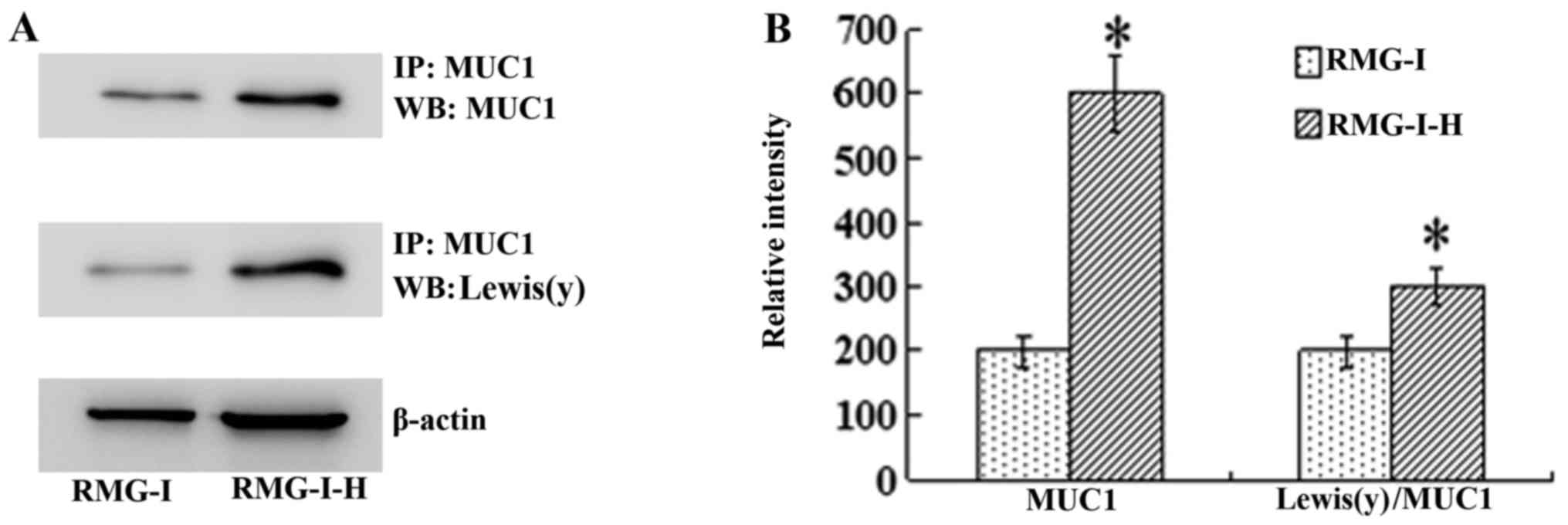
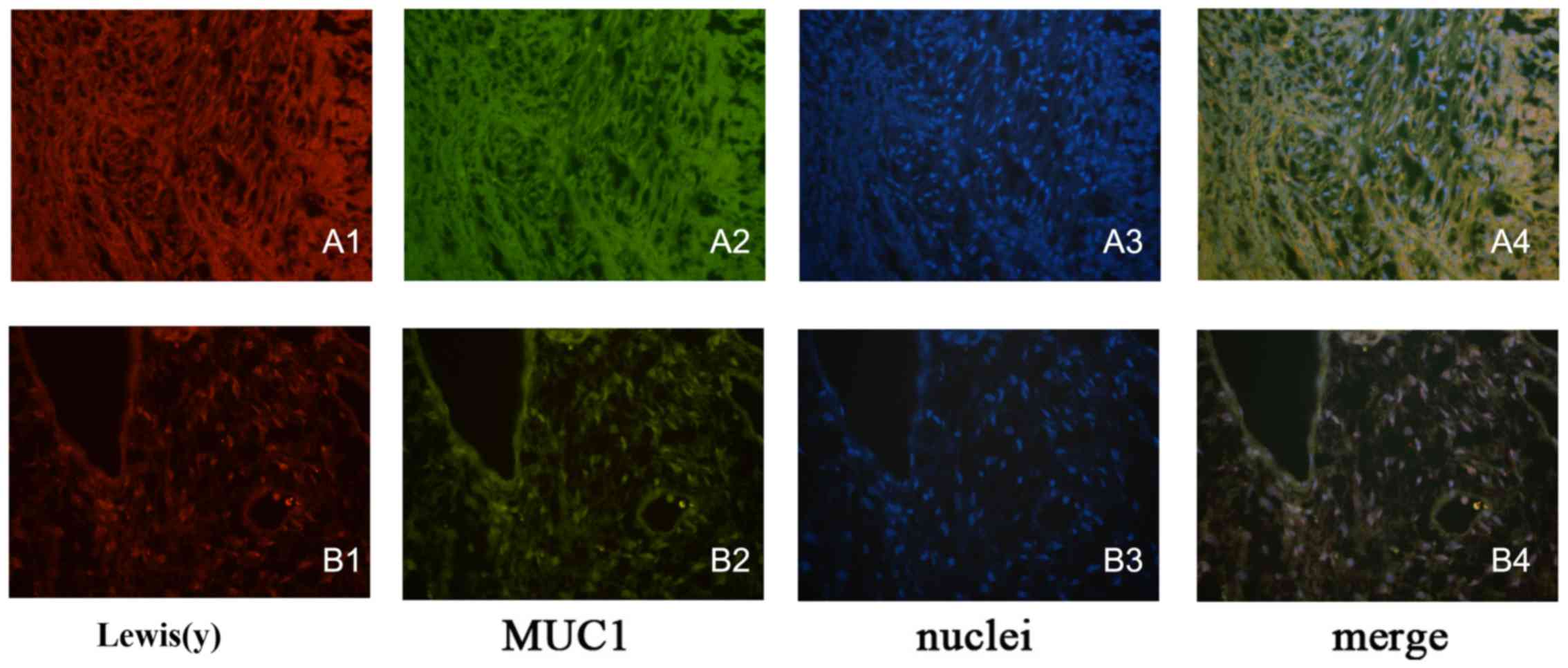
|
1
|
Goupille C, Hallouin F, Meflah K and Le
Pendu J: Increase of rat colon carcinoma cells tumorigenicity by
alpha(1-2)fucosyltransferase gene transfection. Glycobiology.
7:221–229. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hellström I, Garrigues HJ, Garrigues U and
Hellström KE: Highly tumor-reactive, internalizing, mouse
monoclonal antibodies to Le(y)-related cell surface antigens.
Cancer Res. 50:2183–2190. 1990.PubMed/NCBI
|
|
3
|
Madjd Z, Parsons T, Watson NF, Spendlove
I, Ellis I and Durrant LG: High expression of Lewis y/b antigens is
associated with decreased survival in lymph node negative breast
carcinomas. Breast Cancer Res. 7:R780–R787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Z, Wu JH, Kuo HW, Kannagi R and Wu
AM: Expression of sialyl Lex, sialyl Lea, Lex and Ley glycotopes in
secreted human ovarian cyst glycoproteins. Biochimie. 91:423–433.
2009. View Article : Google Scholar
|
|
5
|
Rodríguez-Burford C, Barnes MN, Berry W,
Partridge EE and Grizzle WE: Immunohistochemical expression of
molecular markers in an avian model: A potential model for
preclinical evaluation of agents for ovarian cancer
chemoprevention. Gynecol Oncol. 81:373–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin B, Hao YY, Wang DD, Zhu LC, Zhang SL,
Saito M and Iwamori M: Transfection of alpha1, 2-fucosyltransferase
gene increases the antigenic expression of Lewis y in ovarian
cancer cell line RMG-I. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
30:284–289. 2008.In Chinese. PubMed/NCBI
|
|
7
|
Hao YY, Lin B, Zhao Y, Zhang YH, Li FF,
Diao B, Ou YL and Zhang SL: Alpha1,2-fucosyltransferase gene
transfection influences on biological behavior of ovarian
carcinoma-derived RMG-I cells. Fen Zi Xi Bao Sheng Wu Xue Bao.
41:435–442. 2008.In Chinese.
|
|
8
|
Yin L, Kharbanda S and Kufe D: Mucin 1
oncoprotein blocks hypoxia-inducible factor 1alpha activation in a
survival response to hypoxia. J Biol Chem. 282:257–266. 2007.
View Article : Google Scholar
|
|
9
|
Creaney J, Segal A, Sterrett G, Platten
MA, Baker E, Murch AR, Nowak AK, Robinson BW and Millward MJ:
Overexpression and altered glycosylation of MUC1 in malignant
mesothelioma. Br J Cancer. 98:1562–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Premaratne P, Welén K, Damber JE, Hansson
GC and Bäckström M: O-glycosylation of MUC1 mucin in prostate
cancer and the effects of its expression on tumor growth in a
prostate cancer xenograft model. Tumour Biol. 32:203–213. 2011.
View Article : Google Scholar
|
|
11
|
Iwamori M, Tanaka K, Kubushiro K, Lin B,
Kiguchi K, Ishiwata I, Tsukazaki K and Nozawa S: Alterations in the
glycolipid composition and cellular properties of ovarian
carcinoma-derived RMG-1 cells on transfection of the
alpha1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Engelstaedter V, Fluegel B, Kunze S, Mayr
D, Friese K, Jeschke U and Bergauer F: Expression of the
carbohydrate tumour marker Sialyl Lewis A, Sialyl Lewis X, Lewis Y
and Thomsen-Friedenreich antigen in normal squamous epithelium of
the uterine cervix, cervical dysplasia and cervical cancer. Histol
Histopathol. 27:507–514. 2012.PubMed/NCBI
|
|
14
|
Kim YS, Hwang SY, Kang HY, Sohn H, Oh S,
Kim JY, Yoo JS, Kim YH, Kim CH, Jeon JH, et al: Functional
proteomics study reveals that N-Acetylglucosaminyltransferase V
reinforces the invasive/metastatic potential of colon cancer
through aberrant glycosylation on tissue inhibitor of
metalloproteinase-1. Mol Cell Proteomics. 7:1–14. 2008. View Article : Google Scholar
|
|
15
|
Mejías-Luque R, López-Ferrer A, Garrido M,
Fabra A and de Bolós C: Changes in the invasive and metastatic
capacities of HT-29/M3 cells induced by the expression of
fucosyltransferase 1. Cancer Sci. 98:1000–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chhieng DC, Rodriguez-Burford C, Talley
LI, Sviglin H, Stockard CR, Kleinberg MJ, Barnes MN, Partridge EE,
Khazaeli MB and Grizzle WE: Expression of CEA, Tag-72, and Lewis-Y
antigen in primary and metastatic lesions of ovarian carcinoma. Hum
Pathol. 34:1016–1021. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuemmel A, Single K, Bittinger F, Faldum
A, Schmidt LH, Sebastian M, Taube C, Buhl R and Wiewrodt R: The
prognostic impact of blood group-related antigen Lewis Y and the
ABH blood groups in resected non-small cell lung cancer. Tumour
Biol. 28:340–349. 2007. View Article : Google Scholar
|
|
18
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Liu S, Lin B, Yan L, Wang Y, Wang C
and Zhang S: Expression and correlation of Lewis y antigen and
integrins α5 and β1 in ovarian serous and mucinous carcinoma. Int J
Gynecol Cancer. 20:1482–1489. 2010.PubMed/NCBI
|
|
20
|
Baldus SE, Hanisch FG, Pütz C, Flucke U,
Mönig SP, Schneider PM, Thiele J, Hölscher AH and Dienes HP:
Immunoreactivity of Lewis blood group and mucin peptide core
antigens: Correlations with grade of dysplasia and malignant
transformation in the colorectal adenoma-carcinoma sequence. Histol
Histopathol. 17:191–198. 2002.PubMed/NCBI
|
|
21
|
López-Ferrer A, de Bolós C, Barranco C,
Garrido M, Isern J, Carlstedt I, Reis CA, Torrado J and Real FX:
Role of fucosyltransferases in the association between apomucin and
Lewis antigen expression in normal and malignant gastric
epithelium. Gut. 47:349–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YS, Yuan M, Itzkowitz SH, Sun QB,
Kaizu T, Palekar A, Trump BF and Hakomori S: Expression of LeY and
extended LeY blood group-related antigens in human malignant,
premalignant, and nonmalignant colonic tissues. Cancer Res.
46:5985–5992. 1986.PubMed/NCBI
|
|
23
|
Kuo CH, Chen PK, Chang BI, Sung MC, Shi
CS, Lee JS, Chang CF, Shi GY and Wu HL: The recombinant lectin-like
domain of thrombomodulin inhibits angiogenesis through inter-action
with Lewis Y antigen. Blood. 119:1302–1313. 2012. View Article : Google Scholar
|
|
24
|
Wang C, Yan L, Wang Y, Lin B, Liu S, Li Q,
Gao L, Zhang S and Iwamori M: Overexpression of Lewis(y) antigen
protects ovarian cancer RMG-1 cells from carboplatin-induced
apoptosis by the upregulation of Topo-I and Topo-II β. Anat Rec
(Hoboken). 294. pp. 961–969. 2011, View
Article : Google Scholar
|
|
25
|
Kawano T, Ahmad R, Nogi H, Agata N,
Anderson K and Kufe D: MUC1 oncoprotein promotes growth and
survival of human multiple myeloma cells. Int J Oncol. 33:153–159.
2008.PubMed/NCBI
|
|
26
|
Kufe DW: Functional targeting of the MUC1
oncogene in human cancers. Cancer Biol Ther. 8:1197–1203. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pająk J, Liszka L, Mrowiec S, Gołka D and
Lampe P: MUC1 immunoexpression is a virtually constant feature of
clear cell renal cell carcinoma metastatic to the pancreas. Adv
Anat Pathol. 19:125–127. 2012. View Article : Google Scholar
|
|
28
|
Khodarev NN, Pitroda SP, Beckett MA,
MacDermed DM, Huang L, Kufe DW and Weichselbaum RR: MUC1-induced
transcriptional programs associated with tumorigenesis predict
outcome in breast and lung cancer. Cancer Res. 69:2833–2837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blixt O, Bueti D, Burford B, Allen D,
Julien S, Hollingsworth M, Gammerman A, Fentiman I,
Taylor-Papadimitriou J and Burchell JM: Autoantibodies to
aberrantly glycosylated MUC1 in early stage breast cancer are
associated with a better prognosis. Breast Cancer Res. 13:R252011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng H, Ghazizadeh M, Konishi H and Araki
T: Expression of MUC1 and MUC2 mucin gene products in human ovarian
carcinomas. Jpn J Clin Oncol. 32:525–529. 2002. View Article : Google Scholar
|
|
31
|
Li FF, Liu JJ, Liu DW, Lin B, Hao YY, Cong
JP, Zhu LC, Gao S, Zhang SL and Iwamori M: Lewis Y regulates
signaling molecules of the transforming growth factor β pathway in
ovarian carcinoma-derived RMG-I cells. Int J Oncol. 40:1196–1202.
2012.
|
|
32
|
Rong Y, Jin D, Wu W, Lou W, Wang D, Kuang
T, Ni X and Qin X: Induction of protective and therapeutic
anti-pancreatic cancer immunity using a reconstructed MUC1 DNA
vaccine. BMC Cancer. 9:1912009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Q, Guo X, Nash GB, Stone PC, Hilkens
J, Rhodes JM and Yu LG: Circulating galectin-3 promotes metastasis
by modifying MUC1 localization on cancer cell surface. Cancer Res.
69:6799–6806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hattrup CL and Gendler SJ: Structure and
function of the cell surface (tethered) mucins. Annu Rev Physiol.
70:431–457. 2008. View Article : Google Scholar
|