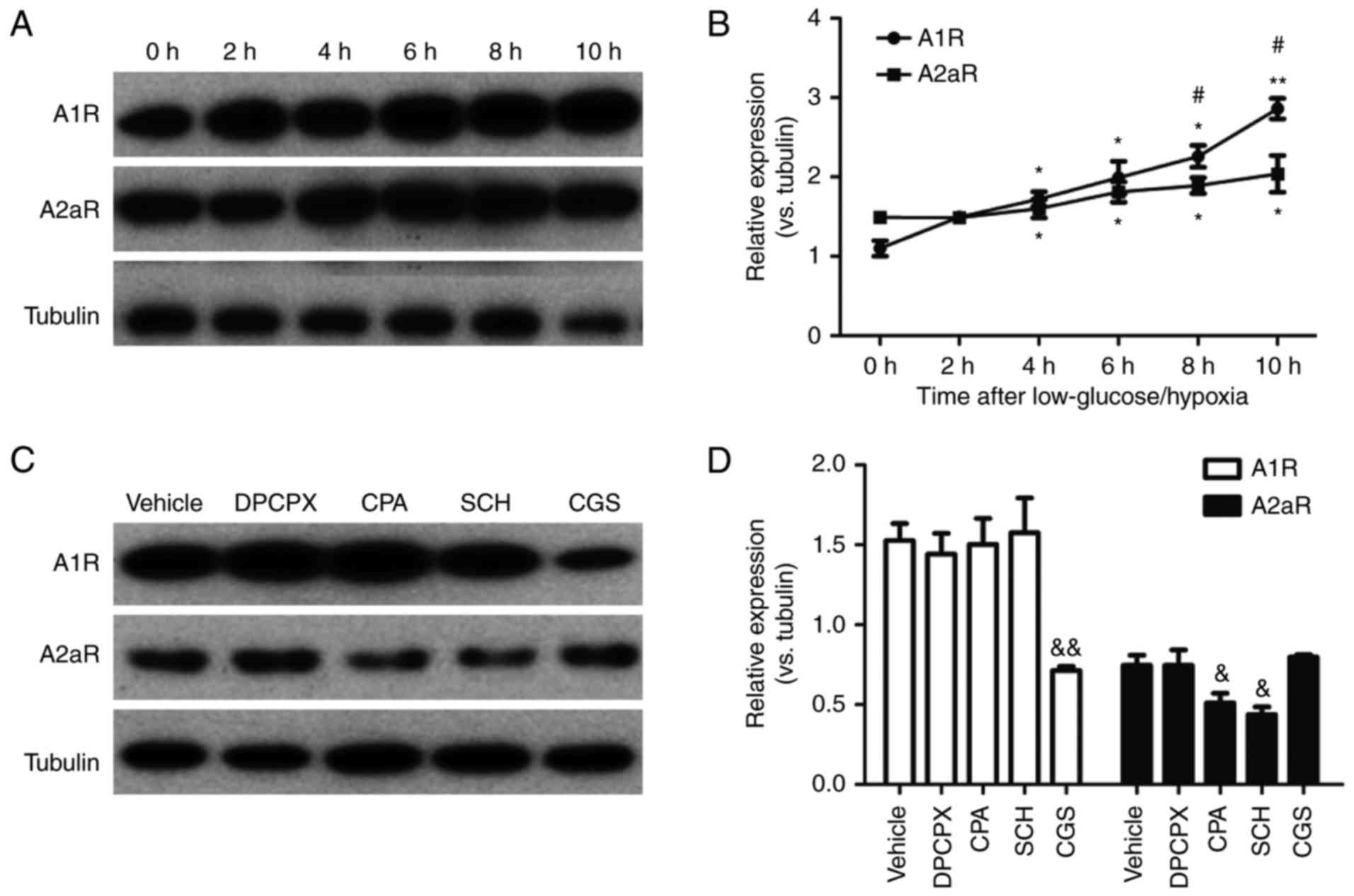
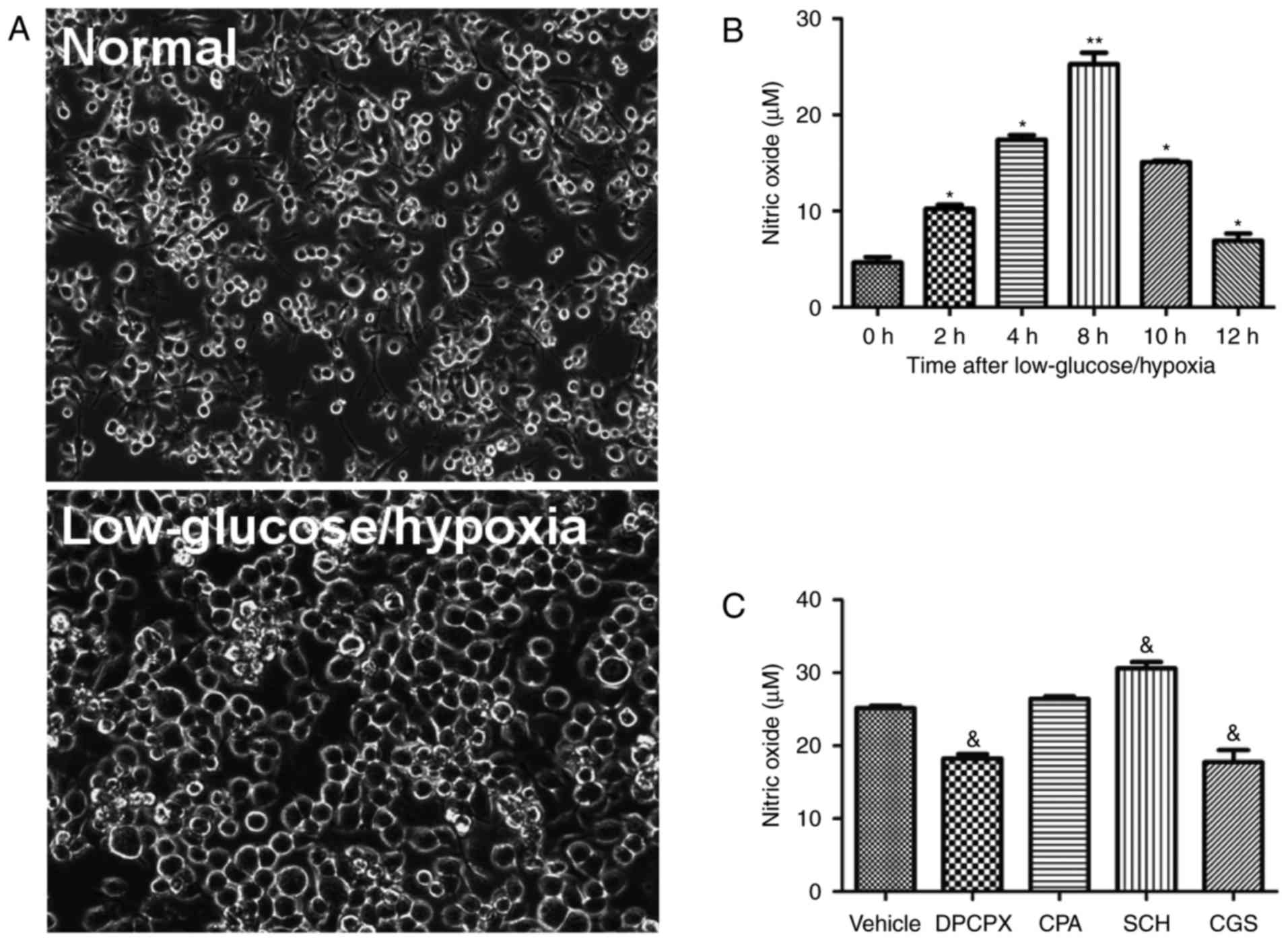
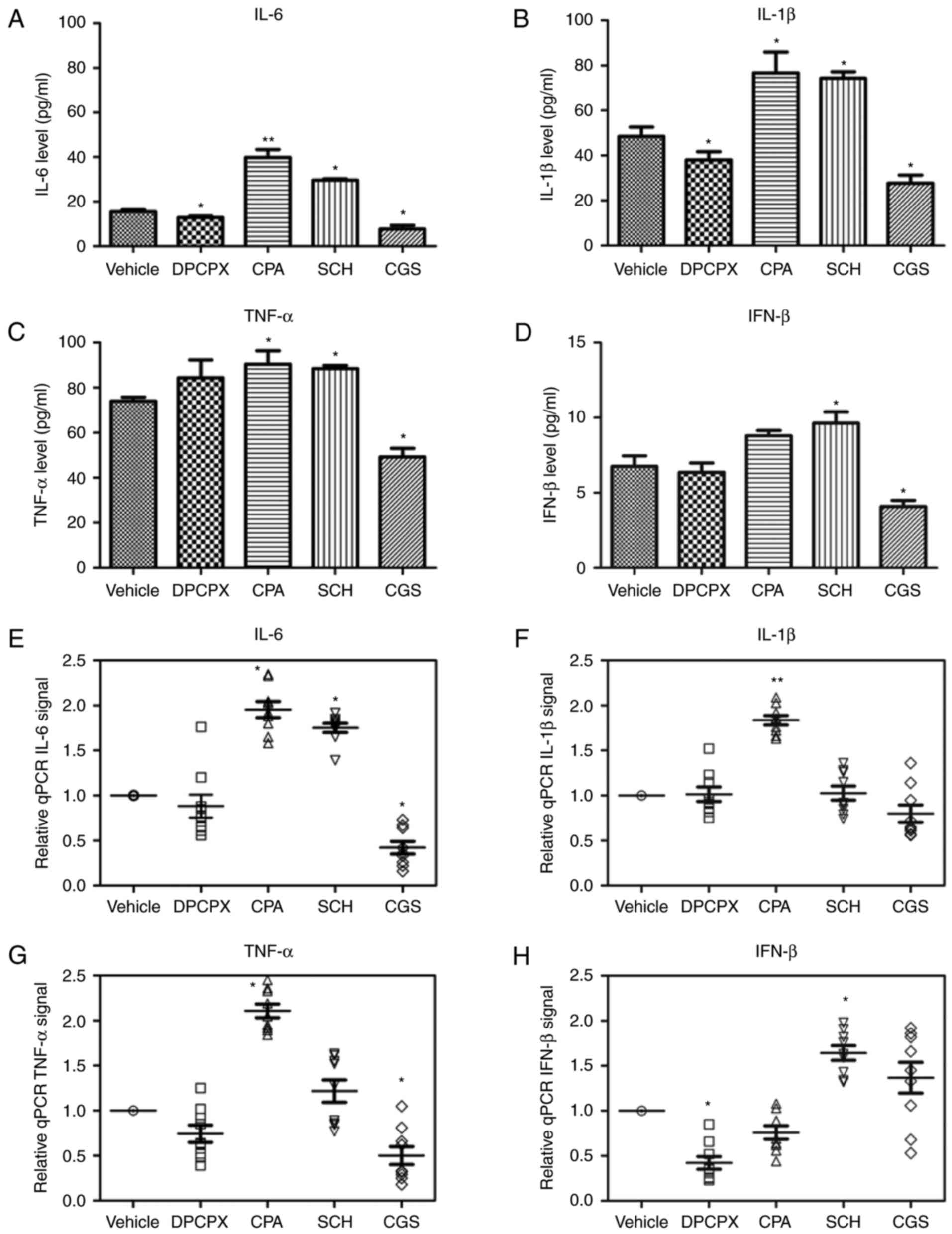
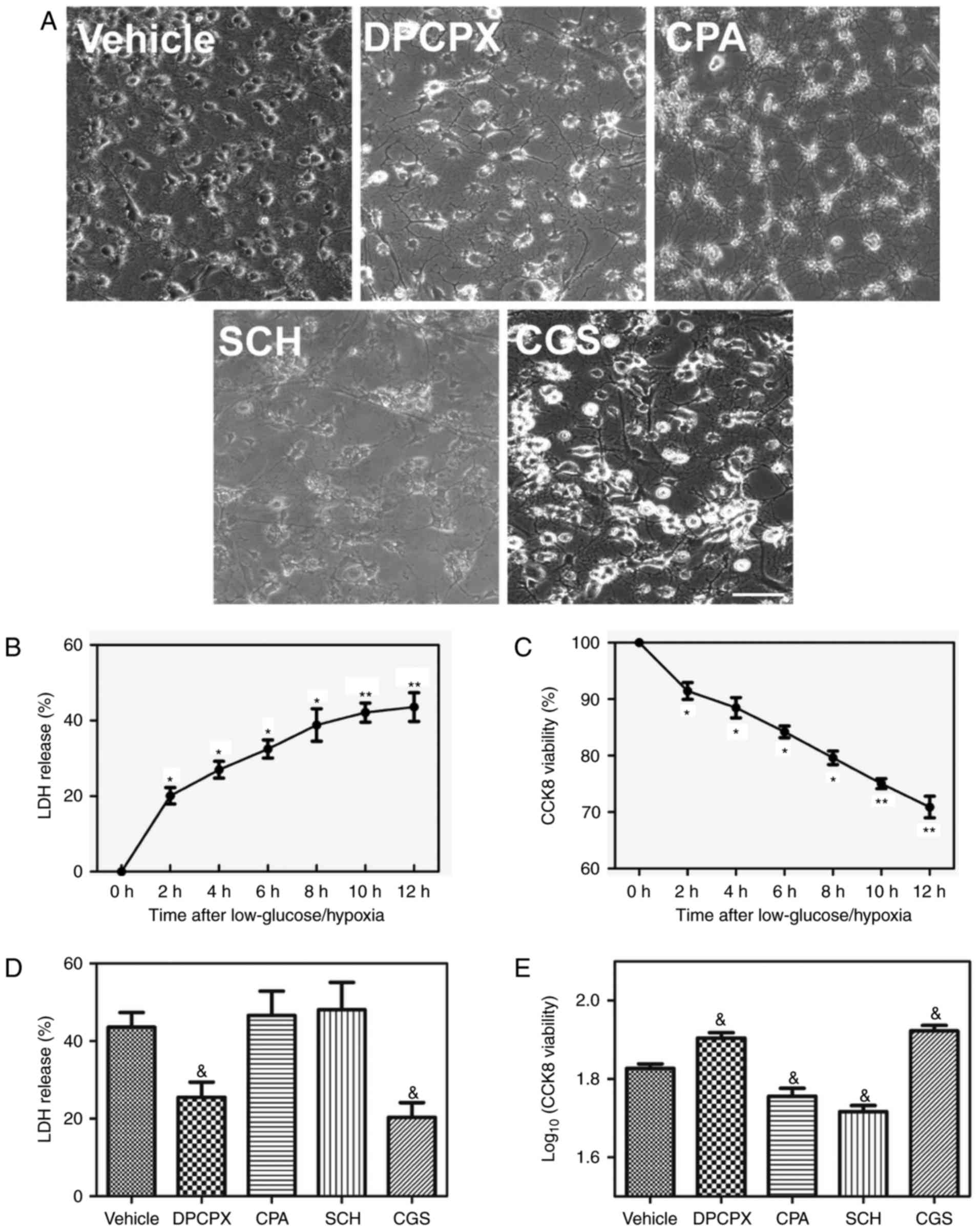
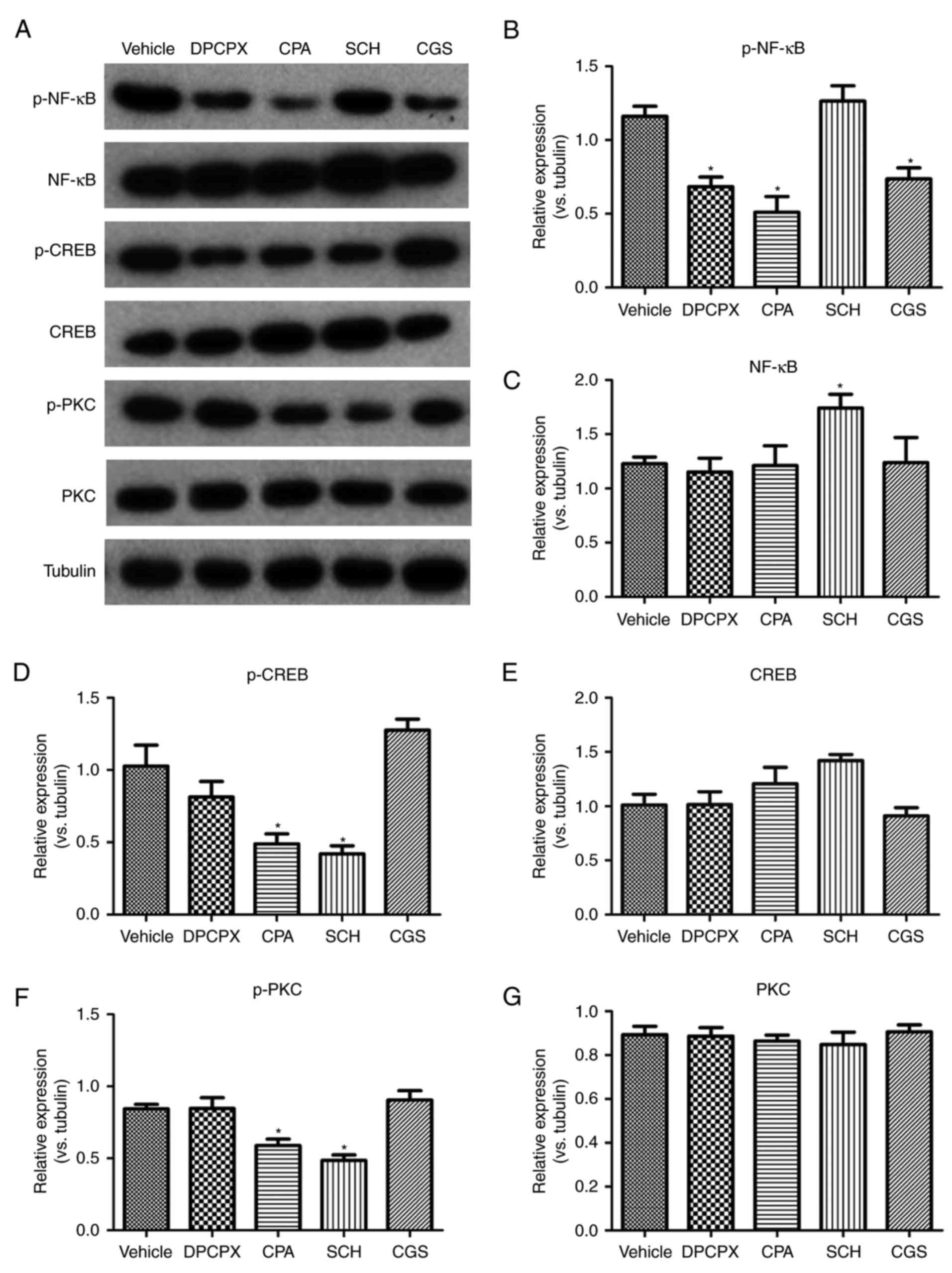
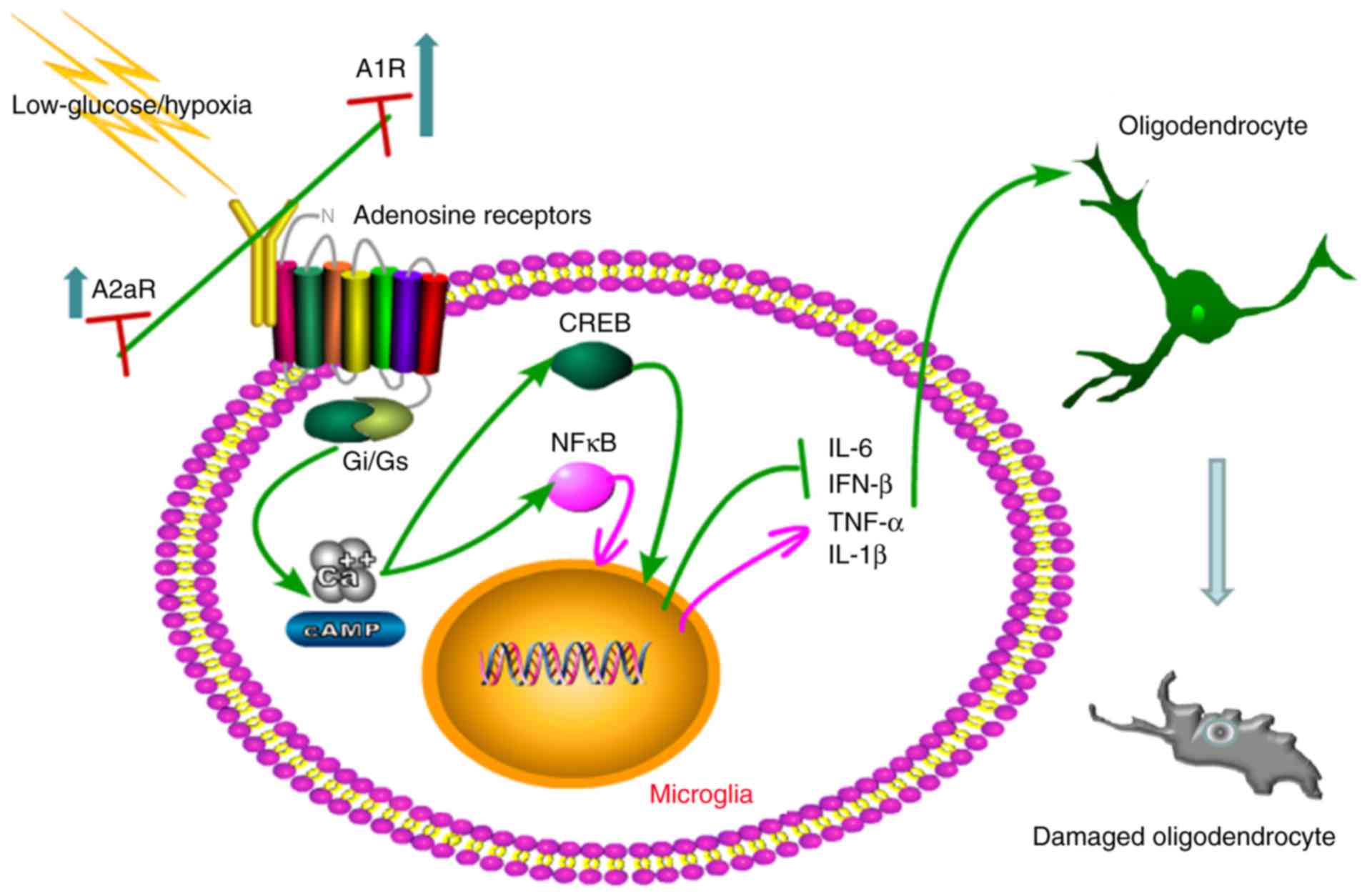
|
1
|
Kaur C and Ling EA: Periventricular white
matter damage in the hypoxic neonatal brain: Role of microglial
cells. Prog Neurobiol. 87:264–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pang Y, Campbell L, Zheng B, Fan L and Cai
Z: Rhodes P. Lipopolysaccharide-activated microglia induce death of
oligodengroctye progenitor cells and impede their development.
Neuroscience. 166:464–475. 2010. View Article : Google Scholar
|
|
3
|
Steinman L: No quiet surrender: Molecular
guardians in multiple sclerosis brain. J Clin Invest.
125:1371–1378. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mittelbronn M, Dietz K, Schluesener HJ and
Meyermann R: Local distribution of microglia in the normal adult
human central nervous system differs by up to one order of
magnitude. Acta Neuropathol. 101:249–255. 2001.PubMed/NCBI
|
|
5
|
Yenari MA, Kauppinen TM and Swanson RA:
Microglial activation in stroke: Therapeutic targets.
Neurotherapeutics. 7:378–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu Y, Yu Z, Xie M, Wang W and Luo X: Hv1
proton channel facilitates production of ROS and pro-inflammatory
cytokines in microglia and enhances oligodendrocyte progenitor
cells damage from oxygen-glucose deprivation in vitro. Biochem
Biophys Res Commun. Jul 1–2017.Epub ahead of print.
|
|
7
|
Espinoza-Rojo M, Iturralde-Rodríguez KI,
Chánez-Cárdenas ME, Ruiz-Tachiquín ME and Aguilera P: Glucose
transporters regulation on ischemic brain: Possible role as
therapeutic target. Cent Nerv Syst Agents Med Chem. 10:317–235.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Butt AM: ATP: A ubiquitous gliotransmitter
integrating neuron-glial networks. Semin Cell Dev Biol. 22:205–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takenouchi T, Tsukimoto M, Iwamaru Y,
Sugama S, Sekiyama K, Sato M, Kojima S, Hashimoto M and Kitani H:
Extracellular ATP induces unconventional release of
glyceraldehyde-3-phosphate dehydrogenase from microglial cells.
Immunol Lett. 167:116–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonioli L, Blandizzi C, Pacher P and
Haskó G: Immunity, inflammation and cancer: A leading role for
adenosine. Nat Rev Cancer. 13:842–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Serchov T, Clement HW, Schwarz MK,
Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A,
Normann C, Biber K and van Calker D: Increased signaling via
adenosine A1 receptors, sleep deprivation, imipramine, and ketamine
inhibit Depressive-like behavior via induction of Homer1a. Neuron.
87:549–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Virgilio F, Ceruti S, Bramanti P and
Abbracchio MP: Purinergic signalling in inflammation of the central
nervous system. Trends Neurosci. 32:79–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cristóvão-Ferreira S, Navarro G,
Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis
C, Ribeiro JA, McCormick PJ, et al: A1R-A2AR heteromers coupled to
Gs and Gi/0 proteins modulate GABA transport into astrocytes.
Purinergic Signal. 9:433–449. 2013. View Article : Google Scholar
|
|
14
|
Ran H, Duan W, Gong Z, Xu S, Zhu H, Hou X,
Jiang L, He Q and Zheng J: Critical contribution of adenosine A2a
receptors in bone marrow-derived cells to white matter lesions
induced by chronic cerebral hypoperfusion. J Neuropathol Exp
Neurol. 74:305–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burnstock G, Fredholm BB and Verkhratsky
A: Adenosine and ATP receptors in the brain. Curr Top Med Chem.
11:973–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodrigues RJ, Tomé AR and Cunha RA: ATP as
a multi-target danger signal in the brain. Front Neurosci.
9:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Mazumder A, Wilder T and Cronstein
BN: Adenosine regulates bone metabolism via A1, A2A, and A2B
receptors in bone marrow cells from normal humans and patients with
multiple myeloma. FASEB J. 27:3446–3454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seki Y, Kato TA, Monji A, Mizoguchi Y,
Horikawa H, Sato-Kasai M, Yoshiga D and Kanba S: Pretreatment of
aripiprazole and minocycline, but not haloperidol, suppresses
oligodendrocyte damage from interferon-γ-stimulated microglia in
co-culture model. Schizophr Res. 151:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomes CV, Kaster MP, Tomé AR, Agostinho PM
and Cunha RA: Adenosine receptors and brain diseases:
Neuroprotection and neurodegeneration. Biochim Biophys Acta.
1808:1380–1399. 2011. View Article : Google Scholar
|
|
20
|
Latini S and Pedata F: Adenosine in the
central nervous system: Release mechanisms and extracellular
concentrations. J Neurochem. 79:463–484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Rudolphi KA, Schubert P, Parkinson FE and
Fredholm BB: Adenosine and brain ischemia. Cerebrovasc Brain Metab
Rev. 4:346–369. 1992.PubMed/NCBI
|
|
23
|
Boison D: Adenosine kinase, epilepsy and
stroke: Mechanisms and therapies. Trends Pharmacol Sci. 27:652–658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dauer W and Przedborski S: Parkinson's
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masino SA, Li T, Theofilas P, Sandau US,
Ruskin DN, Fredholm BB, Geiger JD, Aronica E and Boison D: A
ketogenic diet suppresses seizures in mice through adenosine A1
receptors. J Clin Invest. 121:2679–2683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winerdal M, Winerdal ME, Wang YQ, Fredholm
BB, Winqvist O and Adén U: Adenosine A1 receptors contribute to
immune regulation after neonatal hypoxic ischemic brain injury.
Purinergic Signal. 12:89–101. 2016. View Article : Google Scholar :
|
|
27
|
Naamani O, Chaimovitz C and Douvdevani A:
Pharmacological preconditioning with adenosine A1
receptor agonist suppresses cellular immune response by an
A2A receptor dependent mechanism. Int Immunopharmacol.
20:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Bannon NM, Ilin V, Volgushev M
and Chistiakova M: Adenosine effects on inhibitory synaptic
transmission and excitation-inhibition balance in the rat
neocortex. J Physiol. 593:825–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fenton RA and Dobson JG Jr: Adenosine
A1 and A2A receptor effects on G-protein
cycling in beta-adrenergic stimulated ventricular membranes. J Cell
Physiol. 213:785–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morello S, Ito K, Yamamura S, Lee KY,
Jazrawi E, Desouza P, Barnes P, Cicala C and Adcock IM: IL-1 beta
and TNF-alpha regulation of the adenosine receptor (A2A)
expression: Differential requirement for NF-kappa B binding to the
proximal promoter. J Immunol. 177:7173–7183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loram LC, Taylor FR, Strand KA, Harrison
JA, Rzasalynn R, Sholar P, Rieger J, Maier SF and Watkins LR:
Intrathecal injection of adenosine 2A receptor agonists reversed
neuropathic allodynia through protein kinase (PK)A/PKC signaling.
Brain Behav Immun. 33:112–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Wang H, Lv X, Wang Q, Zhao H, Yang
F, Yang Y and Li J: Involvement of cAMP-PKA pathway in adenosine
A1 and A2A receptor-mediated regulation of
acetaldehyde-induced activation of HSCs. Biochimie. 115:59–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lima FO, Souza GR, Verri WJ Jr, Parada CA,
Ferreira SH, Cunha FQ and Cunha TM: Direct blockade of inflammatory
hypernociception by peripheral A1 adenosine receptors: Involvement
of the NO/cGMP/PKG/KATP signaling pathway. Pain. 151:506–515. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haack KK, Mitra AK and Zucker IH: NF-κB
and CREB are required for angiotensin II type 1 receptor
upregulation in neurons. PLoS One. 8:e786952013. View Article : Google Scholar
|
|
35
|
Ciruela F, Casadó V, Rodrigues RJ, Luján
R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J,
et al: Presynaptic control of striatal glutamatergic
neurotransmis-sion by adenosine A1-A2A receptor heteromers. J
Neurosci. 26:2080–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|