Introduction
Alopecurus aequalis Sobol. (A.
aequalis) is an annual or biennial herb that belongs to the
Gramineae family. It is a dominant resource that grows in winter,
and is commonly found in Korean wetlands and rice fields. Four
Alopecurus species have been reported in Korea:
Alopecurus aequalis, Alopecurus myosuroides Huds.,
Alopecurus paratensis L. and Alopecurus japonicus
Steud. Among these, A. myosuroides, A. paratensis,
and A. japonicus are indigenous plants, whereas A.
aequalis is an endemic species (1,2).
A. aequalis is a weed that causes problems
during the cultivation of barley during winter. A. aequalis
accounts for 95% of all weeds growing during barley cultivation
(3,4). Moreover, A. aequalis grows
before the harvesting of rice, and can compete against the crop.
Thus, it causes the most damage when cultivating barley in rice
fields as an aftercrop (5,6).
A. aequalis sprouts and roots reproduce from nodes on the
ground, even after trimming by plowing or the harrowing of
fields.
Although the eradication of A. aequalis has
been well studied, research evaluating the utilization of A.
aequalis is limited, with the exception of its use in cover
crop research (7,8). Additionally, there is no known use
for A. aequalis plants in food or medicine.
However, A. aequalis has been used as an
effective treatment for anasarca, chickenpox, stomachache and
diarrhea (9). As it has been used
to treat inflammatory diseases, such as anasarca and diarrhea, and
active bacterial diseases, we hypothesized that A. aequalis
may exhibit anti-inflammatory activity.
Inflammation is a defensive response produced by
bio-organisms against external stimuli, including toxic substances,
chemical stimulation and bacterial infection. Dysregulated
inflammatory responses promote mucosal damage, thereby promoting
the development of various diseases, including cancer (10,11). Lipopolysaccharide (LPS), an
outer-membrane component of Gram-negative bacteria, triggers
diverse reactions, including local inflammation, antibody
production and septicemia (12).
Macrophages respond to the early stages of LPS infection, playing a
central role in host defense and the maintenance of homeostasis.
However, high concentrations of LPS can induce the secretion of
pro-inflammatory mediators from macrophages, including tumor
necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and nitric
oxide (NO), leading to host fatality (13–15). NO is generally produced by
inducible NO synthase (iNOS) when macrophages are activated, and
has an antibacterial effect that inhibits several viruses and
parasites (16). However, the
excessive production of NO is known to induce inflammation, tissue
damage, genetic mutation and nerve damage (17,18). The expression of pro-inflammatory
cytokines, such as TNF-α, IL-1β and IL-6 is regulated by
mitogen-activated protein kinases (MAPKs), including extracellular
signal-regulated kinase 1/2 (ERK1/2), p38 kinase (p38), c-Jun
NH2-terminal kinase (JNK) and nuclear factor-κB (NF-κB) (19). In particular, NF-κB plays an
important role in the expression of immunity-associated and
inflammatory genes (20). The
dissociation of the inhibitor of κB-α (IκB-α), which binds and
inhibits NF-κB, allows for the activation and translocation of
NF-κB from the cytoplasm to the nucleus to act as a transcription
factor for cytokines, including TNF-α, IL-12 and IL-6 (21,22).
The aim of this study was to identify the major
compounds involved in the anti-inflammatory activity of A.
aequalis through bioassay-guided fractionation. We examined the
effects of the ethanol (EtOH) extract from A. aequalis and
its different solvent-soluble fractions on the production of the
pro-inflammatory mediator, NO, using RAW 264.7 macrophages. The
most active fraction was further fractionated to identify the
active compounds. The major components were identified by
high-performance liquid chromatography (HPLC) and NMR spectra, and
the anti-inflammatory activity of the individual components was
confirmed by measuring the production of inflammatory mediators,
the protein expression of enzymes involved in their production and
the scavenging of reactive oxygen species (ROS) in LPS-stimulated
macrophages.
Materials and methods
Plant material
A. aequalis was collected from an open field
located at Jangheung (latitude, 34.68; longitude, 126.90),
Jeollanamdo, Korea, in May 2014. A voucher specimen (TKM-2014-55)
has been deposited at the Medicinal Crop Seed Supply Center,
Jeollanamdo Development Institute of Traditional Korean Medicine,
Republic of Korea.
General procedures
Optical rotations were measured on a Jasco P-1020
polarimeter in EtOH (Jasco, Easton, MD, USA). UV spectra were
recorded using a Shimadzu UV-1601 UV-Visible spectrophotometer
(Shimadzu, Tokyo, Japan). High resolution fast-atom bombardment
(HR-FAB) and electrospray ionization (ESI) mass spectra were
obtained on a LC/MS IT-TOF hybrid mass spectrometer (Shimadzu). NMR
spectra, including COSY, HMQC and HMBC experiments were recorded on
a Varian NMR System 600 MHz (Agilent Technologies, Inc., Santa
Clara, CA, USA) NMR spectrometer with chemical shifts given in ppm
(Varian, Palo Alto, CA, USA). Preparative HPLC (Agilent
Technologies, Inc.) was conducted using a Gilson 306 pump (Gilson,
Middleton, WI, USA) with a diode array index detector (DAD). Silica
gel 60 and RP-C18 silica gel (230–400 mesh; Merck, Darmstadt,
Germany) were used for column chromatography. The packing material
for molecular sieve column chromatography was Sephadex LH-20
(Sigma, St. Louis, MO, USA). Spots were detected by thin layer
chromatography (TLC) under UV light or by heating after spraying
with 10% H2SO4 in
C2H5OH (v/v).
Extraction and isolation
A. aequalis (2.0 kg shoot dry weight) were
extracted with 95% EtOH (3×60 liters) at room temperature for 3 h.
The ethanol extract was concentrated under reduced pressure to
yield the ethanol extract (392 g). The concentrated ethanol extract
was then suspended in H2O (2.0 liters) and partitioned
successively to the n-hexane-(75 g), dichloromethane
(CH2Cl2-; 1 g), ethyl acetate (EtOAc-; 4 g),
n-butanol (n-BuOH-; 277 g) and H2O-soluble
fractions (14 g). The CH2Cl2-, EtOAc-,
n-BuOH- and H2O-soluble layers were tested on the
NO production inhibition assay. Amongst these, the EtOAc fraction
demonstrated the most potent activity. Thus, this fraction (4 g)
was separated over a silica gel column (CHCl3-MeOH,
10:1) to yield 14 fractions (AAE1-AAE14). Sub-fraction AAE1 (100
mg) was purified by preparative HPLC (40% MeCN) to yield compound 1
(50 mg).
Compound 1, light yellow in color, showed
1H-NMR (600 MHz, DMSO-d6) δ: 7.33 (2H,
s, H-2′ and H-5′), 6.99 (1H, s, H-3), 6.56 (1H, d, J=2.1 Hz,
H-8), 6.20 (1H, d, J=2.1 Hz, H-6), 3.88 (6H, s, 3′ and 5′
-OCH3)13 C-NMR (150 MHz,
DMSO-d6) δ: 181.79 (C-4), 163.63 (C-2 and 7),
161.39 (C-5), 157.35 (C-9), 148.18 (C-3′ and 5′), 139.84 (C-4′),
120.36 (C-1′), 104.33 (C-2′ and 6′), 103.67 (C-10), 103.58 (C-3),
98.87 (C-6), 94.24 (C-8), 56.36 (C-3′ and C-5′, -OCH3),
ESI-MS m/z 343.09[M-H]−.
Cell culture and MTS assay for cell
viability
RAW 264.7 cells [Korean Cell Line Bank (KCLB); KCLB
no. 40071] were cultured as previously described (23). Briefly, the cells were grown in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco, Invitrogen, Carlsbad, CA, USA) and 1%
penicillin-streptomycin. The cells were cultured at 37°C in a
humidified 5% CO2 incubator. The fractions and isolated
compounds from A. aequalis were dissolved in dimethyl
sulfoxide (DMSO) prior to their use in cell culture; the final
concentrations of DMSO were 0.1% or less. DMSO (0.1%, v/v) was used
as a control. The effects of the EtOH extract and bioactive
compounds from A. aequalis on cell viability were determined
by a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) assay (CellTiter 96 Aqueous One Solution; Promega,
Madison, WI, USA), according to the manufacturer's instructions.
Briefly, the cells were seeded in 96-well plates at a density of
3×105 cells/well, and 24 h after the cells were seeded,
the extracts or their bioactive compounds were added. Following 2 h
of incubation, a solution of MTS was added, and the absorbance at
490 nm was measured using a microplate reader (Infinite 200 PRO;
Tecan, Grödig, Austria) to determine the formazan
concentration.
Measurement of NO production
Nitrite is a stable end-product of NO generated by
activated macrophages. We measured the nitrite accumulation in the
culture supernatant as an indicator of NO production (14). The RAW 264.7 cells were seeded in
12-well plates at a density of 3×105 cells/well and
incubated for 24 h. The cells were treated with the extracts or its
bioactive compounds for 1 h before LPS (500 ng/ml) was added to the
cells. Following incubation for 24 h, 100 µl of cell culture
medium were mixed with an equal volume of Griess reagent [1%
sulfanilamide in 5% phosphoric acid, 0.1% N-(1-naphthyl)
ethylenediamine in H2O] and incubated at room
temperature for 10 min. The absorbance was measured at 540 nm using
an ELISA microplate reader (Infinite 200 PRO; Tecan). The nitrite
concentrations were determined by extrapolation from a standard
sodium nitrite curve.
Measurement of prostaglandin
E2 (PGE2) production
The effect of the bioactive compound on the
LPS-induced release of PGE2, a pro-inflammatory
mediator, was determined. The RAW264.7 cells were seeded in 6-well
plates (1×106 cells/well) and incubated for 24 h. The
cells were treated with the bioactive compounds (5, 10, 50 and 100
µg/ml) for 1 h before LPS (500 ng/ml) was added to the
cells. Following incubation for 18 h, the concentrations of
PGE2 in the conditioned culture medium were determined
using the PGE2 EIA kit (R&D systems, Minneapolis,
MN, USA) according to the manufacturer's instructions.
Western blot analysis
The effects of the bioactive compounds on the
expression of iNOS and COX-2 were examined. The RAW 264.7 cells
were seeded in 6-well plates at a density of 1×106
cells/well, incubated for 24 h and then pre-treated with the
bioactive compounds (5, 10, 50 and 100 µg/ml) for 1 h.
Following stimulation with LPS (500 ng/ml) for 3 h in the presence
of the bioactive compounds, the cells were collected and washed
twice with cold phosphate-buffered saline (PBS). The cells were
lysed in cold RIPA buffer (pH 7.4) containing 20 mM Tris-HCl, 150
mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% NP-40, 1% sodium
deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate,
1 mM Na3VO4 and 1 lg/ml leupeptin. The cell
lysates were centrifuged at 15,000 × g for 30 min at 4°C. The
protein concentrations were determined by the Bradford method
(Bio-Rad, Richmond, CA, USA). Forty-five micrograms of protein were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto PVDF membranes (Bio-Rad). The
membranes were blocked with 5% skim milk in PBS containing 0.1%
Tween-20 (pH 7.2). The membranes were then incubated overnight with
primary antibodies [iNOS (1:100; #13120); COX-2 (1:5,000; #4842);
all from Cell Signaling Technology, Inc., Danvers, MA, USA] at 4°C.
After washing, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (anti-rabbit lgG,
1:2,000; #7074; all from Cell Signaling Technology, Inc.) for at
least 1 h at room temperature. A band of interest was detected with
an ECL system (Ab Frontier, Seoul, Korea) and the intensities were
analyzed and quantified using a FluorChem densitometer and the
ImageJ program (National Institute of Health, Bethesda, MD,
USA).
Measurement of ROS production
Intracellular ROS production was measured using the
cell-permeable fluorogenic probe, dichlorofluorescin diacetate
(DCFH-DA). DCFH-DA is hydrolyzed to DCFH by a deacetylase within
the cells and oxidized by a variety of intracellular ROS to DCF, a
highly fluorescent compound. The cells were seeded in a 60-mm
culture dish at a density of 1.5×106 cells/dish and
incubated for 24 h. The cells were pre-treated with the bioactive
compounds (5, 10, 50 and 100 µg/ml) for 1 h. The cells were
then treated with the bioactive compounds in the presence of LPS
(500 ng/ml) for 18 h. Following treatment, the cells were washed
with SF medium and treated with 20 µM DCFH-DA for 30 min at
37°C in a CO2 incubator. The DCFA level was measured
using a flow cytometer (Cytomics FC500; Beckman Coulter, Brea, CA,
USA).
Statistical analysis
Each experiment was performed at least in
triplicate. All the values are expressed as the means ± SD, and the
data were analyzed using the SPSS 19.0 software. The data were
analyzed by one-way ANOVA followed by Duncan's multiple range test
to determine the differences among the treatment groups. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Effects of MeOH extract and 5 soluble
fractions from A. aequalis on LPS-induced NO production
The RAW 264.7 cells were treated with various
concentrations (10, 50, 100, 200 and 500 µg/ml) of the EtOH
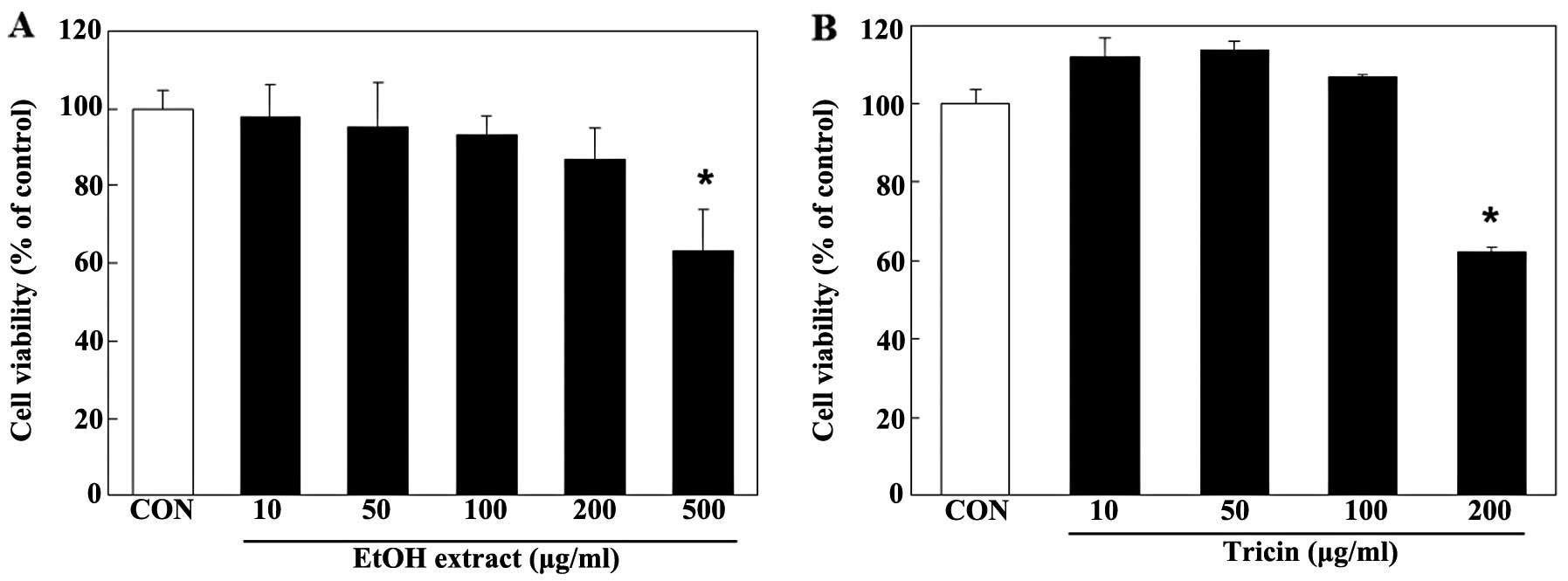
extract to test its cellular cytotoxicity. Cell viability was not
significantly affected by the EtOH extract at a concentration of up
to 200 µg/ml, as determined by MTS assay (Fig. 1A). Thus, the cells were treated
with the EtOH extract of A. aequalis at concentrations in
the range of 0–200 µg/ml. The stimulation of RAW 264.7 cells
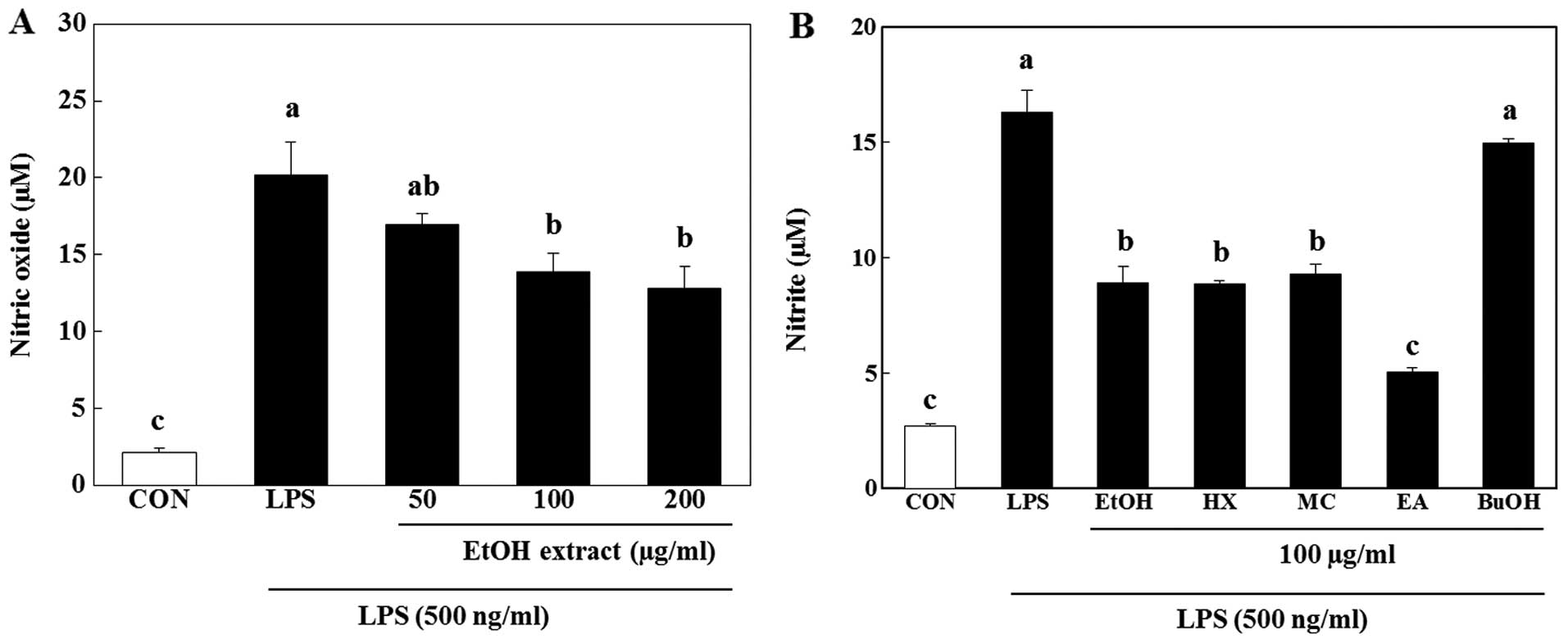
with 500 ng/ml LPS significantly increased NO production. The EtOH
extract of A. aequalis inhibited the LPS-stimulated NO
production in a dose-dependent manner (p<0.05), with a 33%
inhibition observed at a concentration of 200 µg/ml
(Fig. 2A). Among the soluble
fractions, the EtOAc-soluble fraction was comparable to the EtOH
extract at a concentration of 100 µg/ml (Fig. 2B).
Identification of isolated compounds from
the anti-inflammatory soluble fraction
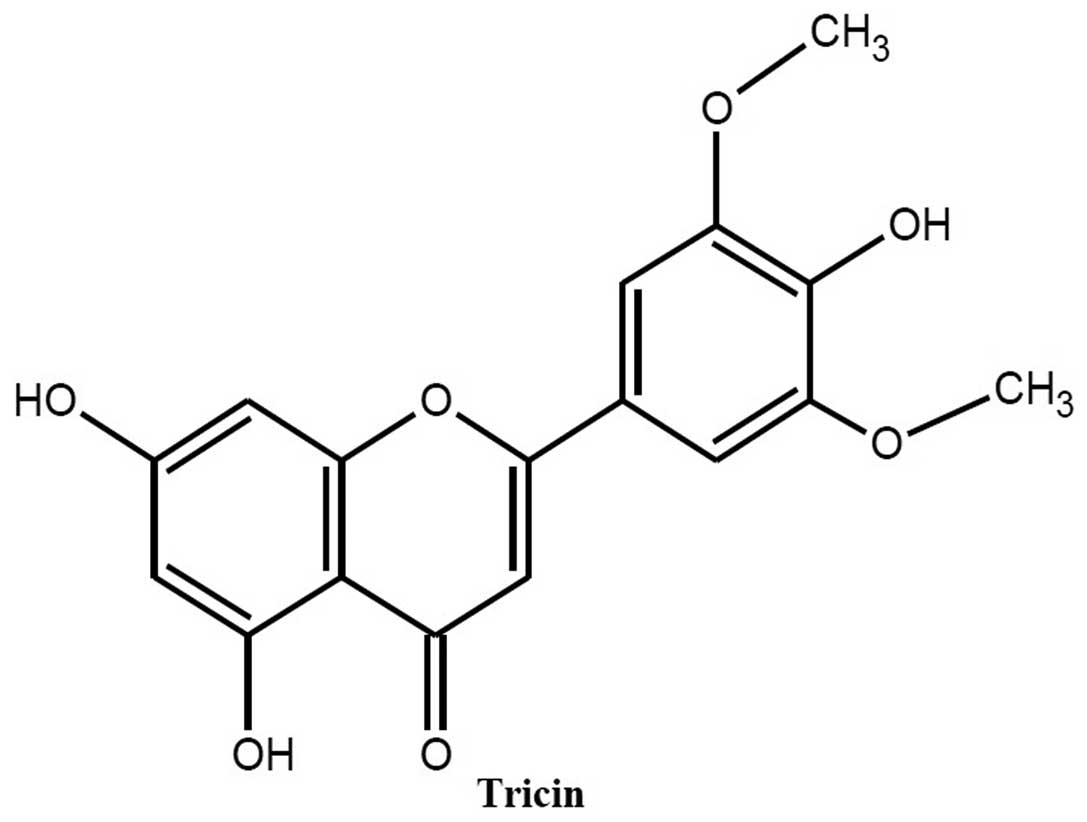
The most active EtOAc-soluble fraction was purified
by reversed-phase chromatography, and one major compound was
separated. The structure of the compound was identified as tricin
through a comparison of the reported spectroscopic data. The
chemical structure of the compound is illustrated in Fig. 3.
Effects of tricin isolated from A.
aequalis on pro-inflammatory mediators
The RAW 264.7 cells were treated with tricin (10,
50, 100 and 200 µg/ml) to test its cellular cytotoxicity.
Tricin did not significantly decrease cell viability (Fig. 1B). To confirm the
anti-inflammatory activity of tricin, we examined the effect of
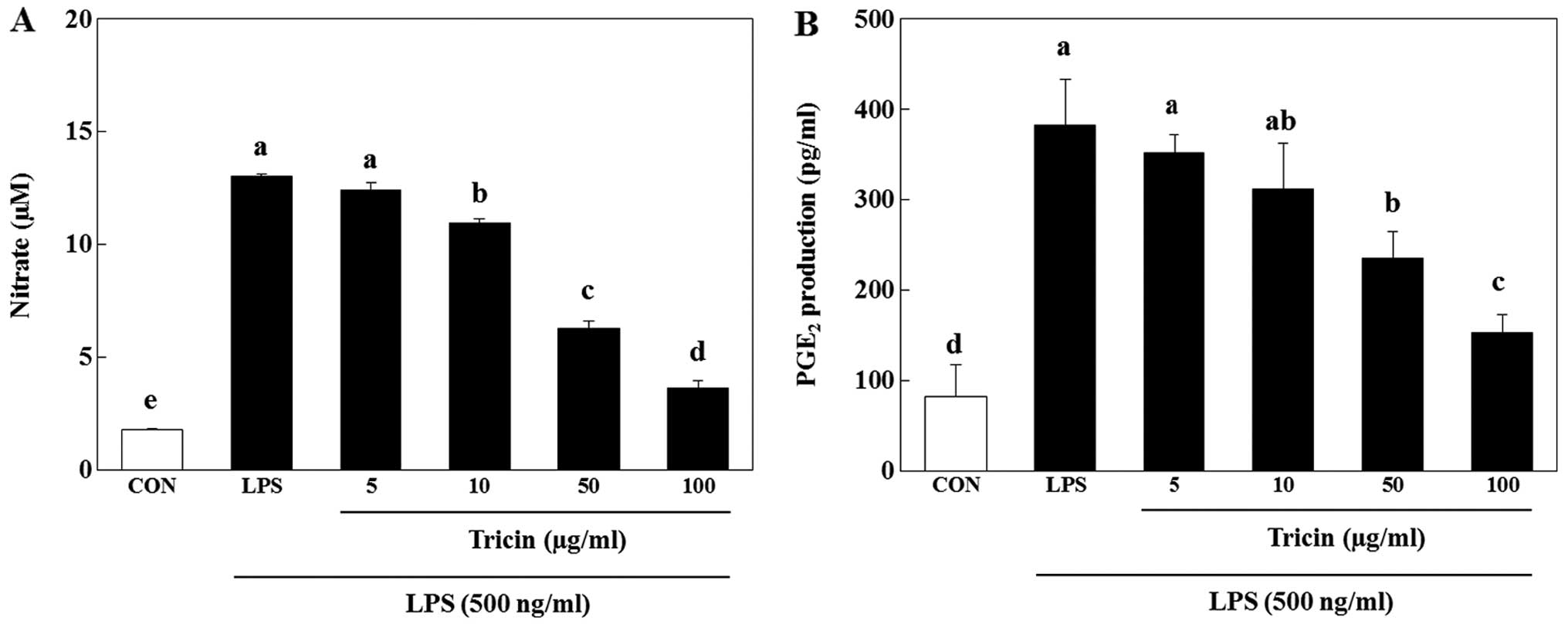
tricin on LPS-induced NO production. The nitrite concentration
decreased by 4.5, 15.8, 51.8 and 72.0% following treatment with 5,
10, 50 and 100 µg/ml tricin, respectively compared with the
LPS-stimulated cells (Fig. 4A).
The concentrations used in this study were based on other studies
that examined the anti-inflammatory effects of tricin in RAW cells
(24,25). Thus, treatment with tricin
inhibited NO production in a dose-dependent manner (p<0.05;
Fig. 4A). Tricin also inhibited
the LPS-induced PGE2 production in a dose-dependent
manner (p<0.05). PGE2 production was decreased by
8.1, 11.2, 25.2 and 35.6% in the cultures containing 5, 10, 50 and
100 µg/ml tricin, respectively (Fig. 4B).
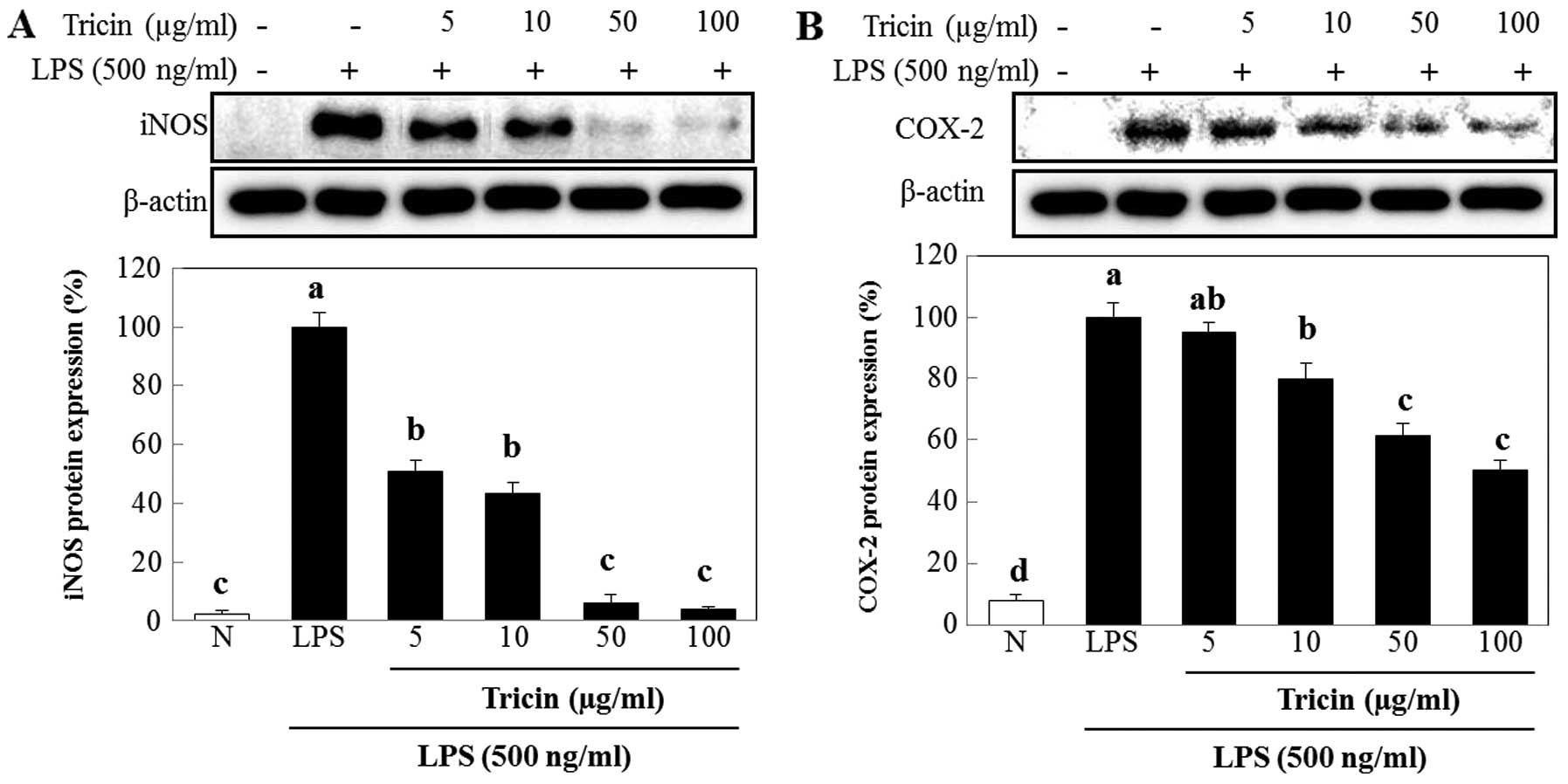
Effect of tricin isolated from A.
aequalis on the protein expression of iNOS and COX-2
We used western blot analysis to determine whether
the inhibitory effect of tricin on NO production was related to the
modulation of iNOS and COX-2 expression. The protein expression
levels of iNOS and COX-2 were upregulated in response to LPS, and
treatment with tricin markedly inhibited these increases
(p<0.05; Fig. 5). The protein
expression of iNOS was decreased by 49.4, 57.0, 94.0 and 96.2% in
cultures containing 5, 10, 50 and 100 µg/ml of tricin,
respectively (p<0.01; Fig.
5A). Similarly, treatment with tricin at 5, 10, 50 and 100
µg/ml suppressed COX-2 expression by as much as 4.7, 20.3,
38.8 and 49.8%, respectively (p<0.01; Fig. 5B).
Effect of tricin isolated from A.
aequalis on ROS production
To investigate whether tricin influences ROS
production, we measured ROS generation based on the DCF
fluorescence intensity in LPS-stimulated RAW 264.7 cells. Exposure
to LPS resulted in an increase in the DCF fluorescence intensity,
and this increase decreased by treatment with tricin in a
dose-dependent manner (p<0.05; Fig. 6). Decreases of 15.7, 34.5 and
75.3% were obtained with concentrations of 10, 50 and 100
µg/ml tricin, respectively.
Discussion
A. aequalis has been traditionally used for
the treatment of inflammatory ailments, such as rheumatic pain,
wounds, ulcers and fever. Although the anti-inflammatory effects of
A. aequalis ethanol extracts have been reported (23), the compounds responsible for these
anti-inflammatory effects and the mechanisms involved have not yet
been fully explored. Therefore, in this study, we isolated and
determined the major anti-inflammatory compounds of the A.
aequalis extract through activity-guided fractionation. We
concluded that tricin is the active component of A. aequalis
responsible for the observed anti-inflammatory effect.
In vitro assays to measure the inhibition of
NO production were used to screen the active soluble phase and
various compounds isolated from the A. aequalis extract. NO
is a major well-known pro-inflammatory mediator, and the excess
production of NO is one of the characteristics of inflammation,
such as autoimmune disease and septic shock (26). It is known that NO contributes to
the inflammatory cascade by increasing vascular permeability and
the extravasation of fluids and proteins at inflammatory sites
(27,28). For these reasons, the suppression
of NO production has been emphasized as a pharmaceutical strategy
for the treatment of inflammatory diseases (29). In this study, we confirmed the
anti-inflammatory effect of A. aequalis ethanol extracts
(23), by the inhibition of NO
production in LPS-stimulated RAW 264.7 cells, which is consistent
with the results of previous studies. In the following experiment,
we compared the ability of the EtOH extract and its four
solvent-soluble fractions to inhibit LPS-induced NO production, and
the bioassay-guided purification resulted in the isolation of one
compound, namely tricin. Tricin is a flavonoid, is found
ubiquitously in plants, but there has been no report on the
biological activity of tricin isolated from A. aequalis.
In this study, the analysis of compound 1 isolated
from the most effective EtOAc fraction revealed that the amount of
tricin was higher than that of the other compounds. Moreover,
tricin significantly suppressed NO production in LPS-stimulated RAW
264.7 cells. These results indicate that tricin is a major
anti-inflammatory compound in A. aequalis EtOAc extract.
The inhibitory effect of the EtOH extract of A.
aequalis and tricin on NO production was not due to treatment
cytotoxicity as the concentrations that suppressed NO production
did not affect cell viability, as determined by MTS assay. NO is
synthesized from L-arginine by the three major NOS isoforms, namely
neuronal NOS (nNOS), endothelial NOS (eNOS) and iNOS. Although the
constitutive isoforms (nNOS and eNOS) controlled by
Ca2+/calmodulin produce small amounts of NO, iNOS
produces markedly higher amounts of NO and is expressed only during
inflammation (30). iNOS is
highly induced by various inflammatory stimuli, such as bacterial
LPS and inflammatory cytokines, in macrophages (31,32). As iNOS inhibitors attenuate
various chronic inflammatory diseases, the inhibition of iNOS
expression has significant meaning as a therapeutic target for
inflammation-related disease. It has been reported that the A.
aequalis extract suppresses the expression and activity of iNOS
in LPS-stimulated RAW264.7 cells. In this study, we found that
tricin isolated from A. aequalis extract inhibited the
protein expression of iNOS. These results suggest that tricin
inhibits NO production in LPS-induced RAW 264.7 cells by
suppressing iNOS expression. PGE2 is also an important
mediator in inflammatory diseases, such as rheumatic arthritis and
osteoarthritis, by promoting local vasodilation and local
attraction and activating neutrophils, macrophages and mast cells
at the early stages of inflammation (33). PGE2 is produced by the
enzyme, COX-2, in response to inflammatory stimuli. Lowering the
production of PGE2 by the inhibition of COX-2 is another
therapeutic approach to suppress the inflammatory response. In this
study, we demonstrated that tricin contributed to the inhibitory
effect of the A. aequalis extract on PGE2
production and COX-2 expression.
Tricin has been characterised by the Oryza
sativa species (34). Tricin
has been proposed to have anti-viral, immunomodulatory,
anti-tubercular, anti-ulcerogenic, anti-mutagenic, mildly
estrogenic, chemopreventive, antioxidant and potent anticancer
effects (24,25,35–38). In this study, the exposure of RAW
264.7 cells to LPS resulted in an accumulation of intracellular
peroxide. Tricin significantly attenuated the LPS-induced
intracellular ROS increase. These results suggest that the
inhibition of NO production by A. aequalis extract is, at
least in part, related to the antioxidant activity of A.
aequalis. Our study was limited in that a single cell line was
used and we did not use an in vivo model. In addition, we
did not investigate the hexane fraction, the second effective
fraction, which was comparable to the EtOH extract. However, to the
best of our knowledge, this study provides the first demonstration
of the compounds responsible for the anti-inflammatory activity of
A. aequalis.
Finally, our obtained data suggested that tricin
could be considered as a lead compound for the development of
agents against NO production. Moreover, the caffeoylglycerol
ester-enrich extracts from the leaf and stem of A. aequalis
may be applied as supplemental and/or functional foods having a
beneficial effect against inflammation as well.
Acknowledgments
This study was supported by the Jeollanamdo
Development Institute of Traditional Korean Medicine, Research Fund
2014.
References
|
1
|
Lee TB: Colored Flora of Korea.
Hyangmunsa; pp. p5011980
|
|
2
|
Park SH: Unrecorded naturalized plants of
Korea. Korean J Pl Taxon. 23:464–468. 2009.
|
|
3
|
Ahn DJ, Park SG, Son CK, Kim CR and Choi
BS: Growth characteristics and yield of wheat as affected by sowing
methods of seed broadcasting over rice plants and rotavating after
seed broadcasting. J Crop Sci. 39:20–26. 1997.
|
|
4
|
Kim DH, Son BY, Kim SK, Shon GM and Kang
DJ: Effect of over-sowing for labor-saving and on growth response
as affected by different barley and wheat. J Crop Sci. 38:106–116.
1996.
|
|
5
|
Chin MS, Park CS and Ham YS: Ecological
analysis of the water foxtail (Alopecurus aequalis) damage in
barley cultivation on drained paddy fields. J Crop Sci. 19:157–170.
1977.
|
|
6
|
Kim DH, Kim SK, Kim ES, Son BY and Kang
DJ: Weed occurrence and control in simultaneous wheat sowing
culture with rice harvest under no-tilled paddy field. Korean J
Weed Sci. 18:186–190. 1998.
|
|
7
|
Jung JS, Lee JS, Choi CD and Cheung JD: A
study on sod culture using water foxtail (Alopecurus aequalis) in
apple orchard. Kor J Weed Sci. 18:128–135. 1998.
|
|
8
|
Seong KY, Park TS, Cho HS, Seo MC and Jeon
WT: Weed occurrence according to the density of water foxtail in
No-tillage seeding rice paddy fields. Kor J Weed Sci. 32:280–284.
2012. View Article : Google Scholar
|
|
9
|
State Administration of Traditional
Chinese medicine (SATC): The encyclopedia of oriental herbal
medicine. State Adm Tradit Chinesemedicine Shanghai China.
8:7393–7394. 1999.
|
|
10
|
Cho W, Nam JW, Kang HJ, Windono T, Seo EK
and Lee KT: Zedoarondiol isolated from the rhizoma of Curcuma
heyneana is involved in the inhibition of iNOS, COX-2 and
pro-inflammatory cytokines via the downregulation of NF-kappaB
pathway in LPS-stimulated murine macrophages. Int Immunopharmacol.
9:1049–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willoughby DA: Heberden Oration, 1974.
Human arthritis applied to animal models Towards a better therapy.
Ann Rheum Dis. 34:471–478. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dobrovolskaia MA and Vogel SN: Toll
receptors, CD14, and macrophage activation and deactivation by LPS.
Microbes Infect. 4:903–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McDaniel ML, Kwon G, Hill JR, Marshall CA
and Corbett JA: Cytokines and nitric oxide in islet inflammation
and diabetes. Proc Soc Exp Biol Med. 211:24–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DH, Park SJ, Jung JY, Kim SC and Byun
SH: Antiinflammatory effects of the aqueous extract of
Hwangnyenhaedok-tang in LPS-activated macrophage cells. Korean J
Herbology. 24:47–39. 2009.
|
|
15
|
Willeaume V, Kruys V, Mijatovic T and Huez
G: Tumor necrosis factor-alpha production induced by viruses and by
lipopoly-saccharides in macrophages: Similarities and differences.
J Inflamm. 46:1–12. 1996.
|
|
16
|
Moncada S and Higgs EA: Endogenous nitric
oxide: Physiology, pathology and clinical relevance. Eur J Clin
Invest. 21:361–374. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YO, Lee SW, Sohn SH, Kim SY, Oh MS and
Kim SK: Anti-inflammatory effects of water extract of Eucommia
ulmoides oliver on the LPS-induced RAW 264.7 cells. Hanguk Yakyong
Changmul Hakhoe Chi. 20:381–386. 2012.
|
|
18
|
McCartney-Francis N, Allen JB, Mizel DE,
Albina JE, Xie QW, Nathan CF and Wahl SM: Suppression of arthritis
by an inhibitor of nitric oxide synthase. J Exp Med. 178:749–754.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng GJ, Goodridge HS, Harnett MM, Wei XQ,
Nikolaev AV, Higson AP and Liew FY: Extracellular signal-related
kinase (ERK) and p38 mitogen-activated protein (MAP) kinases
differentially regulate the lipopolysaccharide-mediated induction
of inducible nitric oxide synthase and IL-12 in macrophages:
Leishmania phosphoglycans subvert macrophage IL-12 production by
targeting ERK MAP kinase. J Immunol. 163:6403–6412. 1999.PubMed/NCBI
|
|
20
|
Anest V, Hanson JL, Cogswell PC,
Steinbrecher KA, Strahl BD and Baldwin AS: A nucleosomal function
for IkappaB kinase-alpha in NF-kappaB-dependent gene expression.
Nature. 423:659–663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bohuslav J, Kravchenko VV, Parry GC, Erlic
JH, Gerondakis S, Mackman N and Ulevitch RJ: Regulation of an
essential innate immune response by the p50 subunit of NFkappaB. J
Clin Invest. 102:1645–1652. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beinke S and Ley SC: Functions of
NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J.
382:393–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung HK, Kang BM, Jang JH, Ahn BK, Yeo JH,
Jung WS, Cho JH, Kuk YI, Kyun KH and Cho HW: Inhibitory effect of
Alopecurus aequalis Sobol ethanol extracts on LPS-induced
inflammatory response in RAW 264.7 cells. Hanguk Yakyong Changmul
Hakhoe Chi. 22:98–104. 2014.
|
|
24
|
Zhou J and Ibrahim RK: Tricin-a potential
multifunctional nutraceutical. Phytochem Rev. 9:413–424. 2010.
View Article : Google Scholar
|
|
25
|
Cai H, Al-Fayez M, Tunstall RG, Platton S,
Greaves P, Steward WP and Gescher AJ: The rice bran constituent
tricin potently inhibits cyclooxygenase enzymes and interferes with
intestinal carcinogenesis in ApcMin mice. Mol Cancer Ther.
4:1287–1292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
MacMicking JD, Nathan C, Hom G, Chartrain
N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson
N, et al: Altered responses to bacterial infection and endotoxic
shock in mice lacking inducible nitric oxide synthase. Cell.
81:641–650. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.
|
|
28
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
29
|
Tsao LT, Tsai PS, Lin RH, Huang LJ, Kuo SC
and Wang JP: Inhibition of lipopolysaccharide-induced expression of
inducible nitric oxide synthase by phenolic
(3E)-4-(2-hydroxyphenyl)but-3-en-2-one in RAW 264.7 macrophages.
Biochem Pharmacol. 70:618–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lowenstein CJ and Padalko E: iNOS (NOS2)
at a glance. J Cell Sci. 117:2865–2867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levy D, Höke A and Zochodne DW: Local
expression of inducible nitric oxide synthase in an animal model of
neuropathic pain. Neurosci Lett. 260:207–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guastadisegni C, Nicolini A, Balduzzi M,
Ajmone-Cat MA and Minghetti L: Modulation of PGE(2) and TNFalpha by
nitric oxide and LPS-activated RAW 264.7 cells. Cytokine.
19:175–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012.
View Article : Google Scholar :
|
|
34
|
Mohanlal S, Parvathy R, Shalini V, Helen A
and Jayalekshmy A: Isolation, characterization and quantification
of tricin and flavonolignans in the medicinal rice Njavara (Oryza
sativa L.), as compared to staple varieties. Plant Foods Hum Nutr.
66:91–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Havsteen B: Flavonoids, a class of natural
products of high pharmacological potency. Biochem Pharmacol.
32:1141–1148. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Verschoyle RD, Greaves P, Cai H, Borkhardt
A, Broggini M, D'Incalci M, Riccio E, Doppalapudi R, Kapetanovic
IM, Steward WP and Gescher AJ: Preliminary safety evaluation of the
putative cancer chemopreventive agent tricin, a naturally occurring
flavone. Cancer Chemother Pharmacol. 57:1–6. 2006. View Article : Google Scholar
|
|
37
|
Oyama T, Yasui Y, Sugie S, Koketsu M,
Watanabe K and Tanaka T: Dietary tricin suppresses
inflammation-related colon carcinogenesis in male Crj: CD-1 mice.
Cancer Prev Res (Phila). 2:1031–1038. 2009. View Article : Google Scholar
|
|
38
|
Chang CL, Wang GJ, Zhang LJ, Tsai WJ, Chen
RY, Wu YC and Kuo YH: Cardiovascular protective flavonolignans and
flavonoids from Calamus quiquesetinervius. Phytochemistry.
71:271–279. 2010. View Article : Google Scholar
|