Introduction
Bone mass homeostasis is regulated by a delicate
balance between osteoclast-mediated bone degradation and
osteoblast-induced bone formation, a process termed bone remodeling
(1). Osteoclasts are large cells
with multiple nuclei, which form from hematopoietic progenitors of
the mono-cyte/macrophage lineage. The cells are specialized in bone
resorption (2). Several
pathological bone-related diseases, including postmenopausal
osteoporosis, rheumatoid arthritis, periodontitis and lytic bone
metastasis, are associated with excessive bone breakdown by an
increase in the number and activity of osteoclasts (3–5).
Macrophage-colony stimulating factor (M-CSF) and
receptor activator of nuclear factor (NF)-κB ligand (RANKL) are two
major cytokines, which regulate osteoclast differentiation
(6–8). M-CSF is responsible for the
proliferation and survival of osteoclast precursors and stimulates
the expression of receptor activator of NF-κB (RANK), a receptor
for RANKL, with RANKL inducing osteoclast differentiation (7). The binding of RANKL to its receptor,
RANK, triggers several downstream signaling pathways, including the
p38, extracellular signal-regulated kinase (ERK) and c-Jun
N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) and
NF-κB pathways, which lead to the activation of c-Fos and nuclear
factor of activated T cells cytoplasmic 1 (NFATc1) (9–14).
NFATc1, a master transcription factor of osteoclast formation,
induces several genes responsible for osteoclast differentiation
and function, including matrix metalloproteinase-9 (MMP-9),
tartrate-resistant acid phosphatase (TRAP) and cathepsin K (CtsK)
(15,16).
There are many natural compounds that can be
exploited in the development of novel drugs (17). Several compounds from natural
products suppress osteoclast formation and function. These products
have potential therapeutic value for bone-related diseases
characterized by the hyperactivation of osteoclast activity
(18). Rheum undulatum is
a perennial plant that belongs to the Polygonaceae family and is
mainly distributed in Korea. The rhizomes of R. undulatum
and other Rheum species, commonly known as rhubarb, have
been used for the prevention of a number of diseases in traditional
medicines (19,20). Anthraquinones and stilbenes are
the primary constituents from the rhizomes of R. undulatum.
Anthraquinones have a laxative effect (21), whereas the stilbenes from this
plant exhibit various pharmacological activities, which include
antioxidant, anti-inflammatory and hepatoprotective effects
(22,23). Desoxyrhapontigenin (DRG) is a
stilbene compound isolated from the rhizomes of R. undulatum
and has an anti-inflammatory effect via activating the nuclear
factor erythroid 2-related factor 2/heme oxygenase-1 pathway and
attenuating the NF-κB and MAPK pathways in macrophages (24). However, the anti-osteoclastogenic
effect of DRG and its underlying mechanisms remain to be fully
elucidated. On the basis of the association between chronic
inflammatory and bone diseases (25), the present study aimed to
determine the pharmacological effects of DRG on RANKL-induced
osteoclast formation and function in mouse bone marrow macrophages
(BMMs) and on bone loss induced by lipopolysaccharide (LPS) in an
in vivo animal model. It was demonstrated that DRG
suppressed RANKL-induced osteoclast formation at an early stage of
osteoclastogenesis in BMMs and prevented LPS-induced bone
destruction in the animal model.
Materials and methods
Isolation of stilbene derivatives
Dried R. undulatum L. rhizomes were purchased
from the Yakryoung-si folk medicine market in Daegu, Korea, in May
2015. Botanical identification was performed by Professor Byung-Sun
Min (College of Pharmacy, Catholic University of Daegu, Hayang,
Korea). A voucher specimen (CUD-1188-1) was stored at the College
herbarium. The MeOH extract of the dried rhizomes of R.
undulatum L. was concentrated to yield a residue (4.5 kg),
which was suspended in water and then successively partitioned with
n-hexane, ethyl acetate and n-butanol to afford
n-hexane-, EtOAc-, and n-BuOH-soluble fractions, and
a water (H2O) layer. The EtOAc-soluble fraction (658 g)
was subjected to silica gel column chromatography and eluted with
n-hexane-EtOAc (7:1→0:1) to yield six fractions (E1-E6).
Fraction E2 was rechromatographed on a silica gel column eluted
with chloroform (CHCl3)-MeOH (7:1→0:1) to yield six
sub-fractions (E2-1-E2-6). Subsequently, the E2-1 sub-fraction was
chromatographed on an RP-18 silica gel column with
MeOH-H2O (1:1→1:0) as the mobile phase to yield five
fractions (E2-1-1-E2-1-5). Sub-fraction E2-1-3 was subjected to
silica gel column chromatography using a stepwise gradient elution
of CHCl3-MeOH (14:1→0:1), to yield rhapontigenin (2.1 g)
and resveratrol (200 mg). Sub-fraction E2-1-4 was rechromatographed
on a silica gel column with
CH2Cl2-MeOH-H2O (5:1:1) to obtain
DRG (500 mg). Fraction E3 was fractionated on a silica gel column
using CHCl3-MeOH (20:1→0:1) as the solvent system to
obtain piceatannol (3 g). Sub-fraction E3-3 was purified using
high-performance liquid chromatography with a stepwise gradient of
H2O-MeOH (1:1→3:1) to yield resveratroloside (15 mg).
The structures of the stilbene derivatives are shown in Fig. 1. The purity of these stilbenes was
assessed using 1H and 13C nuclear magnetic
resonance spectra. The spectra revealed signals with a high level
of purity with no impurities.
Cell culture
RAW264.7 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and were cultured in
Dulbecco’s modified essential medium with 10% heat-inactivated
fetal bovine serum (FBS; Cambrex, Charles City, IA, USA) and
penicillin (100 U/ml)-streptomycin (100 μg/ml). The cells
were cultured at 37°C in a humidified 5% CO2
atmosphere.
Isolation of BMMs and osteoclast
differentiation
Bone marrow cells were isolated from the femurs and
tibias of 6-week-old male ICR mice weighing 22–25 g (DBL, Emseong,
Chungbuk, Korea) housed at a temperature of 24±2°C and humidity of
55±10% controlled colony room under a 12 h light and 12 h dark
cycle. All mice were allowed ad libitum access to a standard
chow diet and water prior to all experiments. The bone marrow cells
were then cultured in α-MEM (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% FBS, 100 U/ml penicillin, 100
μg/ml streptomycin and 10 ng/ml M-CSF (Prospec-Tany
TechnoGene Ltd., East Brunswick, NJ, USA) overnight in a humidified
incubator with 5% CO2 at 37°C. The floating cells were
harvested and maintained for 3 days with 30 ng/ml M-CSF. The cells
adhered to the culture dish were characterized as BMMs and used for
subsequent experiments. The BMMs (5×104 cells/well) were
cultured in 96-well plates and maintained with RANKL (100 ng/ml)
and M-CSF (30 ng/ml) for 7 days in the presence or absence of the
indicated compounds. The medium was replaced every 2 days. The
cells were then fixed for 15 min in 3.7% formalin, permeabilized
with 0.1% Triton X-100, and stained for TRAP with an acid
phosphatase leukocyte kit (Sigma-Aldrich; EMD Millipore, Billerica,
MA, USA). TRAP-positive multinucleated cells with more than five
nuclei were defined as osteoclasts.
Antibodies and reagents
Recombinant mouse RANKL and M-CSF were obtained from
R&D Systems, Inc. (Minneapolis, MN, USA). Antibodies targeting
NFATc1 (cat no. 8032S), c-Fos (cat no. 2250S), c-Src (cat no.
2019), ERK1/2 (cat no. 4695S), p38 (cat no. 9212), JNK (cat no.
9252), phospho-p38 (cat no. 9216S), phospho-ERK1/2 (cat no. 9101S)
and phospho-JNK (cat no. 4668S) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Fluorescein isothiocyanate
(FITC)-conjugated palloidin (cat no. A12379) was from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Antibodies
targeting α-tubulin (cat no. SC-5546) and CtsK (cat no. SC48353)
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Cytotoxicity assay
Cytotoxicity was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT)-based assay. The BMMs were cultured into 96-well plates
(1×104 cells/well) and supplemented with 30 ng/ml M-CSF
in the presence of the indicated concentrations of DRG. After 72 h,
0.5 mg/ml of MTT was added to each well for 3 h. At the end of the
incubation, the insoluble formazan products were dissolved in
dimethyl sulfoxide, and absorbance at 540 nm was determined.
Bone resorption assay
The BMMs (5×104 cells/well) were seeded
in OsteoAssay Surface 96-well plates (Corning Incorporated,
Corning, NY, USA) in α-MEM supplemented with 10% FBS, 1% penicillin
and streptomycin, 30 ng/ml M-CSF, 100 ng/ml RANKL, and various
concentrations of DRG. The culture medium was replaced every 2 days
and culture continued for 7 days. The differentiated BMMs were
washed with tap water and images of the surface of resorption pits
were captured using a model H550L microscope (Nikon Corporation,
Tokyo, Japan) and quantified via ImageJ software [Java 1.6.0_20
(64-bit); NIH, Bethesda, MD, USA].
Actin ring formation and
immunofluorescence
The BMMs (106 cells/ml) were seeded on a
cover glass and cultured for 7 days with 30 ng/ml M-CSF and 100
ng/ml RANKL in the presence of the indicated concentrations of DRG.
The BMMs were washed and fixed in 15 min with 4% paraformaldehyde.
Following permeabilization with 0.1% Triton X-100, the BMMs were
stained for 10 min with FITC-phalloidin at room temperature.
Following washing with phosphate buffered saline (PBS), the BMMs
were mounted and images were captured using an LSM510 META NLO
inverted confocal laser scanning microscope (Zeiss GmbH, Jena,
Germany; Korea Basic Science Institute Chuncheon Center, Chuncheon,
Korea) equipped with an external Argon, HeNe laser and HeNe laser
II.
Western blot analysis
The cells were harvested and lysed in a lysis buffer
containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1%
NP-40, 5 mM sodium orthovanadate and protease inhibitor cocktail
(BD Biosciences, Franklin Lakes, NJ, USA), and then centrifuged for
10 min at 4°C and 22,000 × g. Protein concentration was determined
using the Bradford method (26).
A total of 30 μg of cellular extracts was separated via 8%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred onto a Hybond-P membrane (GE Healthcare Life Sciences,
Chalfont, UK). The membranes were blocked with 5% nonfat skim milk
at room temperature for 1 h and probed for 2 h with the indicated
primary antibodies (1:1,000 dilution). Following washing with PBS
containing 0.1% Tween-20, the membranes were incubated with the
secondary antibody (1:2,000 dilution) at room temperature for 2 h.
The signal was detected using the enhanced chemiluminescence system
(Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The BMMs were collected and total RNA was extracted
using an RNeasy Mini kits according to the manufacturer’s protocol
(Qiagen, Inc., Valencia, CA, USA). The first-strand cDNA was
synthesized using 1 μg of total RNA. RT-qPCR analysis was
performed using TOPreal qPCR 2X PreMIX (SYBR-Green; Enzynomics,
Inc., Daejon, Korea) and the Rotor-Gene Q real-time PCR cycler
(Qiagen, Inc.). The primers sequence were as follows: MMP-9,
forward, 5′-TGGGCAAGCAGTACTCTTCC-3′ and reverse,
5′-AACAGGCTGTACCCTTGGTC-3′; CtsK, forward,
5′-GACACCCAGTGGGAGCTATG-3′ and reverse, 5′-AGAGGCCTCCAGGTTATGGG-3′;
TRAP, forward, 5′-ACTTGCGACCATTGTTAGCC-3′ and reverse,
5′-TTCGTTGATGTCGCACAGAG-3′; β-actin, forward,
5′-GGGAAATCGTGCGTGACATCAAAG-3′ and reverse,
5′-AACCGCTCCTTGCCAATAGT-3′. The PCR conditions were as follows:
95°C for 10 min, followed by 40 cycles at 95°C for 10 sec, 60°C for
15 sec and 72°C for 20 sec. All reactions were performed in
triplicate and β-actin was used as an internal control.
Quantification of the relative gene expression was computed using
the 2−ΔΔCq method (27).
LPS-induced bone loss in vivo
All experimental protocols were approved by the
Institutional Animal Care and Use Committee (IACUC) of Kangwon
National University (IACUC approval No. KW-180119-3). To
investigate the effect of DRG on inflammation-induced bone loss,
the mice were randomly divided into control vehicle-treated,
LPS-treated, DRG-treated, and DRG + LPS-treated groups (n=4/group).
The mice in the control group were treated with control vehicle
(dimethyl sulfoxide: cremophor-EL in PBS; 1:1:8). The mice were
injected intraperitoneally with DRG (50 mg/kg) solubilized in
dimethyl sulfoxide: Chremophore-EL in PBS (1:1:8 by volume) or
control vehicle for 1 h prior to the first LPS (5 mg/kg) injection
and then every other day for 8 days. LPS was injected on days 2 and
6. All mice were sacrificed on day 9. Intact left femoral
metaphysic regions of each mouse were evaluated by high-resolution
micro-computed tomography (micro-CT) analysis using an
NFR-Polaris-S160 apparatus (Nanofocus Ray; Korea Basic Science
Institute Chuncheon Center) with a 90 μA current, source
voltage of 45 kVp and 7 μm isotropic resolution. Femoral
scans were performed over 2 mm from the growth plate, with a total
of 350 sections per scan. Following three-dimensional (3D)
reconstruction, trabecular number (Tb.N), bone volume per tissue
volume (BV/TV), trabecular separation (Tb.Sp) and bone surface/bone
volume (BS/BV) were examined with quantitative analyses using
INFINITT-Xelis software (version 1.7; INFINITT Healthcare Co.,
Ltd., Seoul, Korea).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS (version
14.0; SPSS Inc., Chicago, IL, USA). Statistical significance was
assessed by one-way analysis of variance and the difference between
the experimental groups was compared. P<0.05 was considered to
indicate a statistically significant difference.
Results
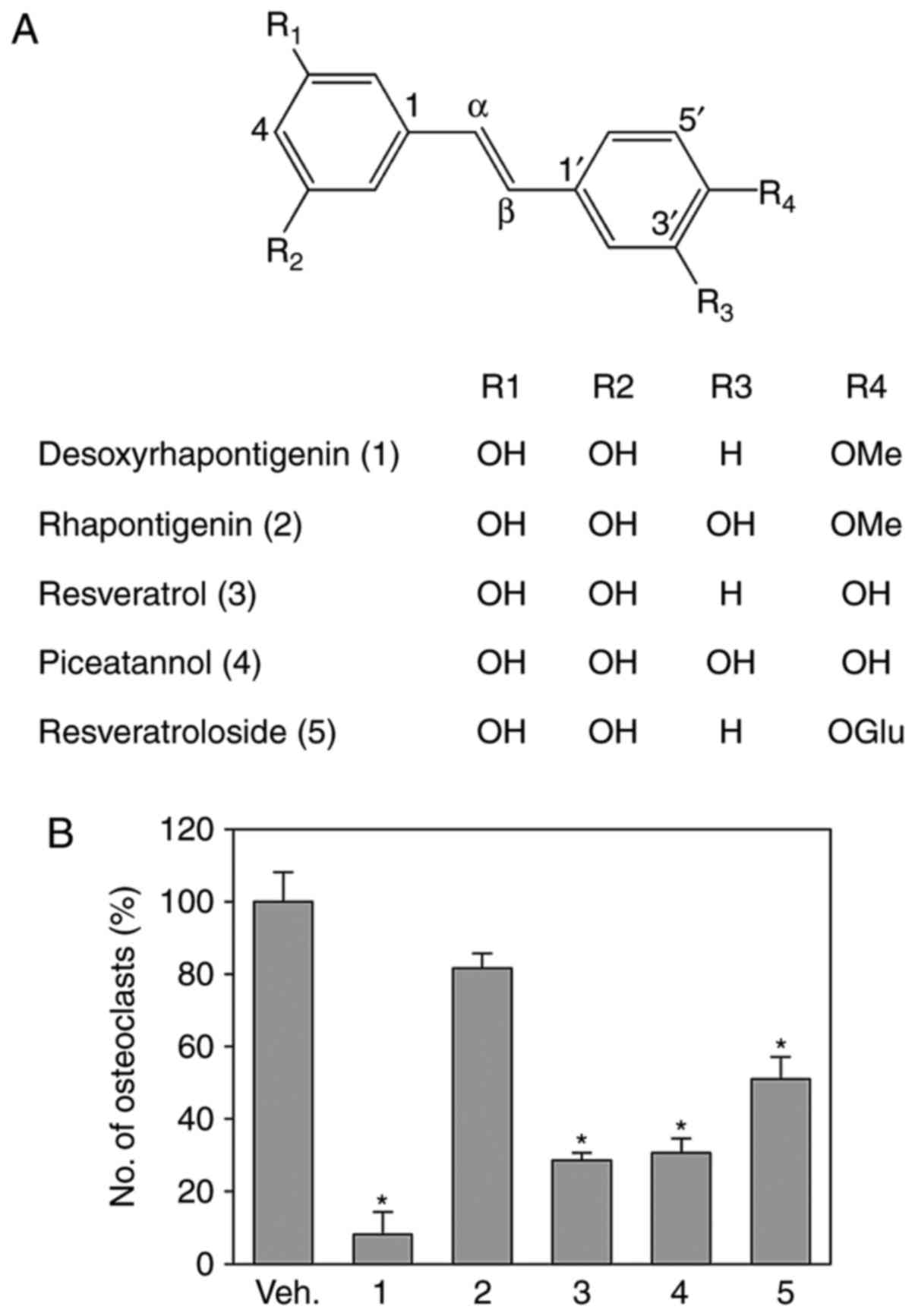
Isolation of the stilbene derivatives
from R. undulatum and their anti-osteoclastogenic effects in
BMMs
In order to identify novel anti-osteoclastogenic
compounds from the natural products, the stilbene derivatives were
isolated from R. undulatum. Repeated column chromatography
of the CHCl3-soluble fraction of R. undulatum on
a silica gel and RP-C18 led to the isolation of five
stilbenes. These stilbenes were identified as DRG, rhapontigenin,
resveratrol, piceatannol and resveratroloside, by comparison with
the published spectroscopic data (28–30). Their structures are shown in
Fig. 1A. To investigate the
effects of these stilbenes on osteoclast formation, the effects of
these stilbenes on RANKL-induced osteoclast formation were
investigated in mouse BMMs. At 10 μM, DRG, resveratrol,
piceatannol and resveratroloside significantly reduced the
RANKL-induced formation of osteoclasts as characterized by
TRAP-positive multinucleated cells. However, rhapontigenin did not
significantly inhibit osteoclast formation (Fig. 1B). As resveratrol and piceatannol
are reported to have anti-osteoclastogenic activities (31,32) and DRG exhibited potent
anti-osteoclastogenic activity, the present study further
investigated the anti-osteoclastogenic effect of DRG.
DRG inhibits RANKL-induced formation in
mouse BMMs and RAW264.7 cells
To investigate the effects of DRG on RANKL-induced
osteoclast formation in detail, the anti-osteoclastogenic activity
of DRG was evaluated using mouse BMMs and RAW264.7 cells. The mouse
BMMs were incubated with RANKL and M-CSF for 7 days, and the
RAW264.7 cells were stimulated with RANKL for 4 days. Treating BMMs
or RAW264.7 cells with DRG reduced the formation of osteoclasts in
a dose-dependent manner, as measured by TRAP-positive
multinucleated cells (Fig. 2A and
B). Subsequently, whether DRG down-regulated the RANKL-induced
expression of osteoclast markers, including c-Src and CtsK, in the
BMMs was evaluated. Western blot analysis revealed that DRG
effectively suppressed the RANKL-induced upregulation of these
markers (Fig. 2C), suggesting
that DRG suppressed RANKL-induced osteoclast formation. To
investigate whether DRG inhibited RANK-induced osteoclastogenesis
due to the potential cytotoxicity of DRG to BMMs, the cytotoxic
effect of DRG on BMMs was examined. The MTT assay results showed
that DRG was not cytotoxic towards BMMs up to 30 μM
(Fig. 2D). These results
suggested that DRG suppressed RANKL-induced osteoclastogenesis
without affecting the viability of BMMs.
 | Figure 2DRG inhibits RANKL-induced osteoclast
formation at the early stage. (A) BMMs were induced to
differentiate into osteoclasts by incubating with M-CSF (30 ng/ml)
and RANKL (100 ng/ml) in the presence or absence of the indicated
concentrations of DRG for 7 days, and then a TRAP assay was
performed. The quantities of TRAP-positive multinucleated (>5
nuclei) osteoclasts were determined following image capture
(magnification, ×40). Data are presented as the mean ± standard
error of the mean (*P<0.01, compared with
vehicle-treated control; n=3). (B) RAW264.7 cells were induced to
differentiate into osteoclasts by incubating with RANKL (100 ng/ml)
in the presence or absent of the indicated concentrations of DRG
for 4 days, and then a TRAP assay was performed. The quantities of
TRAP-positive multinucleated (>5 nuclei) osteoclasts were
determined following image capture (magnification, ×40). Data are
presented as the mean ± standard error of the mean
(*P<0.01, compared with vehicle-treated control;
n=3). (C) BMMs were stimulated with M-CSF (30 ng/ml) and RANKL (100
ng/ml), in the presence or absence of DRG (30 μM) for 7
days. Total lysates were prepared and the expression levels of
c-Src and cathepsin K were determined by western blot analysis. (D)
BMMs were seeded into 96-well plates with M-CSF (30 ng/ml),
followed by incubation with the indicated concentrations of DRG for
3 days. Cell viability was measured using the MTT assay. Data are
presented as mean ± standard error of the mean
(*P<0.01, compared with vehicle-treated control;
n=3). (E) Effect of DRG on RANKL-induced osteoclast formation at
different time points. BMMs were induced to differentiate into
osteoclasts by incubating with M-CSF (30 ng/ml) and RANKL (100
ng/ml), and then treated with 30 μM DRG at the indicated
periods of time. The cells were cultured for 7 days, and then fixed
and the TRAP staining assay was performed. The quantities of
TRAP-positive multinucleated (>5 nuclei) osteoclasts were
determined following image capture (magnification, ×40). Data are
presented as the mean ± standard error of the mean
(*P<0.01, compared with Con; n=3). BMMs, bone marrow
macrophages; DRG, desoxyrhapontigenin; RANKL, receptor activator of
nuclear factor-κB ligand; TRAP, tartrate-resistant acid
phosphatase; Con, control; M-CSF, macrophage-colony stimulating
factor. |
To determine whether DRG inhibits the early or late
stage of osteoclast formation, the present study investigated the
effects of DRG on RANKL-induced osteoclast precursor
differentiation by treating at different times between days 1 and 5
post-RANKL stimulation (Fig. 2E).
DRG significantly reduced RANKL-induced osteoclast formation at day
1 of treatment. However, DRG was not effective in the suppression
of osteoclast formation at day 5 of treatment, suggesting that DRG
suppresses RANKL-induced osteoclastogenesis at an early stage.
DRG inhibits actin-ring formation and
bone resorption in RANKL-stimulated BMMs
To further investigate the effect of DRG on
osteoclast differentiation, the present study determined whether
DRG inhibited F-actin ring formation, which is a critical indicator
of the bone resorption activity of osteoclasts and is a
characteristic feature of mature osteoclasts (33). Under the stimulation of RANKL,
BMMs formed the F-actin ring structures, as evidenced by
FITC-phalloidin staining (Fig.
3A). Treating the BMMs with DRG significantly decreased the
number and size of actin-ring structures in a
concentration-dependent manner, suggesting that DRG inhibited the
formation of mature osteoclasts. To confirm whether DRG suppressed
the bone-resorbing activity of osteoclasts, the effect of DRG on
RANKL-induced bone resorption in BMMs was investigated (Fig. 3B). In the presence of RANKL,
osteoclasts formed bone-resorption pits. However, DRG markedly
reduced the formation of pits formed in the overall osteoassay
surface in a concentration-dependent manner. These results
demonstrated that DRG suppressed RANKL-induced bone resorption
ability.
DRG suppresses LPS-induced bone loss in
vivo
The involvement of DRG in the inhibition of
osteoclastogenesis in vivo was assessed using an
inflammation-induced bone loss mouse model. The mice were treated
with LPS in the presence or absence of DRG. After 9 days, the
femurs were examined by micro-CT. 3D analysis revealed that LPS
administration appeared to cause trabecular bone loss in the
femurs. However, LPS-induced bone loss was considerably reduced in
mice who received DRG (Fig. 4A).
In correlation with micro-CT images, the reductions of Tb. N and
BV/TV by LPS injection were rescued in DRG-treated mice (Fig. 4B). The LPS-induced changes in
Tb.Sp and BS/BV were also attenuated by DRG administration
(Fig. 4B). These results
indicated that DRG suppressed inflammation-induced osteoclast
formation in vivo.
 | Figure 4DRG suppresses LPS-induced bone loss
in vivo. (A) At 9 days following the first LPS injection,
mice were sacrificed, their femurs were collected and their
three-dimensional images were generated using micro-computed
tomography. (B) BV/TV, Tb. N, Tb. Sp, and BS/BV were obtained using
the CTAn software. n=4 (eight legs) in each group.
*P<0.05, compared with vehicle-treated control. DRG,
desoxyrhapontigenin; LPS, lipopolysaccharide; BV/TV, bone volume
per tissue volume; Tb. N, trabecular number; Tb. Sp, trabecular
separation; BS/BV, bone surface to bone volume. |
DRG inhibits the RANKL-induced activation
of ERK, c-Fos and NFATc1
The finding that DRG suppressed RANKL-induced
osteoclast formation at an early stage prompted an experiment
designed to examine the effect of DRG on RANKL-induced early
signaling events, including MAPK pathways. BMMs showed increased
phosphorylation levels of ERK, p38 and JNK MAPKs in the BMMs upon
stimulation with RANKL. Pretreatment with DRG did not decrease the
phosphorylation levels of p38 and JNK. However, DRG suppressed the
RANKL-induced phosphorylation of ERK (Fig. 5A). Activation of MAPK signaling
pathways induces the expression of NFATc1 via activator protein-1
(AP-1) transcription factor (14), which is a heterodimer of the c-Jun
and c-Fos transcription factors. Therefore, the present study
determined whether DRG suppresses the RANKL-induced expression of
c-Fos and NFATc1. Stimulation of the BMMs with RANKL increased the
expression levels of c-Fos and NFATc1. However, the RANKL-induced
expression of c-Fos and NFATc1 was attenuated by DRG treatment
(Fig. 5B), suggesting that DRG
suppressed RANKL-induced osteoclastogenesis, at least in part by
inhibiting the MAPK/AP-1 signaling pathway.
DRG inhibits the expression of NFATc1
target genes
To further examine the role of DRG in the activation
of NFATc1, the present study examined the effects of DRG on the
expression of marker genes associated with osteoclastogenesis,
including CtsK, MMP-9 and TRAP. These three genes are downstream
target genes of the NFATc1 pathway. DRG significantly inhibited the
expression of these NFATc1 target genes in a time- and
concentration-dependent manner during RANKL-induced
osteoclastogenesis (Fig. 6).
 | Figure 6DRG impairs the RANKL-induced
expression of NFATc1 target genes. (A) BMMs were incubated with
RANKL (100 ng/ml) in the presence or absence of 30 μM DRG
for the indicated periods of time. RT-qPCR analysis was performed
to quantify the mRNA expression levels of CtsK, TRAP and MMP-9.
Data are presented as the mean ± standard error of the mean
(*P<0.01, compared with the RANKL-only treated
control; n=5). (B) BMMs were pretreated with the indicated
concentration of DRG under the stimulation of RANKL (100 ng/ml) for
4 days. RT-qPCR analysis was performed to quantify the mRNA
expression levels of CtsK, TRAP and MMP-9. Data are presented as
the mean ± standard error of the mean (*P<0.01,
compared with the RANKL-only treated control; n=5). BMMs, bone
marrow macrophages; DRG, desoxyrhapontigenin; RANKL, receptor
activator of nuclear factor-κB ligand; TRAP, tartrate-resistant
acid phosphatase; CtsK, cathepsin K; MMP-9, matrix
metalloproteinase-9; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
Discussion
Osteoclasts are specialized multinucleated cells,
which are able to resorb the collagen matrix of bone. An aberrant
increase in RANKL/RANK signaling results in increased osteoclast
formation and activity, as reported in various bone loss-related
diseases, including postmenopausal osteoporosis, autoimmune
arthritis, bone tumors and periodontitis. Therefore, the inhibition
of osteoclast differentiation and/or its function may be a
potential approach to treat or prevent pathological bone loss. To
this end, attention has turned to stilbenes. Stilbenes are a class
of plant polyphenols and, due to the potential in therapeutic
applications, their derivatives are promising for drug research and
development (34). Resveratrol, a
naturally occurring stil-bene, and/or a variety of plant food
containing resveratrol has a therapeutic potential for treatment or
prevention of age-related degenerative diseases such as
osteoporosis (35,36). DRG is a stilbene isolated from
R. undulatum and has the anti-inflammatory activity
(24). However, the effect of DRG
on osteoclast differentiation and its underlying molecular
mechanism(s) in osteoclastogeneis remain to be elucidated. In the
present study, it was demonstrated that DRG suppressed
RANKL-induced osteoclastogenesis at the early stages, but had no
cytotoxic effect on BMMs. DRG also prevented inflammation-induced
bone destruction in the in vivo model. At the molecular
level, DRG suppressed the RANKL-induced activation of ERK and
NFATc1, and the expression of NFATc1 target genes, including TRAP,
CtsK and c-Src. These collective findings support the therapeutic
potential of DRG for bone resorption-associated diseases.
M-CSF regulates the proliferation and survival of
BMMs, and the differentiation of BMMs to the osteoclast precursor,
whereas the differentiation of osteoclast precursors to mature
osteoclasts is regulated by RANKL (9). In the present study, it was
demonstrated that DRG did not show cytotoxic activity towards the
M-CSF-stimulated BMMs. However, DRG did inhibit the RANKL-induced
formation of mature osteoclasts from osteoclast precursors,
suggesting that DRG suppressed RANKL-induced osteoclastogenesis at
the early stage by modulating the RANKL/RANK signaling pathway.
The ERK, JNK, and p38 MAPK pathways are involved in
the RANKL/RANK signaling pathway and are important in
osteoclastogenesis through their regulation of the expression of
NFATc1 via AP-1 transcription factors (9,37).
Treating BMMs with JNK- or p38-specific inhibitors suppresses
RANKL-induced osteoclast differentiation (38,39). Activation of the ERK signaling
pathway is also important for RANKL-induced osteoclastogenesis.
Treatment with PD98059, an ERK inhibitor, attenuates osteoclast
differentiation (40). In the
present study, DRG suppressed the RANKL-induced phosphorylation of
ERK and c-Fos, and the expression of NFATc1, suggesting that DRG
suppressed RANKL-induced osteoclast differentiation via inhibiting
the MAPK/AP-1 signaling pathway.
The present study demonstrated the protective effect
of DRG on LPS-induced bone loss in vivo. The administration
of DRG at 50 mg/kg suppressed LPS-induced bone loss in a mouse
model, as conformed by micro-CT analysis, suggesting that DRG may
be effective in treating or preventing inflammation-induced bone
loss in vivo by suppressing osteo-clast formation. R.
undulatum is a rich source of stilbenes, including DRG and
resveratrol. DRG is a major constituent of R. undulatum
(28), and the content of DRG in
ethanol extract from the dried rhizomes of R. undulatum is
~0.1% (28). The findings in the
present study suggested that DRG may be considered as a novel lead
compound for the development of a therapeutic or preventive agent
against inflammation-induced bone loss. In addition, the
DRG-enriched extracts from the rhizomes of R. undulatum may
be applied as a supplemental or functional food, having a
beneficial effect on inflammation-induced bone.
In conclusion, DRG, a stilbene compound, can
suppress RANKL-induced osteoclast differentiation in BMMs and
LPS-induced bone destruction in an in vivo model. DRG
impaired the RANKL-induced activation of NFATc1 via the MAPK/AP-1
signaling pathway. These findings indicated that DRG may be a
valuable stilbene compound for the prevention and/or treatment of
osteoclast-associated bone diseases, including rheumatoid arthritis
and osteoporosis.
Acknowledgments
The authors would like to thank Mr. Kim Song-Rae
(Korea Basic Science Institute, Chuncheon Center, Chuncheon,
Gangwon-Do, Republic of Korea) for technical assistance.
References
|
1
|
Robling AG, Castillo AB and Turner CH:
Biomechanical and molecular regulation of bone remodeling. Annu Rev
Biomed Eng. 8:455–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyce BF, Rosenberg E, de Papp AE and
Duong LT: The osteoclast, bone remodelling and treatment of
metabolic bone disease. Eur J Clin Invest. 42:1332–1341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar
|
|
4
|
Hienz SA, Paliwal S and Ivanovski S:
Mechanisms of bone resorption in periodontitis. J Immunol Res.
2015:6154862015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crockett JC, Rogers MJ, Coxon FP, Hocking
LJ and Helfrich MHL: Bone remodelling at a glance. J Cell Sci.
124:991–998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|
|
8
|
Chambers TJ: Regulation of the
differentiation and function of osteoclasts. J Pathol. 192:4–13.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
10
|
He Y, Staser K, Rhodes SD, Liu Y, Wu X,
Park SJ, Yuan J, Yang X, Li X, Jiang L, et al: Erk1 positively
regulates osteoclast differentiation and bone resorptive activity.
PLoS One. 6:e247802011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto A, Miyazaki T, Kadono Y,
Takayanagi H, Miura T, Nishina H, Katada T, Wakabayashi K, Oda H,
Nakamura K and Tanaka S: Possible involvement of IkappaB kinase 2
and MKK7 in osteoclastogenesis induced by receptor activator of
nuclear factor kappaB ligand. J Bone Miner Res. 17:612–621. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto M, Sudo T, Saito T, Osada H and
Tsujimoto M: Involvement of p38 mitogen-activated protein kinase
signaling pathway in osteoclastogenesis mediated by receptor
activator of NF-kappa B ligand (RANKL). J Biol Chem.
275:31155–31161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Gu J, Wang Y, Yuan Y, Liu X, Bian J
and Liu Z: Involvement of the mitogenactivated protein kinase
signaling pathway in osteoprotegerininduced inhibition of
osteoclast differentiation and maturation. Mol Med Rep.
12:6939–6945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Logar DB, Komadina R, Prezelj J, Ostanek
B, Trost Z and Marc J: Expression of bone resorption genes in
osteoarthritis and in osteoporosis. J Bone Miner Metab. 25:219–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An J, Hao D, Zhang Q, Chen B, Zhang R,
Wang Y and Yang H: Natural products for treatment of bone erosive
diseases: The effects and mechanisms on inhibiting
osteoclastogenesis and bone resorption. Int Immunopharmacol.
36:118–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He ZH, He MF, Ma SC and But PP:
Anti-angiogenic effects of rhubarb and its anthraquinone
derivatives. J Ethnopharmacol. 121:313–317. 2009. View Article : Google Scholar
|
|
20
|
Matsuda H, Tewtrakul S, Morikawa T and
Yoshikawa M: Anti-allergic activity of stilbenes from Korean
rhubarb (Rheum undulatum L.): Structure requirements for inhibition
of antigen-induced degranulation and their effects on the release
of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem.
12:4871–4876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paneitz A and Westendorf J: Anthranoid
contents of rhubarb (Rheum undulatum L.) and other Rheum species
and their toxicological relevance. Eur Food Res Technol.
210:97–101. 1999. View Article : Google Scholar
|
|
22
|
Matsuda H, Morikawa T, Toguchida I, Park
JY, Harima S and Yoshikawa M: Antioxidant constituents from
rhubarb: Structural requirements of stilbenes for the activity and
structures of two new anthraquinone glucosides. Bioorg Med Chem.
9:41–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi RJ, Chun J, Khan S and Kim YS:
Desoxyrhapontigenin, a potent anti-inflammatory phytochemical,
inhibits LPS-induced inflammatory responses via suppressing NF-κB
and MAPK pathways in RAW 264.7 cells. Int Immunopharmacol.
18:182–190. 2014. View Article : Google Scholar
|
|
24
|
Choi SZ, Lee SO, Jang KU, Chung SH, Park
SH, Kang HC, Yang EY, Cho HJ and Lee KR: Antidiabetic stilbene and
anthraquinone derivatives from Rheum undulatum. Arch Pharm Res.
28:1027–1030. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Redlich K and Smolen JS: Inflammatory bone
loss: Pathogenesis and therapeutic intervention. Nat Rev Drug
Discov. 11:234–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta deltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Ngoc TM, Minh PT, Hung TM, Thuong PT, Lee
I, Min BS and Bae K: Lipoxygenase inhibitory constituents from
Rhubarb. Arch Pharm Res. 31:598–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi B, Kim S, Jang BG and Kim MJ:
Piceatannol, a natural analogue of resveratrol, effectively reduced
beta-amyloid levels via activation of alpha-secretase and matrix
metalloproteinase-9. J Funct Foods. 23:124–134. 2016. View Article : Google Scholar
|
|
30
|
Pawlus AD, Sahli R, Bisson J, Rivière C,
Delaunay JC, Richard T, Gomès E, Bordenave L, Waffo-Téguo P and
Mérillon JM: Stilbenoid profiles of canes from vitis and
muscandinia species. J Agric Food Chem. 61:501–511. 2013.
View Article : Google Scholar
|
|
31
|
He X, Andersson G, Lindgren U and Li Y:
Resveratrol prevents RANKL-induced osteoclast differentiation of
murine osteoclast progenitor RAW 264.7 cells through inhibition of
ROS production. Biochem Biophys Res Commun. 401:356–362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamasaki T, Ariyoshi W, Okinaga T, Adachi
Y, Hosokawa R, Mochizuki S, Sakurai K and Nishihara T: The dectin 1
agonist curdlan regulates osteoclastogenesis by inhibiting nuclear
factor of activated T cells cytoplasmic 1 (NFATc1) through Syk
kinase. J Biol Chem. 289:19191–19203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilson SR, Peters C, Saftig P and Brömme
D: Cathepsin K activity-dependent regulation of osteoclast actin
ring formation and bone resorption. J Biol Chem. 284:2584–2592.
2009. View Article : Google Scholar
|
|
34
|
Shen T, Wang XN and Lou HX: Natural
stilbenes: An overview. Nat Prod Rep. 26:916–935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chachay VS, Kirkpatrick CM, Hickman IJ,
Ferguson M, Prins JB and Martin JH: Resveratrol-pills to replace a
healthy diet? Br J Clin Pharmacol. 72:27–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tou JC: Resveratrol supplementation
affects bone acquisition and osteoporosis: Pre-clinical evidence
toward translational diet therapy. Biochim Biophys Acta.
1852:1186–1194. 2015. View Article : Google Scholar
|
|
37
|
Kim JH and Kim N: Regulation of NFATc1 in
osteoclast differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ikeda F, Nishimura R, Matsubara T, Tanaka
S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, et
al: Critical roles of c-Jun signaling in regulation of NFAT family
and RANKL-regulated osteoclast differentiation. J Clin Invest.
114:475–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Böhm C, Hayer S, Kilian A, Zaiss MM,
Finger S, Hess A, Engelke K, Kollias G, Krönke G, Zwerina J, et al:
The alpha-isoform of p38 MAPK specifically regulates arthritic bone
loss. J Immunol. 183:5938–5947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SE, Woo KM, Kim SY, Kim HM, Kwack K,
Lee ZH and Kim HH: The phosphatidylinositol 3-kinase, p38, and
extracellular signal-regulated kinase pathways are involved in
osteoclast differentiation. Bone. 30:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|