Introduction
In recent years, accumulating evidence in both the
clinic and in experimental animal models have shown that
microinflammation characterized by inflammatory cell proliferation,
inflammatory cytokine overexpression and subsequent extracellular
matrix (ECM) expansion in glomeruli is a common pathway for the
progression of diabetic nephropathy (DN). Anti-microinflammatory
strategies may thus offer approaches of great interest for early DN
patients (1,2). Utimura et al (3) reported that mycophenolate mofetil
(MMF), an anti-inflammatory drug, inhibited glomerular macrophage
infiltration and improved glomerulosclerosis (GS) in streptozotocin
(STZ)-induced and uninephrectomized diabetic rats. Correspondingly,
pentoxifylline (PTF), which possesses significant anti-inflammatory
properties, has recently been proven to reduce urinary protein
excretion in diabetic subjects, both with normal renal function and
with renal insufficiency. Moreover, these beneficial effects are
related to a reduction in the concentration of tumor necrosis
factor (TNF)-α, one of the most important pro-inflammatory
cytokines (4,5). Therefore, these findings also
indicate that inhibition of microinflammation may be a therapeutic
target and a pharmacological mechanism for protecting glomerular
lesions in DN.
It is well-known that microinflammation is regulated
by inflammation-associated signaling pathways. The p38
mitogen-activated protein kinase (MAPK) signaling pathway becomes
activated by inflammation stress-related signals including
pro-inflammatory cytokines such as TNF-α, interleukin (IL)-1, IL-6
and IL-18 through phosphorylation mediated by upstream kinases. In
turn, phosphorylated p38 MAPK (p-p38 MAPK), as an important active
p38 MAPK signaling molecule, enters the nucleus, as well as
controls the expression of a variety of downstream inflammatory
factors and inflammatory mediators, which lead to the promotion of
renal inflammatory response that exacerbates glomerular damage
(2,6). For these reasons, by modulating the
expression of p-p38 MAPK, transforming growth factor (TGF)-β and
nuclear factor (NF)-κB, it is possible to block inflammatory
signaling pathway reactions and ameliorate relevant inflammatory
injuries in the kidney.
Multi-glycoside of Tripterygium wilfordii
Hook. f. (GTW) is a stable glycoside extracted from Tripterygium
wilfordii Hook. f. (TWHF), also known as 'Lei Gong Teng' (the
local name in China), which is a natural anti-inflammatory
phytomedicine used for various autoimmune and inflammatory diseases
in Eastern and Southern China, America, Korea and Japan (7–9).
GTW, as a Chinese traditional patented medicine, has been approved
by the China State Food and Drug Administration (Z32021007) for the
routine treatment of glomerulonephritis and rheumatoid arthritis
(10,11). For the past 30 years, GTW at the
dose of 60 mg/day has been proven to be clinically effective in
reducing proteinuria and hematuria by suppressing renal
microinflammation, GS and podocyte injury in several types of human
chronic kidney disease (CKD) (12–14). In addition, a single central study
in China found that a large oral dose of GTW (120 mg/day) reduced
proteinuria and alleviated renal dysfunction in 65 DN patients at
early and mid-term stages (15).
More importantly, the therapeutic mechanisms of GTW and its main
bioactive component, triptolide, may act by reducing macrophage
proliferation in glomeruli (16–18). Despite this, its pharmacological
mechanistic link in vivo between anti-microinflammatory
effects and the protection against GS related to diabetes remains
to be elucidated.
Hence, in this study, we examined the ameliorative
effects of GTW on GS, using a modified STZ-induced DN rat model. We
then clarified the anti-microinflammatory mechanisms in vivo
of GTW by inhibiting the activation of inflammatory signaling
pathways and the overexpression of inflammatory factors in the
kidney. These results may provide a novel and effective therapeutic
method for early stage DN patients.
Materials and methods
GTW quantity control
GTW purchased from Jiangsu Meitong Pharmaceutical
Co., Ltd. (Taizhou, China) was composed of extracts from TWHF. One
tablet contained 10 mg of GTW. The extraction method and productive
process of GTW are both subjected to strict quality control, and
the main components are subjected to standardization (19). In addition, GTW is not only
manufactured as granules after dynamic cycle extraction and
concentration by evaporating and spray drying, but is also
monitored for the absence of contaminants (heavy metals,
pesticides, hormone and mycotoxins) prior to formulation. In this
study, GTW (batch no. 150206) was dissolved in distilled water (GTW
suspension) and stored at 4°C before use.
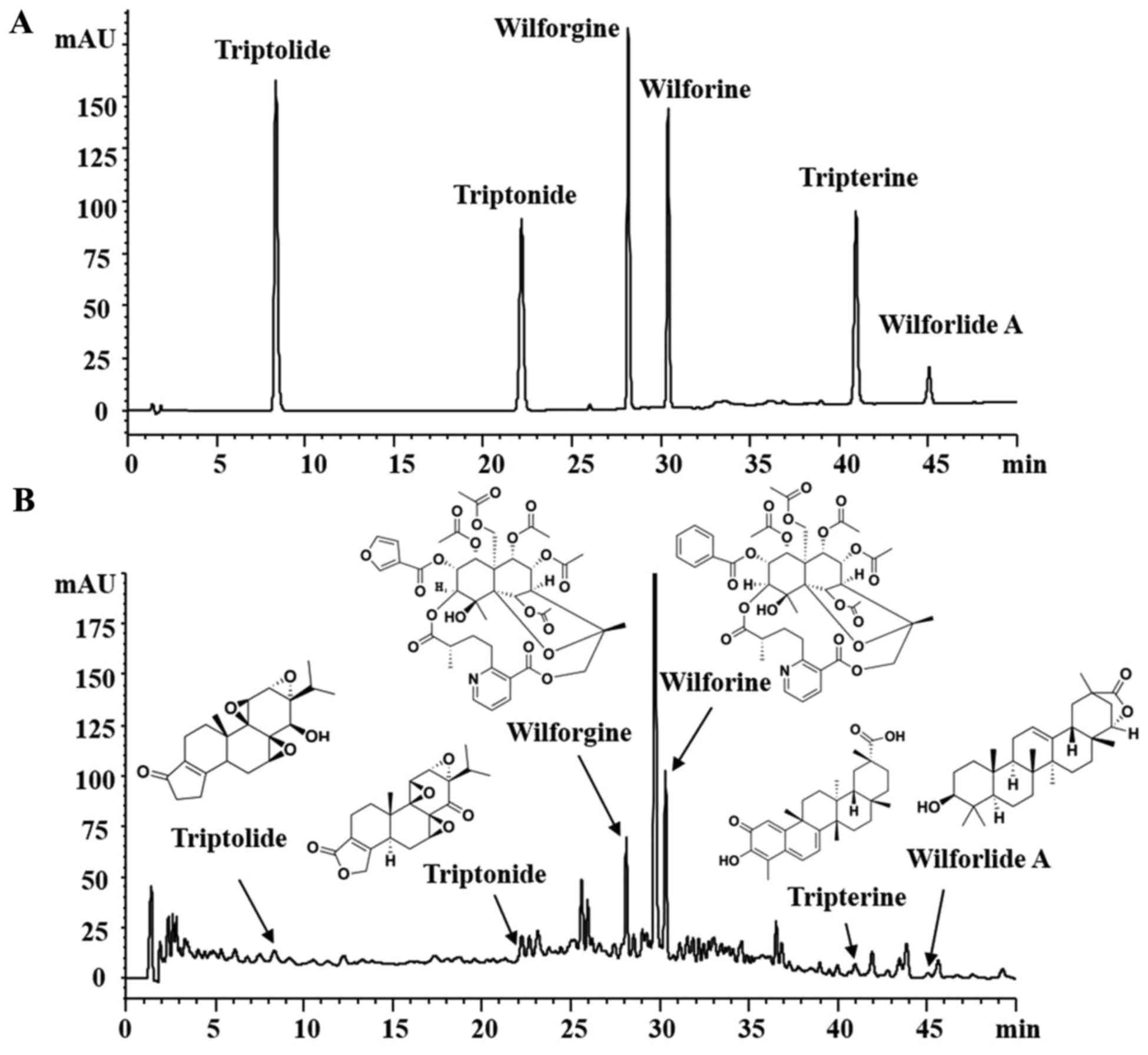
The quality of GTW was examined with fingerprint
analysis by high-performance liquid chromatography (HPLC) in
Jiangsu Province Academy of Chinese Medicine (Nanjing, China). As
shown in Fig. 1, the known
bioactive components including triptolide
(C20H24O6; CAS, 38748-32-2),
triptonide (C20H22O6; CAS,
38647-11-9), wilforgine
(C41H47NO19; CAS, 37239-47-7),
wilforine (C43H49O18; CAS,
11088-09-8), tripterine (C29H38O4;
CAS, 34157-83-0) and wilforlide A
(C30H46O3; CAS, 84104-71-2)
(20,21) (Fig.
1A) in 5 batches exhibited high stability.
Animals, drugs and reagents
All experiments were performed using male
Sprague-Dawley (SD) rats weighing from 180 to 200 g, purchased from
the Animal Center of Nanjing Military District General Hospital
(Nanjing, China). The surgical procedures and experimental protocol
were approved by the Animal Ethics Committee of Nanjing University
Medical School. STZ was purchased from Sigma-Aldrich (St. Louis,
MO, USA). Antibodies against TGF-β1, TNF-α, IL-1β and horseradish
peroxidase (HRP)-labeled IgG were purchased from Abcam (Cambridge,
UK). Antibodies against p38 MAPK, p-p38 MAPK, p-IκB and NF-κB (p65)
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). The antibody against glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was obtained from Bioworld Technology, Inc. (Louis Park,
MN, USA).
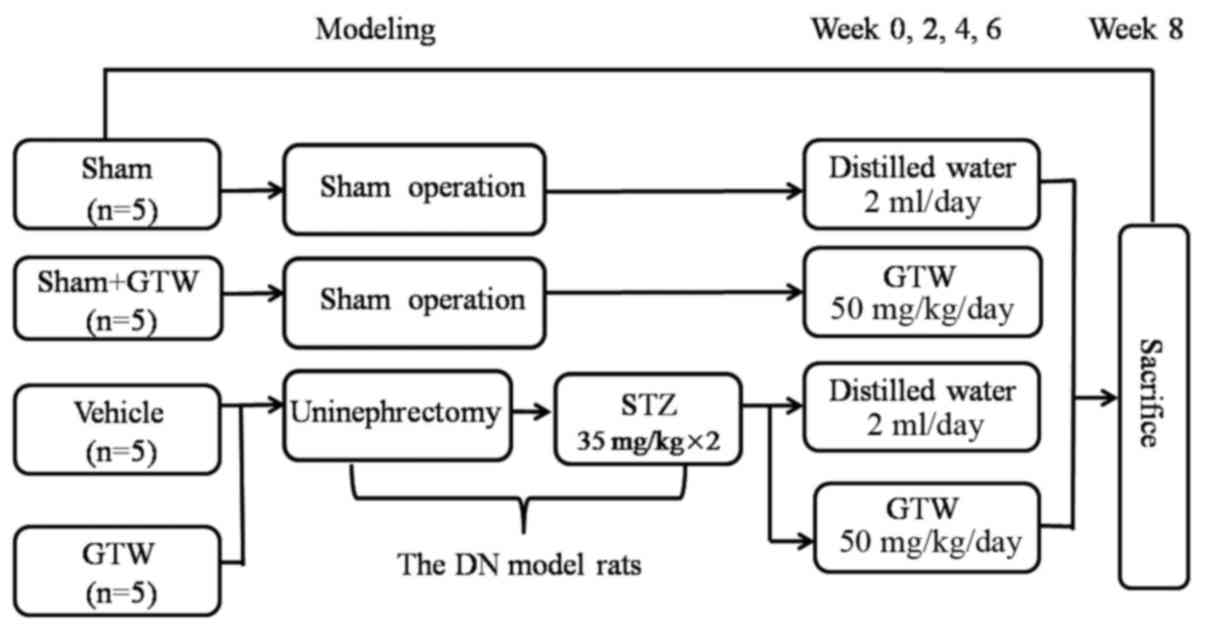
Experimental protocol
The experimental procedure is illustrated in
Fig. 2. The DN model was
established as described in our previous study (22). Twenty rats were divided into 4
groups: the sham group (sham operation + distilled water), the sham
+ GTW group (sham operation + GTW), the vehicle group (DN +
distilled water) and the GTW group (DN + GTW). In the clinic, GTW
at a dose of 60 mg/day is used to treat a patient weighing 60 kg
(11,15), which is equivalent to 50 mg/kg/day
in rats. Following the second injection of STZ, the GTW suspension
was administered to the rats in the 2 GTW-treated groups by gastric
gavage once a day for 8 weeks, while rats in the vehicle and sham
group were treated with 2 ml distilled water. Eight weeks after
administration, all rats were anesthetized and sacrificed through
cardiac puncture. Blood samples and kidneys were collected for
detection of various indicators.
General status and biochemical parameters
of the rats
Energy level, diet, water intake, fur color and
activities of the rats in each group were observed daily. Body
weight (BW), blood glucose (BG) and urinary albumin (UAlb) of the
rats were detected respectively before and every 2 weeks after
modeling. The right kidney of rats in each group was removed and
weighed after cardiac puncture. At the end of week 8 after
drug-intervention, the rats were anesthetized and blood samples (2
ml) were drawn from the heart. Indices including serum creatinine
(Scr), blood urea nitrogen (BUN), serum alanine transaminase (ALT)
and serum aspartate transaminase (AST) were detected.
Renal histomorphometry
Periodic acid-Schiff (PAS) staining, Masson staining
and electron microscopic assessment were performed as previously
described (22). Cell numbers,
ECM and collagen rates in the glomerulus were calculated with
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). The results were confirmed by a professional
pathologist.
Immunohistochemistry
Macrophages were detected in 4-µm thick
paraffin-embedded renal sections. For immunostaining of
macrophages, monoclonal anti-ED1 antibody (AbD Serotec, Oxford, UK)
was used. Quantitative analysis of ED1+ cells was
performed in a blinded fashion and expressed as cells/glomerular
cross section (gcs). The results were also confirmed by the
pathologist professional.
Semi-quantitative western blot
analysis
Western blot analysis was performed as previously
described (22). Renal tissues
from the rats were isolated with phosphate-buffered saline (PBS)
including protease inhibitors (PI) and sequentially solubilized
with 1% Triton X-100, RIPA buffer (0.1% SDS, 1% sodium
deoxycholate, 1% Triton X-100, 0.15 mol/l NaCl and 0.01 mol/l
ethylenediaminetetraacetic acid (EDTA) in 0.025 mol/l Tris-HCl, pH
7.2) with PI, and separated into Triton X-100-soluble (T),
RIPA-soluble (R) and RIPA-insoluble (S) fractions. The
RIPAI-insoluble fraction was solubilized with SDS-PAGE sample
buffer (2% SDS, 10% glycerol, and 5% 2-mercaproethanol in 0.0625
mol/l Tris-HCl, pH 6.8) (Sfractions). Equal amounts of theses
equentially solubilized fractions were subjected to SDS-PAGE with
7.5 or 10% acrylamide gel, and transferred onto a PVDF membrane
(Bio-Rad, Hercules, CA, USA) by electrophoretic trans-blotting for
30 min using Trans-Blot SD (Bio-Rad). After blocking with BSA, the
strips of membrane were exposed to anti-p38 MAPK, p-p38 MAPK, IκB,
p-IκB, NF-κB (p65), TGF-β1, TNF-α, IL-1β and GAPDH antibodies,
respectively. They were washed and incubated with
peroxidase-conjugated secondary antibodies for 1 h at room
temperature. The bands were visualized by employing analkaline
phosphatase chromogen kit (5-bromo-4-chloro-3-indolil phosphate
p-toluidine salt/nitro blue tetrazolium; Biosynth AG, Staad,
Switzerland). The density of the positive bands was quantitated by
Densitograph (ATTO, Tokyo, Japan). This procedure was carried out 3
times. The ratio of the densitometric signal of the molecules
examined to that of GAPDH was determined. Data are shown as ratios
relative to control findings and expressed as mean ± SE. of 3
independent experiments.
Statistical analysis
The differences among groups were analyzed by
one-way analysis of variance (ANOVA), and LSD method was used for
multiple comparison. Qualitative data were analyzed using Fisher's
exact test as indicated. P<0.05 was considered statistically
significant.
Results
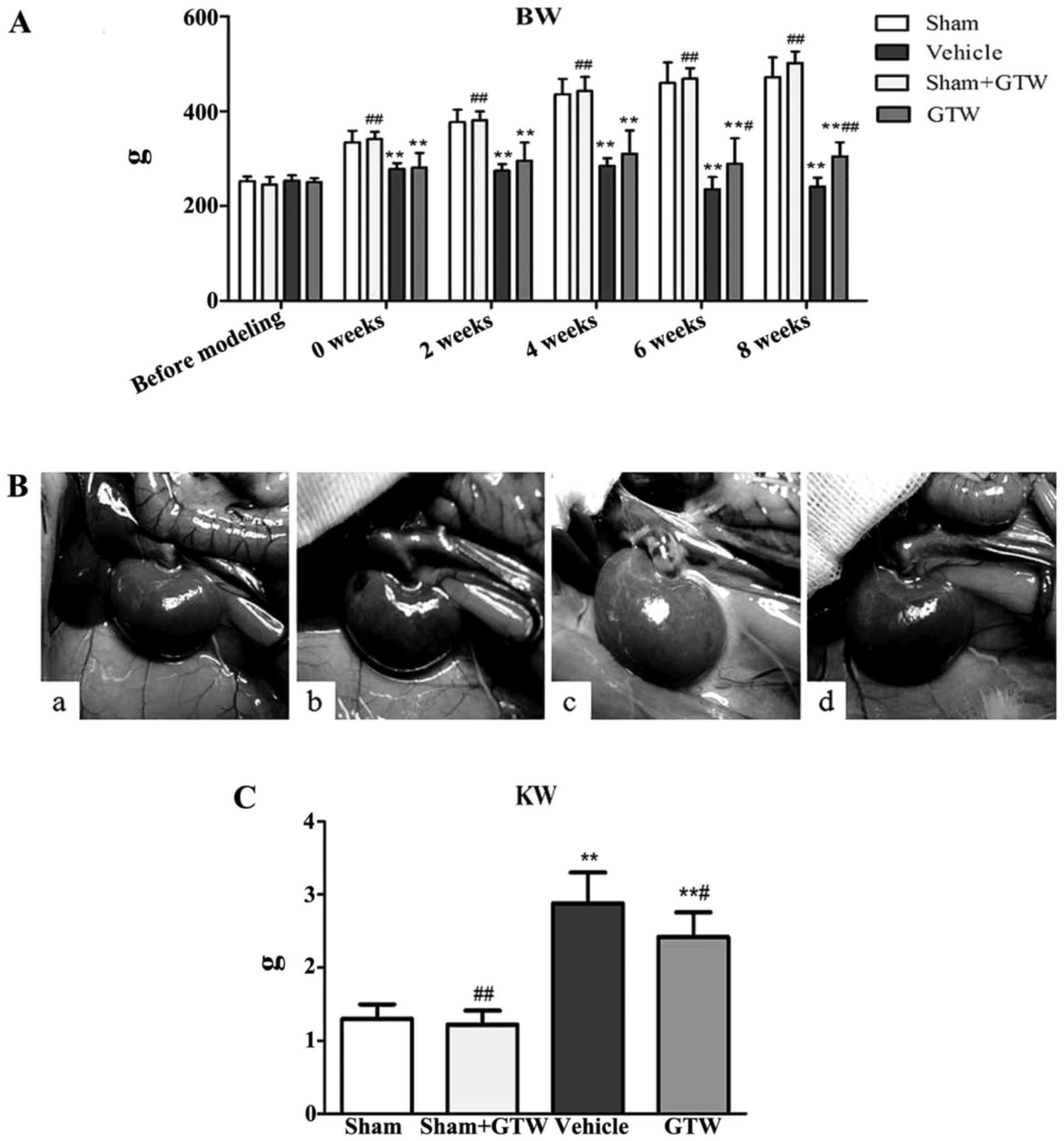
GTW improves the general condition and
biochemical parameters of the rats, but does not lower BG
During the experiment, the rats in the vehicle group
showed increased diet, water intake and urine volume, low activity,
dull fur and BW loss at different degrees. BW of rats in the
vehicle group increased slowly from week 2. After GTW-intervention,
BW of rats in the GTW group increased gradually. At the end of week
6 and 8, BW of rats in the GTW group was obviously higher than that
in the vehicle group, and the differences were statistically
significant (Fig. 3A). After
sacrifice, we found that renal appearance in the sham and sham +
GTW groups was moderated and crimson, while the kidneys in the
vehicle group were swollen and pale. The kidneys of rats treated
with GTW were significantly ameliorated, with less swelling and
ischemia (Fig. 3B). In addition,
kidney weight (KW) of rats in the vehicle group was obviously
higher than that in the sham group. While KW of rats in the GTW
group declined, and the differences were statistically significant
compared with the vehicle group, but they were still higher than
that in the sham group (Fig.
3C).
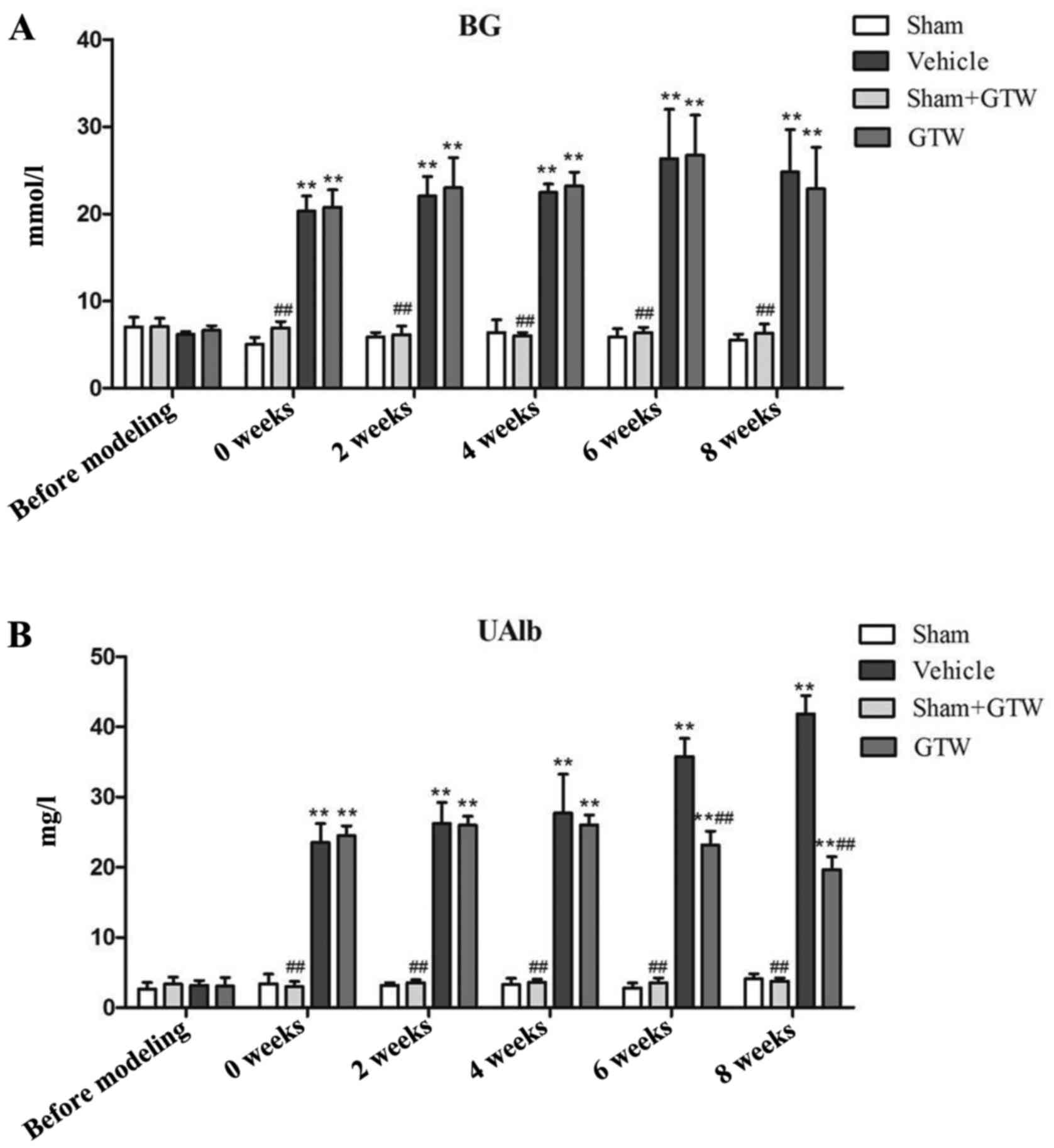
Next, we investigated the effects of GTW on BG and
UAlb in the 4 rat groups. During the entire course, BG of rats in
the sham and sham + GTW groups remained at low levels, while it
increased in the rats of the vehicle group after two injections of
STZ for 72 h, and remained at a high level (random BG, 20.3±1.72
mmol/l) by the consecutive intervention with Novolin N. Moreover,
notably, BG of rats in the GTW group also remained high, and the
differences were statistically significant compared with the sham
group (Fig. 4A). The level of
UAlb in each group was low before modeling, but rapidly increased 2
weeks after modeling, and constantly increased with the extension
of time in the vehicle group. At the end of week 8, UAlb in the
vehicle group reached an abnormal level (41.82±2.61 mg/l), which
was significantly higher than that in the sham and sham + GTW
groups. After GTW-intervention, the level of UAlb was significantly
decreased in the GTW group at week 8, and the difference was
statistically significant compared with the vehicle group (Fig. 4B).
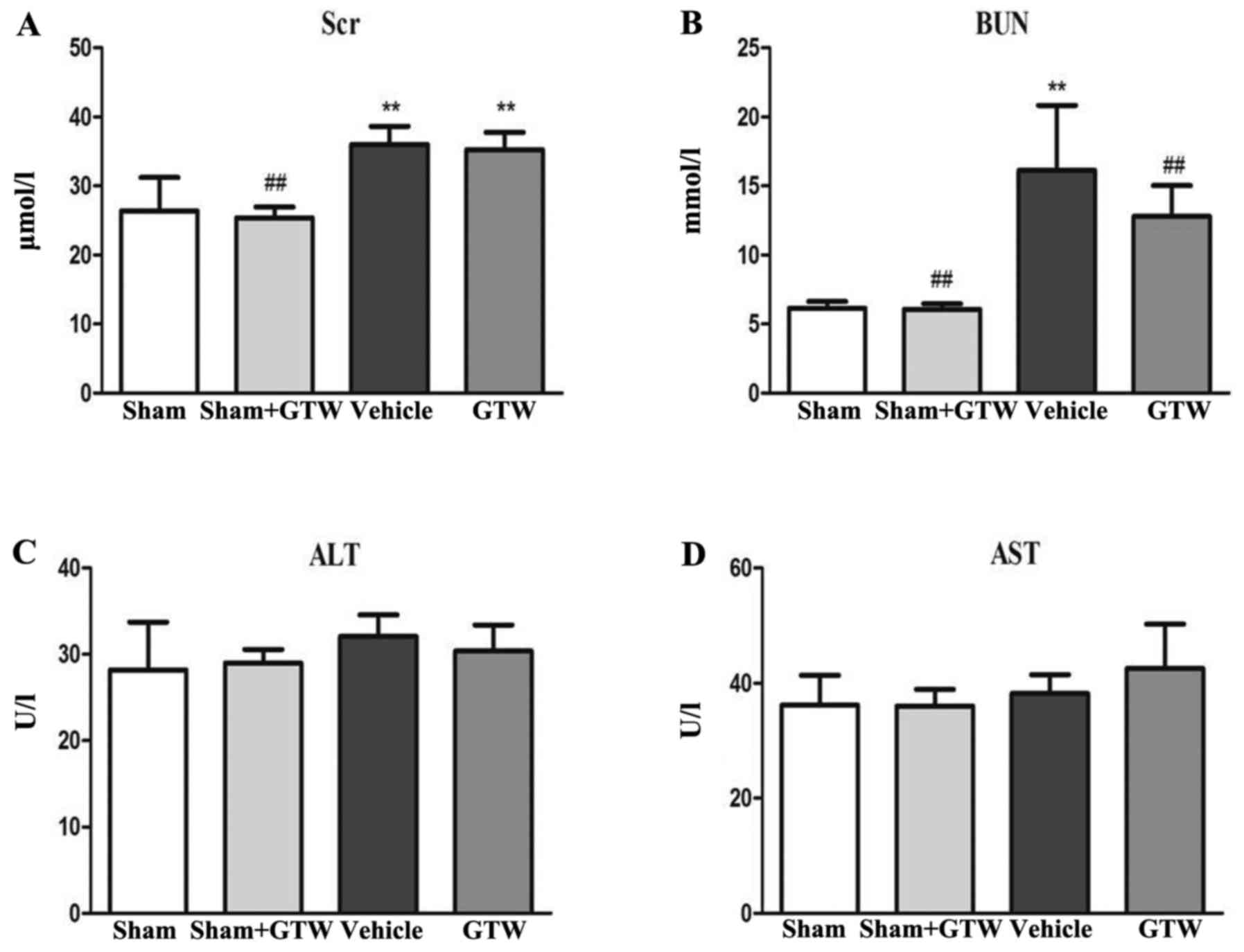
Thirdly, we examined the effects of GTW on blood
biochemical parameters including Scr, BUN, ALT and AST in the 4 rat
groups. At the end of week 8 after GTW-intervention, serum ALT and
AST in each group had no obvious changes, but Scr and BUN in the
vehicle group were increased, and the differences were
statistically significant respectively compared with the sham and
sham + GTW groups. After GTW treatment, the levels of Scr and BUN
were decreased, but the differences were not statistically
significant compared with the vehicle group (Fig. 5).
GTW attenuates GS
We observed glomerular morphological changes by
light microscopy in the 4 rat groups. In the sham and sham + GTW
groups, glomerular capillary loops were well-opened and tubular
epithelial cells were arranged in order. Mesangial tissues had
hyperplasia in the vehicle group including glomerular
mild-hypertrophy, capillary loop area reduction, glomerular cell
proliferation, ECM expansion and collagen deposition. With GTW
treatment, the injurious glomerular morphological changes were
obviously attenuated. When compared with the sham group, cell
numbers, rate of ECM and collagen area in glomerulus were
significantly increased in the vehicle group respectively, but
decreased in the GTW group. However, no significant differences in
glomerular morphological changes were found between the sham and
sham + GTW groups (Fig. 6A–E). We
measured podocytic morphological characteristics and glomerular
basement membrane (GBM) thickness by electron microscopy in the 4
rat groups. Unexpectedly, the injurious morphological changes in
podocytes and GBM including foot process effacement and GBM
thicking in the vehicle group were significantly alleviated in the
GTW group (Fig. 6F).
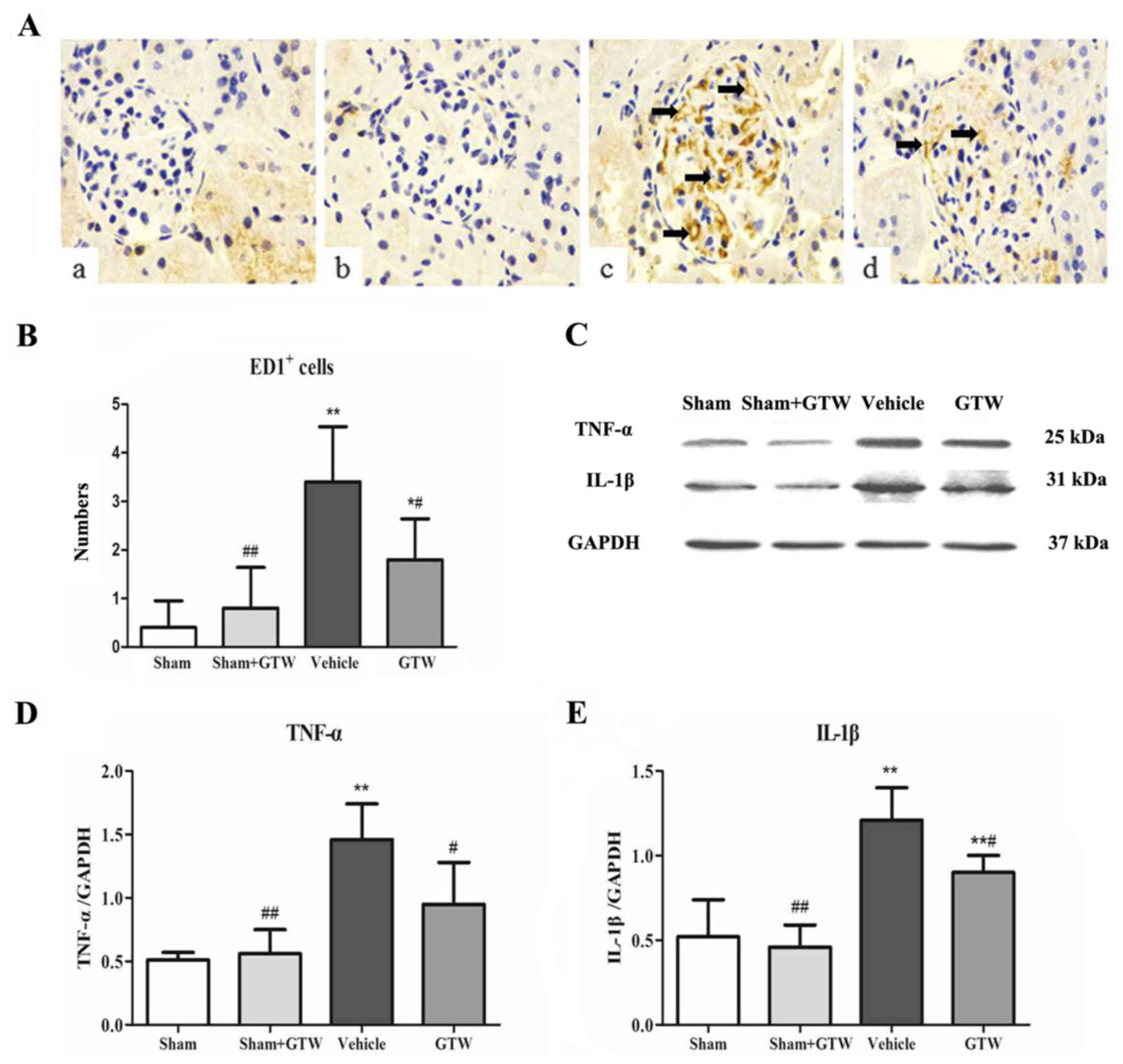
GTW suppresses glomerular
microinflammation
We determined the number of ED1+ cells, a
marker of total macrophages in glomeruli. By means of
immunohistochemical staining, the number of ED1+ cells
in glomeruli was increased notably in the vehicle group, while the
number declined significantly in the GTW group (Fig. 7A and B). No obvious infiltrated
macrophages in the glomeruli were detected in the sham and sham +
GTW groups. We further investigated the protein expression of
inflammatory cytokines in the kidney including TNF-α and IL-1β in 4
rat groups. Western blot analysis of renal tissues revealed that
the protein expression of TNF-α and IL-1β was markedly upregulated
in the vehicle group. Compared with the vehicle group, the protein
overexpression of TNF-α and IL-1β in the kidney was significantly
reduced in the GTW group (Fig.
7C–E).
GTW inhibits activation of p38 MAPK and
canonical NF-κB signaling pathways in the kidney
Semi-quantitative western blot analysis showed that
the expression level of p-p38 MAPK, p-IκB, NF-κB (p65) and TGF-β1
at the protein levels in the vehicle group was significantly higher
than those in the sham group. Compared with the vehicle group, the
protein overexpression of p-p38 MAPK, p-IκB, NF-κB (p65) and TGF-β1
was significantly reduced in the GTW group (Fig. 8).
 | Figure 8Effects of Tripterygium
wilfordii Hook. f. (GTW) on the protein expression of p-p38
mitogen-activated protein kinase (MAPK), p38 MAPK, p-IκB, IκB,
nuclear factor (NF)-κB (p65) and transforming growth factor
(TGF)-β1 in the kidney in the 4 rat groups. (A) Western blot
analysis of p-p38 MAPK, p38 MAPK, p-IκB, IκB, NF-κB (p65) and
TGF-β1 protein expression. (B–E) Quantitative analysis of p-p38
MAPK, p-IκB, NF-κB (p65) and TGF-β1 protein expression. Data are
expressed as mean ± SE. *P<0.05 vs. the sham group;
**P<0.01 vs. the sham group; ##P<0.01
vs. the vehicle group at week 8 after drug-intervention. |
Discussion
In the present study, we demonstrated that GTW, a
natural anti-inflammatory phytomedicine, can improve GS and
glomerular microinflammation in vivo. Nevertheless, GTW had
no effect on hyperglycemia in these DN model rats. To clarify the
therapeutic effects of phytomedicines on renal damage related with
diabetes in vivo, it is necessary to establish an
appropriate animal model that simulates human DN patients. In this
study, we used a modified DN rat model by unilateral nephrectomy
combined with STZ intraperitoneal injections with low doses of 35
mg/kg BW at a 72-h interval. Our results showed that these DN model
rats not only had stable hyperglycemia and a certain degree of
UAlb, but also had typical GS characteristics. Therefore, we
believed that this DN rat model was conducive to discovering novel
therapeutic phytomedicines for human DN.
It has been reported that microinflammation plays a
pivotal role in promoting glomerular injury in several studies of
human and experimental DN induced by STZ injection (23). During microinflammation in renal
parenchyma, macrophages can be attracted/activated directly by
mechanical stress and may produce pro-inflammatory cytokines such
as TNF-α, IL-1, IL-6 and IL-18, thus recruiting additional
inflammatory cells that contribute to the propagation of
inflammation and GS (24). Hence,
to investigate whether the anti-microinflammatory effects of GTW
could be related to the improvement of GS in vivo, we
examined the number of infiltrated ED1+ cells in
glomeruli, a marker of total macrophages, and the protein
expression of TNF-α and IL-1β in the kidney, representative
inflammatory cytokines, at the end of week 8 after two STZ
injections in the GTW and vehicle groups, respectively. Our results
showed that macrophage infiltration, affecting almost exclusively
glomeruli, was evident at the end of week 8 after modeling in the
vehicle group. Furthermore, the intensity of macrophage
infiltration in glomeruli and the levels of inflammatory cytokine
expression in the kidney were strongly correlated with the
injurious characteristics of GS in these DN model rats. In addition
to these, GTW suppressed glomerular microinflammation together with
the amelioration of GS. Consequently, GTW, as an anti-inflammatory
phytomedicine, can alleviate GS in vivo, which is possibly
connected with anti-microinflammatory action.
To the best of our knowledge, the therapeutic action
in DN animal models of various anti-inflammatory agents such as
thiazolidinediones, 1,25-dihydroxyvitamin D3,
cilostazol, curcumin (23) and
Huangkui capsule (22) are
directly related to controlling activation of intracellular
signaling pathways associated with renal microinflammation, in
which p38 MAPK, Janus kinase/signal transducers and activators of
transcription (JAK/STAT) and NF-κB-dependent pathways occupy the
key positions in DN at the early stage. Thus, we proposed that
regulating the activation of p38 MAPK and/or canonical NF-κB
pathways in this DN rat model are the successful means with which
to identify anti-inflammatory mechanisms of GTW on attenuating GS
in vivo. Our data clearly indicated that enhanced protein
expression of p-p38 MAPK, p-IκB, NF-κB (p65) and TGF-β1 in the
kidney were obviously detected in these DN model rats, concomitant
with deterioration of GS induced by microinflammation. These
results indicated that the p38 MAPK and canonical NF-κB pathways
were activated in this DN rat model, and there is a strong
causality between key signaling molecular overexpression and GS
in vivo. More importantly, we also found that GTW
simultaneously downregulated the protein overexpression of p-p38
MAPK, p-IκB, NF-κB (p65) and TGF-β1 in the kidney of these DN model
rats. For these reasons, we suggest that GTW in vivo can
attenuate GS by inhibiting p38 MAPK pathway activation and the
release of NF-κB dimer p50/p65 in the canonical NF-κB pathway.
Here, unfortunately, we could not assess whether GTW in vivo
directly blocks the p38 MAPK signaling pathway based upon this DN
rat model, without the signaling inhibitors. Further detailed
analyses in vitro of p38 MAPK signaling molecules are needed
to address this hypothesis.
Finally, we need to discuss three additional points.
First, GTW, a natural anti-inflammatory phytomedicine, did not
affect hyperglycemia in this DN rat model induced by STZ injection.
We unavoidably think of the cause-and-effect of the relationship
among hyperglycemia, microinflammation and GS. Some studies have
shown that activation of the immune system and microinflammation
are both involved in the pathogenesis of GS in DN and of course
play an important role in vivo (25). Thus, we firmly believe that GTW
has renoprotective action similar to other CKD models such as the
anti-Thy1.1 glomerulonephritis rat model (26) and the adriamycin-induced GS rat
model (27), completely
independent of affecting hyperglycemia. Second, GTW has been proven
clinically effective in suppressing microinflammation in various
CKDs; however, its clinical application is often limited by side
effects to the liver (28). To
exclude the side effects of GTW on hepatic damage in this DN rat
model, we compared the levels of serum ALT and AST in the 4 rat
groups. Our results revealed that serum ALT and AST in the groups
had no obvious changes, especially between the sham and sham + GTW
groups (Fig. 5), thereby indicate
that GTW at the suitable dose of 50 mg/kg BW daily had no negative
effect on liver function in these DN model rats. Third, although
GTW affected the activation of the p38 MAPK and canonical NF-κB
pathways in vivo, we could not draw a clear conclusion
regarding the key molecular mechanisms of p38 MAPK and NF-κB
pathways in this DN rat model. It may be worth pursing whether p38
MAPK at the upstream controls NF-κB transcriptional activation both
in vivo and in vitro since NF-κB is activated by a
wide variety of stimuli such as cytokines, oxygen radicals,
bacterial products and metabolic abnormalities, which are related
with p38 MAPK activation (29). A
diabetic knockout mouse (30) and
a special test of NF-κB (p65) DNA-binding activity (31) in the kidney should be necessary in
future studies to clarify this point.
In conclusion, in the present study, we demonstrated
that GTW, as a natural regulator in vivo, alleviated GS
without affecting hyperglycemia using modified DN model rats, by
exerting anti-microinflammatory effects, including reducing
macrophage infiltration in glomeruli, suppressing TNF-α, IL-1β and
TGF-β1 overexpression in the kidney and inhibiting p38 MAPK and
NF-κB signaling activities. To the best of our knowledge, the
findings of this study provide the first evidence in vivo
that GTW directly contributes to the prevention of DN by exerting
anti-microinflammatory effects.
Acknowledgments
This study was supported by two grants from the
National Natural Science Foundation of China (81374030 and
81573903) (to Y.-G.W.) and a grant from Nanjing Medical Science and
Technique Development Foundation (to W.W.). The authors thank Dr
Xun-Yang Luo and Dr Le Zhang (Department of Laboratory Medicine,
Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing
University Medicine School, Nanjing, China) for their technical
assistance and instructions. The authors also thank Professor Jian
Yao (Division of Molecular Signaling, Department of Advanced
Biomedical Research, Interdisciplinary Graduate School of Medicine
and Engineering, University of Yamanashi, Yamanashi, Japan) and
Professor Yan Chen (Key Laboratory of New Drug Delivery System of
Chinese Materia Medica, Jiangsu Provincial Academy of Chinese
Medicine, Nanjing, China) for their helpful discussions and
technical assistance.
References
|
1
|
Agrawal NK and Kant S: Targeting
inflammation in diabetes: newer therapeutic options. World J
Diabetes. 5:697–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Navarro-González JF, Mora-Fernández C,
Muros de Fuentes M and García-Pérez J: Inflammatory molecules and
pathways in the pathogenesis of diabetic nephropathy. Nat Rev
Nephrol. 7:327–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utimura R, Fujihara CK, Mattar AL,
Malheiros DM, Noronha IL and Zatz R: Mycophenolate mofetil prevents
the development of glomerular injury in experimental diabetes.
Kidney Int. 63:209–216. 2003. View Article : Google Scholar
|
|
4
|
Navarro JF, Mora C, Muros M and García J:
Additive antiproteinuric effect of pentoxifylline in patients with
type 2 diabetes under angiotensin II receptor blockade: a
short-term, randomized, controlled trial. J Am Soc Nephrol.
16:2119–2126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navarro-González JF, Mora-Fernández C,
Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del
Castillo N, Rivero A, Getino MA, et al: Effect of pentoxifylline on
renal function and urinary albumin excretion in patients with
diabetic kidney disease: The PREDIAN trial. J Am Soc Nephrol.
26:220–229. 2015. View Article : Google Scholar :
|
|
6
|
Schieven GL: The p38alpha kinase plays a
central role in inflammation. Curr Top Med Chem. 9:1038–1048. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brinker AM, Ma J, Lipsky PE and Raskin I:
Medicinal chemistry and pharmacology of genus Tripterygium
(Celastraceae). Phytochemistry. 68:732–766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marks WH: Tripterygium wilfordii Hook F.
versus sulfasalazine in the treatment of rheumatoid arthritis: a
well-designed clinical trial of a botanical demonstrating
effectiveness. Fitoterapia. 82:85–87. 2011. View Article : Google Scholar
|
|
9
|
Wan YG, Gu LB and Shimizu F: Mechanism of
protective effects of effective components in Ttipterygium
wilfordii Hook. f. on glomerulonephritis. Int J Clin Exp Med.
207:285–288. 2003.In Japanese.
|
|
10
|
Jiang M, Zha Q, Zhang C, Lu C, Yan X, Zhu
W, Liu W, Tu S, Hou L, Wang C, et al: Predicting and verifying
outcome of Tripterygium wilfordii Hook F. based therapy in
rheumatoid arthritis: from open to double-blinded randomized trial.
Sci Rep. 5:97002015. View Article : Google Scholar
|
|
11
|
Leishi L: Clinical study of Tripterygium
wilfordii Hook in treating glomerulonephritis (author's transl).
Zhonghua Nei Ke Za Zhi. 20:216–220. 1981.In Chinese. PubMed/NCBI
|
|
12
|
Liu ZH, Li SJ, Wu Y, Zuo K, Wang B, Zeng
CH and Li LS: Treatment of membranous nephropathy with Tripterygium
wilfordii and steroid: a prospective randomized control trial. J
Nephrol Dialy Transpl. 18:303–309. 2009.In Chinese.
|
|
13
|
Ma R, Xu Y, Jiang W and Zhang W:
Combination of Tripterygium wilfordii Hook F and angiotensin
receptor blocker synergistically reduces excretion of urinary
podocytes in patients with type 2 diabetic kidney disease.
Biotechnol Biotechnol Equip. 29:139–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu B, Wang Y, Jardine M, Jun M, Lv JC,
Cass A, Liyanage T, Chen HY, Wang YJ and Perkovic V: Tripterygium
preparations for the treatment of CKD: a systematic review and
meta-analysis. Am J Kidney Dis. 62:515–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge Y, Xie H, Li S, Jin B, Hou J, Zhang H,
Shi M and Liu Z: Treatment of diabetic nephropathy with
Tripterygium wilfordii Hook F extract: a prospective, randomized,
controlled clinical trial. J Transl Med. 11:1342013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Q, Shen W, Qin W, Zheng C, Zhang M,
Zeng C, Wang S, Wang J, Zhu X and Liu Z: Treatment of db/db
diabetic mice with triptolide: a novel therapy for diabetic
nephropathy. Nephrol Dial Transplant. 25:3539–3547. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang YR, Wan YG, Sun W, Mao ZM, Zhao Q,
Shi XM and Yao J: Effects and mechanisms of multi-glycoside of
Tripterygium wilfordii improving glomerular inflammatory injury by
regulating p38MAPK signaling activation in diabetic nephropathy
rats. Zhongguo Zhong Yao Za Zhi. 39:4102–4109. 2014.In Chinese.
|
|
18
|
Zhang H, Sun W, Wan Y, Che X, He F, Pu H
and Dou C: Preventive effects of multi-glycoside of Tripterygium
wilfordii on glomerular lesions in experimental diabetic
nephropathy. Zhongguo Zhong Yao Za Zhi. 35:1460–1465. 2010.In
Chinese. PubMed/NCBI
|
|
19
|
Li K and Wang S: Fingerprint chromatogram
analysis of extracts from the leaves of Tripterygium wilfordii
Hook. F. by high performance liquid chromatography. J Sep Sci.
28:653–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kupchan SM, Court WA, Dailey RG Jr,
Gilmore CJ and Bryan RF: Triptolide and tripdiolide, novel
antileukemic diter-penoid triepoxides from Tripterygium wilfordii.
J Am Chem Soc. 94:7194–7195. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakano K, Yoshida C, Furukawa W, Takaishi
Y and Shishido K: Terpenoids in transformed root culture of
Tripterygium wilfordii. Phytochemistry. 49:1821–1824. 1998.
View Article : Google Scholar
|
|
22
|
Mao ZM, Shen SM, Wan YG, Sun W, Chen HL,
Huang MM, Yang JJ, Wu W, Tang HT and Tang RM: Huangkui capsule
attenuates renal fibrosis in diabetic nephropathy rats through
regulating oxidative stress and p38MAPK/Akt pathways, compared to
α-lipoic acid. J Ethnopharmacol. 173:256–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar
|
|
24
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan YG, Sun W, Zhen YJ, Che XY, Pu HP,
Wang Y, Li M, Ruan JG and Yan QJ: Multi-glycoside of Tripterygium
wilfordii Hook. f. reduces proteinuria through improving podocyte
slit diaphragm dysfunction in anti-Thy1.1 glomerulonephritis. J
Ethnopharmacol. 136:322–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan YG, Che XY, Sun W, Huang YR, Meng XJ,
Chen HL, Shi XM, Tu Y, Wu W and Liu YL: Low-dose of multi-glycoside
of Tripterygium wilfordii Hook. f., a natural regulator of
TGF-β1/Smad signaling activity improves adriamycin induced
glomerulosclerosis in vivo. J Ethnopharmacol. 151:1079–1089. 2014.
View Article : Google Scholar
|
|
28
|
Wan YG, Zhao Q, Sun W, Zhang HL, Li M, Wei
QX, Wu W, Yue LJ and Wang Q: Contrasting dose-effects of
multi-glycoside of Tripterygium wilfordii HOOK. f. on glomerular
inflammation and hepatic damage in two types of anti-Thy1.1
glomerulonephritis. J Pharmacol Sci. 118:433–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S and Chen ZJ: Expanding role of
ubiquitination in NF-κB signaling. Cell Res. 21:6–21. 2011.
View Article : Google Scholar
|
|
30
|
Nakagawa T, Sato W, Glushakova O, Heinig
M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ
and Croker B: Diabetic endothelial nitric oxide synthase knockout
mice develop advanced diabetic nephropathy. J Am Soc Nephrol.
18:539–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kodera R, Shikata K, Kataoka HU, Takatsuka
T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota
D, et al: Glucagon-like peptide-1 receptor agonist ameliorates
renal injury through its anti-inflammatory action without lowering
blood glucose level in a rat model of type 1 diabetes.
Diabetologia. 54:965–978. 2011. View Article : Google Scholar : PubMed/NCBI
|