development of functional food
materials for healthy life. Korean J Crop Sci. 51:26–39. 2006.In
Korean.
|
|
6
|
Makrantonaki E and Zouboulis CC: Molecular
mechanisms of skin aging: state of the art. Ann NY Acad Sci.
1119:40–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo MY, Chung SY, Choi WK, Seo YK, Jung
SH, Park JM, Seo MJ, Park JK, Kim JW and Park CS: Anti-aging effect
of rice wine in cultured human fibroblasts and keratinocytes. J
Biosci Bioeng. 107:266–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seo JE, Kim S, Shin MH, Kim MS, Eun HC,
Park CH and Chung JH: Ultraviolet irradiation induces
thrombospondin-1 which attenuates type I procollagen downregulation
in human dermal fibroblasts. J Dermatol Sci. 59:16–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang TH, Park HM, Kim YB, Kim H, Kim N, Do
JH, Kang C, Cho Y and Kim SY: Effects of red ginseng extract on UVB
irradiation-induced skin aging in hairless mice. J Ethnopharmacol.
123:446–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukahara K, Nakagawa H, Moriwaki S,
Takema Y, Fujimura T and Imokawa G: Inhibition of
ultraviolet-B-induced wrinkle formation by an elastase-inhibiting
herbal extract: implication for the mechanism underlying
elastase-associated wrinkles. Int J Dermatol. 45:460–468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MJ, Kim JY, Jung TK, Choi SW and Yoon
KS: Skin anti-aging effect of Forsythia viridissima L. extract.
Korean J Biotechnol Bioeng. 21:444–450. 2006.
|
|
12
|
Yeom MH, Lee JY, Kim JS, Park CW, Kim DH
and Kim HK: The anti-aging effects of Korean ginseng berry in the
skin. Korean J Pharmacogn. 41:26–30. 2010.
|
|
13
|
Klippel A, Reinhard C, Kavanaugh WM, Apell
G, Escobedo MA and Williams LT: Membrane localization of
phosphatidylinositol 3-kinase is sufficient to activate multiple
signal-transducing kinase pathways. Mol Cell Biol. 16:4117–4127.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Long AM and Haard NF: The effect of
carotenoid-protein association on pigmentation and growth rates of
rainbow trout (Salmo gairdneri). Bull Aquacult Assoc Can. 2:98–100.
1988.
|
|
16
|
Shahidi F and Synowiecki J: Isolation and
characterization of nutrients and value-added products from snow
crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing
discards. J Agric Food Chem. 39:1527–1532. 1991. View Article : Google Scholar
|
|
17
|
Shahidi F, Naczk M, Pegg R and Synowiecki
J: Chemical composition and nutritional value of processing
discards of cod (Gadus morhua). Food Chem. 42:145–151. 1991.
View Article : Google Scholar
|
|
18
|
Kim YM, Kim EY, Kim IH and Nam TJ: Peptide
derived from desalinated boiled tuna extract inhibits adipogenesis
through the downregulation of C/EBP-α and PPAR-γ in 3T3-L1
adipocytes. Int J Mol Med. 35:1362–1368. 2015.PubMed/NCBI
|
|
19
|
Kim YM, Kim EH, Choi JW, Lee MK and Nam
TJ: The anti-obesity effects of a tuna peptide on 3T3-L1 adipocytes
are mediated by the inhibition of the expression of lipogenic and
adipogenic genes and by the activation of the Wnt/β-catenin
signaling pathway. Int J Mol Med. 36:327–334. 2015.PubMed/NCBI
|
|
20
|
Kang BK, Kim KBWR, Kim MJ, Bark SW, Park
WM, Kim BR, Ahn NK, Choi YU, Bae NY, Park JH, et al: Anti-atopic
activity of tuna heart ethanol extract. J Korean Soc Food Sci Nutr.
44:1–6. 2015.In Korean. View Article : Google Scholar
|
|
21
|
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher
B, Spencer JA, Yanagisawa H, Zuo J and Li T: Elastic fiber
homeostasis requires lysyl oxidase-like 1 protein. Nat Genet.
36:178–182. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patel A, Fine B, Sandig M and Mequanint K:
Elastin biosynthesis: The missing link in tissue-engineered blood
vessels. Cardiovasc Res. 71:40–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suganuma K, Nakajima H, Ohtsuki M and
Imokawa G: Astaxanthin attenuates the UVA-induced up-regulation of
matrix-metalloproteinase-1 and skin fibroblast elastase in human
dermal fibroblasts. J Dermatol Sci. 58:136–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sandhu SV, Gupta S, Bansal H and Singla K:
Collagen in health and disease. J Orofac Res. 2:153–159. 2012.
View Article : Google Scholar
|
|
25
|
Kim YH, Chung CB, Kim JG, Ko KI, Park SH,
Kim JH, Eom SY, Kim YS, Hwang YI and Kim KH: Anti-wrinkle activity
of ziyuglycoside I isolated from a Sanguisorba officinalis root
extract and its application as a cosmeceutical ingredient. Biosci
Biotechnol Biochem. 72:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuji-Naito K, Ishikura S, Akagawa M and
Saeki H: Alpha-Lipoic acid induces collagen biosynthesis involving
prolyl hydroxylase expression via activation of TGF-beta-Smad
signaling in human dermal fibroblasts. Connect Tissue Re. 1–10.
2010.
|
|
27
|
Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun
HC and Chung JH: Photoprotective and anti-skin-aging effects of
eicosapentaenoic acid in human skin in vivo. J Lipid Res.
47:921–930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabe JH, Mamelak AJ, McElgunn PJ, Morison
WL and Sauder DN: Photoaging: mechanisms and repair. J Am Acad
Dermatol. 55:1–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kivirikko KI and Myllyharju J: Prolyl
4-hydroxylases and their protein disulfide isomerase subunit.
Matrix Biol. 16:357–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Park SM, Jung HJ, Kim HS and Yu
TS: Characteristics of Ju-back and effect of Ju-back fertilizer on
growth of crop plants. J Life Sci. 17:1562–1570. 2007. View Article : Google Scholar
|
|
31
|
Tsuji N, Moriwaki S, Suzuki Y, Takema Y
and Imokawa G: The role of elastases secreted by fibroblasts in
wrinkle formation: implication through selective inhibition of
elastase activity. Photochem Photobiol. 74:283–290. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin MO, Ku MJ and Bae SJ: Cytotoxicity
and quinine reductase activity stimulating effects of fin of
Thunnus thynnus extracts in various cancer cells. Korean J Nutr.
40:147–153. 2007.
|
|
33
|
DeWitt DL, Rollins TE, Day JS, Gauger JA
and Smith WL: Orientation of the active site and antigenic
determinants of prostaglandin endoperoxide synthase in the
endoplasmic reticulum. J Biol Chem. 256:10375–10382.
1981.PubMed/NCBI
|
|
34
|
Roth GJ, Siok CJ and Ozols J: Structural
characteristics of prostaglandin synthetase from sheep vesicular
gland. J Biol Chem. 255:1301–1304. 1980.PubMed/NCBI
|
|
35
|
Makrantonaki E and Zouboulis CC; German
National Genome Research Network 2: The skin as a mirror of the
aging process in the human organism - state of the art and results
of the aging research in the German National Genome Research
Network 2(NGFN-2). Exp Gerontol. 42:879–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SN, Lee SH, Lee BK and Jang IS:
Acompositions containing Anthriscus sylvestris Hoffmann extract or
Petroselinum sativum Miller extract for external application having
effects of improving skin wrinkle. Korea Patent 10-2002-0079594.
2002
|
|
37
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs.
photoaging: a comparative histopathological, immunohistochemical,
and ultrastructural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berneburg M, Plettenberg H and Krutmann J:
Photoaging of human skin. Photodermatol Photoimmunol Photomed.
16:239–244. 2000. View Article : Google Scholar : PubMed/NCBI
|