Introduction
Coronary heart disease is one of the leading causes
of mortality globally (1).
Myocardial ischemia/reperfusion (I/R) injury is a common
cardiovascular problem that leads to augmented cardiovascular
dysfunction and further cell death following myocardial ischemia or
cardiac operation in patients with coronary heart disease (2). The mechanisms of I/R injury are
complicated and multifactorial, including excessive reactive oxygen
species (ROS) production, intracellular calcium imbalance,
mitochondrial dysfunction, exaggerated inflammation and/or
programmed cell death (3,4). Notably, excessive intracellular ROS
production associated with apoptotic cell death has a direct effect
on the cellular structure and function in myocardial tissue injury
during myocardial ischemia and in particular, the myocardial
reperfusion phase (5). Therefore,
preventing oxidative stress and cardiomyocyte apoptosis may be an
effective treatment for coronary heart disease.
Myrica rubra (Lour.) S. et Zucc., which is of
high nutritional and medicinal value, is an important subtropical
fruit tree that is widely distributed in China and other Asian
countries (6,7). The fruit is very appealing due to
its pleasant sweet/sour flavor and is also popularly applied in
wine- and juice-making (8).
Additionally, the bark of M. rubra (Myricae cortex) is
traditionally used as a natural drug for treating bruises, swelling
and stomach and duodenal ulcers in Japan and China. Pharmacological
studies have demonstrated that M. rubra extract exhibits
various biological functions, including antioxidant,
anti-inflammatory, antibacterial and anticancer activities
(9,10). Numerous phytochemicals, including
flavonoids, tannins and triterpenes, can be isolated from M.
rubra (11,12). Notably, flavonoids, including
myricetin and quercetin, which are major constituents of M.
rubra, have drawn considerable attention because of their
health-promoting functions (13,14). Previous studies have revealed that
M. rubra flavonoids exhibit strong cellular antioxidant
activity (15) and possess
excellent lipid-lowering activities (14). These results suggest that M.
rubra flavonoids hold immense possibility to be developed as a
novel natural agent for preventing and treating cardiovascular
disease. However, the cardioprotective effects of M. rubra
flavonoids (MRF) against I/R injury to cardiac myocytes remain
unknown.
Therefore, in the present study, the protective
effects of MRF against isoproterenol (ISO)-mediated myocardial
injury were first examined in vivo and hypoxia/reoxygenation
(H/R)-induced cardiomyocyte injuries in vitro. Furthermore,
the role of the phosphoinositide 3-kinase (PI3K)-protein kinase B
(Akt) signaling pathway in the cardioprotection of MRF was
investigated.
Materials and methods
Preparation of MRF
The bark of M. rubra was purchased from the
local market in Ningbo (Zhejiang, China). MRF was provided and
chemically identified at the Institute of Medicinal Plant
Development (Beijing, China) (16). Briefly, the sliced bark of M.
rubra (500 g) was extracted with methanol using reflux
extraction three times (each time for 1 h). The extracts were
combined and evaporated in vacuo. The concentrate was
diluted with distilled water, subjected to a column containing D101
macroporous resin (1 kg; Cangzhou Bon Cang Bon Adsorber Technology
Co., Ltd., Cangzhou, China) and eluted successively with
ethanol-water (1:9, v/v and ethanol-water (7:3, v/v). The ethanol
was evaporated in vacuo to yield a pale yellow residue (35 g) for
further uses.
Ultra-performance liquid chromatography
(UPLC) analysis of MRF
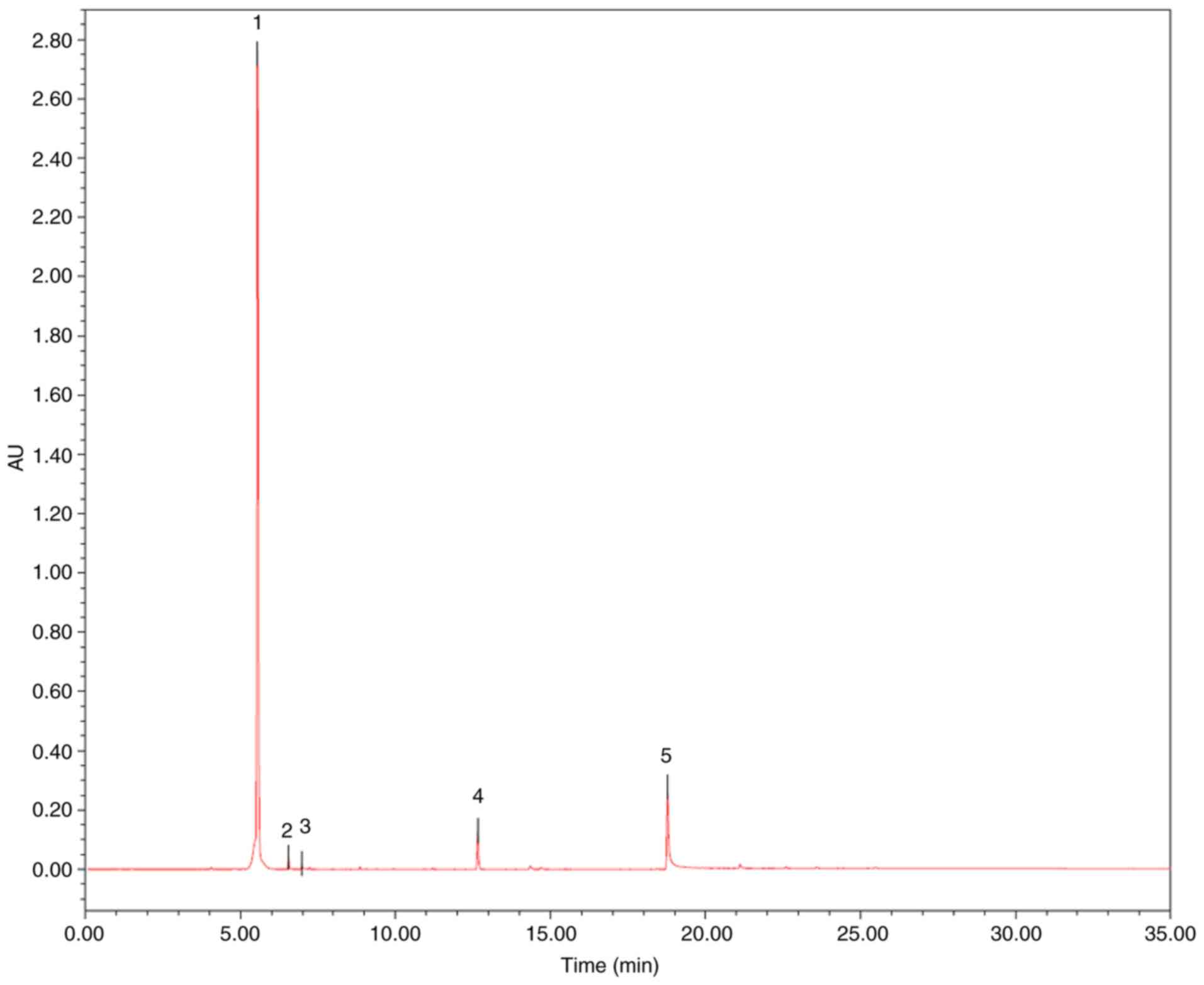
The contents of three markers in MRF were analyzed
using UPLC analysis (16), which
was performed on a Waters ACQUITY UPLC (ELSD) system. An ACQUITY
UPLC BEH C18 (50x2.1 mm ID, 1.7 µm) was used with an injection
volume of 2 µl for UPLC separation. The mobile phases consisted of
(A) acetonitrile and (B) H2O at a flow rate of 0.4
ml/min. The gradient elution was used as follows: 0→30 min, 5-95%
A; 30→35 min, 95% A. Column temperature was set as 40°C. UV
absorption was measured at 354 nm. All solutions were filtered
through a 0.22 µm filter prior to detection. Peak identification in
the samples was performed with the retention time compared with the
standard. The content of each compound was quantified using the
external standard method using the area under the peak. As
presented in Fig. 1 and Table I, the contents of myricitrin,
quercetin-3-O-rhamnoside and quercetin were 85.52, 0.86 and 0.69%,
respectively.
 | Table IUltra-pressure liquid chromatography
quantification of Myrica rubra flavonoids. |
Table I
Ultra-pressure liquid chromatography
quantification of Myrica rubra flavonoids.
| Peak | Chemical name | tR (min) | Area (mv*s) | Height (mv) | % Area |
|---|
| 1 | Myricitrin | 5.545 | 9152352 | 2742517 | 85.52 |
| 2 |
Quercetin-3-O-rhamnoside | 6.552 | 92418 | 40823 | 0.86 |
| 3 | Quercetin | 6.982 | 74023 | 18571 | 0.69 |
| 4 | Myricanol | 12.656 | 378747 | 130856 | 3.54 |
| 5 | Myricanone | 18.779 | 1004491 | 273583 | 9.39 |
Animals
A total of 90 8 week-old male Sprague-Dawley rats
weighing 200-220 g were purchased from the Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). The
animals were housed under standard laboratory conditions (25±1°C,
60% humidity and 12 h photoperiod), provided with standard rodent
chow and allowed free access to water. All procedures were approved
by the Laboratory Animal Ethics Committee of the Institute of
Medicinal Plant Development, Peking Union Medical College (Beijing,
China) and complied with the Guide for the Care and Use of
Laboratory Animals published by the US National Institute of Health
(NIH Publication, 8th edition, 2011).
Experimental protocols
A total of 90 Sprague-Dawley rats were randomly
assigned to six groups: 1, Control; 2, ISO treatment; 3, ISO with
51 mg/kg MRF; 4, ISO with 10 mg/kg MRF; 5, ISO with 20 mg/kg MRF;
and 6, Di-ao-xin-xue-kang capsule (Di-ao, 80 mg/kg) as positive
control. Groups 1 and 2 were intragastrically provided with the
vehicle (1% Tween 80). Groups 3, 4 and 5 were intragastrically
dosed with MRF (5, 10 and 20 mg/kg) for 15 days. Group 6 was
intragastrically administered with Di-ao (80 mg/kg) for 15 days.
Following 1 h of MRF and Di-ao administration on days 14 and 15,
rats in groups 2 to 6 were injected with ISO (4 mg/kg, injectio
hypodermaticus), whereas rats in group 1 received saline
solution.
Preparation of samples and measurement of
biochemical variables
Following intraperitoneally anesthetizing the rats
(320-350 g) with urethane solution (1 g/kg), blood samples (5 ml)
were collected for serum creatine kinase (CK), aspartate
aminotransferase (AST) and lactate dehydrogenase (LDH) measurement
using the appropriate kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Following the preparation of blood
samples, the hearts were excised and myocardial homogenates were
prepared for analyzing malondialdehyde (MDA), superoxide dismutase
(SOD) and catalase (CAT) activities using the corresponding kits
(Nanjing Jiancheng Bioengineering Institute). Experiments were
performed according to the manufacturer's protocol.
Histopathological examination
The heart apex was fixed in 10% formalin for 24 h at
room temperature, routinely processed and embedded in paraffin. The
paraffin sections (3 mm) were cut on glass slides, stained with
hematoxylin for 5 min and eosin for 1 min (H&E) at room
temperature and examined under a light microscope (CKX41; Olympus
Corporation, Tokyo, Japan). Examination was performed by a
pathologist blinded to the experimental groups.
Cell culture and treatment
Rat embryonic cardiomyo-blast-derived H9c2
cardiomyocytes (Cell Bank of the Chinese Academy of Sciences,
Shanghai, China) were cultured as previously described (17). Briefly, H9c2 cells were cultured
in high-glucose Dulbecco's modified Eagles medium (DMEM)
supplemented with 10% (v/v) fetal bovine serum (both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 1%
penicillin/streptomycin (v/v) and 2 mM L-glutamine at 37°C with 5%
CO2 incubation. For all the experiments, the cells were
plated at an appropriate density in accordance with the
experimental design and were grown for 24 h to reach 70-80%
confluence before experimentation.
The H/R model was built according to previously
published methods (17,18). H9c2 cardiomyocytes were cultured
under hypoxia for 6 h and then removed from the anaerobic glove box
(TYPE C; Coy Laboratory Products, Inc., Grasslake, MI, USA) to a
regular incubator with the medium replaced by normal medium to
mimic reperfu-sion. In the MRF-treated group, the H9c2
cardiomyocytes subjected to H/R were treated with MRF (6.25 µg/ml)
for 12 h. In the inhibitor-treated group, the cells were
pre-incubated with 20 µM LY294002 for 1 h prior to treatment with
MRF. The concentration of LY294002, was determined based on data
present in the literature and the authors' preliminary experiments
(19).
Cell viability analysis
Cell viability was determined using an MTT assay as
previously described (20). H9c2
cells were seeded at a density of 1x104 cells/well in
96-well plates. Following the different treatments, the cells were
incubated with 20 µl of MTT (5 mg/ml) each well for 4 h. The
supernatant was subsequently removed and the formazan crystals were
dissolved in dimethyl sulfoxide. The absorbance was detected at 570
nm using a microplate reader (Infinite M1000; Tecan Group, Ltd.,
Mannedorf, Switzerland).
Measurement of LDH and MDA levels and
SOD, CAT and glutathione-peroxidase (GSH-PX) activities
H9c2 cells were cultured in six-well plates at
5x105 cells/well. The cultured cells were incubated with
different MRF concentrations following exposure to hypoxia. The
supernatant and cells were then collected following different
treatments to determine the LDH and MDA levels and SOD, CAT and
GSH-PX activities using the corresponding detection kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) in accordance
with the manufacturers' protocol (21). For the LDH release assay, the cell
medium was removed for the analysis of extracellular LDH activity,
which could catalyze the conversion of lactate to pyruvate and then
reacted with 2,4-dinitrophenylhydrazine to give the brownish red
color in basic solution. After each reaction, the sample was
analyzed and the absorbance was read at wavelength 440 nm. The
results were expressed as U/L. For the lipid peroxidation assay,
MDA was measured as an indicator of lipid peroxidation according to
the thiobarbituric acid (TBA) method. The method was based on the
spectrophotometric measurement of the red color produced during the
reaction to TBA with MDA. The optical density was measured at 532
nm. For the SOD activity assay, the SOD activity was detected with
an assay kit according to xanthine oxidase method. The assay used
the xanthine-xanthine oxidase system to produce superoxide anions,
which react with
2-(4-iodophenyl)-3-(4-ni-trophenol-5-phenyltetrazolium chloride) to
form a red formazan dye and the absorbance at 550 nm was
determined. For the CAT activity assay, the hydrogen peroxide
(H2O2) was catalyzed by CAT for 1 min at 37°C
and then terminated by forming stable complexes with ammonium
molybdate. The CAT level was calculated by recording the visible
absorbance of these complexes at 405 nm. For the GSH-Px activity
assay 1 mmol L-1 GSH was incubated with cells for 5 min
at 37°C and then centrifuged for 10 min at 1,800 x g at room
temperature. The GSH in the supernatant reduced 5,5′-dithiobis
2-nitrobenzoic acid to 2-nitro-5-thiobenzoate anion (NTP). The
GSH-Px level was calculated by recording the absorbance of NTP at
412 nm. Non-enzymatic control was used to eliminate the
interference from endogenous GSH.
Detection of intracellular ROS
production
Intracellular ROS production was monitored using a
total ROS detection kit in accordance with the manufacturer's
protocol (Enzo Life Sciences, Inc., Farmingdale, NY, USA). After
different treatments, the cells were harvested and washed with 1X
wash buffer. Subsequently, the supernatant was discarded and the
cells were incubated with 500 µl of ROS detection solution and
stained in the dark at 37°C for 30 min. The fluorescence was
analyzed using a flow cytometer with CellQuest software, version
5.0 (FACS Calibur™, BD Biosciences; Becton, Dickinson and Company,
Franklin Lakes, NJ, USA) (22).
Flow cytometric detection of cell
apoptosis rate
The percentages of early apoptosis and necrosis were
detected using an Annexin V FITC/PI apoptosis kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Following drug treatment, the
cells were harvested, washed twice with cold PBS and incubated in
the dark with 5 µl of FITC-Annexin V and 1 µl of PI working
solution (100 µg/ml) for 15 min at room temperature. Apoptosis rate
was measured through flow cytometry analysis.
Determination of mitochondrial
transmembrane potential (∆Ψm)
JC-1 (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to determine the effect of MRF on MMP. After treatments,
H9c2 cells (5x105 cells/well) were harvested and
incubated with 2 µM JC-1 at 37°C for 30 min in the dark and washed
twice with PBS. Cells labeled with JC-1 were analyzed by BD
FACSCalibur flow cytometry using 488 nm excitation and green (525
nm) or orange-red (575 nm) emission wavelengths with CellQuest
software, version 5.0 (BD Biosciences; Becton, Dickinson and
Company). The change in mitochondrial membrane potential was
expressed as the ratio of red to green fluorescence intensity.
Analysis of caspase-3 activation
Caspase-3 activity was measured using a fluorescein
active caspase-3 staining kit (BioVision, Inc., Milpitas, CA, USA).
Briefly, 300 µl (1x106 cells/ml) of culture was
incubated with 1 µl of the substrate FITC-DEVD-FMK for 1 h at 37°C.
Then the supernatant was removed and the cells were resuspended in
300 µl buffer following being washed twice with wash buffer and
subjected to a microplate reader (Infinite M1000; Tecan Group,
Ltd.) at 400 nm excitation and 505 nm emission wavelength.
Western blot analysis
Total cell lysate preparation and western blot
analysis were performed as previously described (20). Briefly, H9c2 cardiomyocytes were
lysed in Mammalian Protein Extraction Reagent (CWBioTech, Beijing,
China) containing 1% phenylmethylsulfonyl fluoride. Equal amounts
of protein (20 µg) from each sample were separated by 10% SDS-PAGE
and then transferred onto a nitrocellulose membrane. After being
blocked (2 h, room temperature) with 5% (w/v) non-fat milk powder,
the membranes were incubated overnight at 4°C with appropriate
primary antibodies. The primary antibodies (Abcam, Cambridge, UK)
used were as follows: Rabbit monoclonal anti-Akt1 (phospho S473)
antibody (cat. no. ab81283; 1:2,000), rabbit monoclonal
anti-Akt1/2/3 antibody (cat. no. ab185633; 1:2,000), rabbit
polyclonal anti-GSK3 β (phospho S9) antibody (cat. no. ab131097;
1:1,000), rabbit poly-clonal anti-GSK3 β antibody (cat. no.
ab131356; 1:1,000), rabbit polyclonal anti-Bcl-2 antibody (cat. no.
ab196495; 1:1,000), rabbit polyclonal anti-Bax antibody (cat. no.
ab199677; 1:1,000) and rabbit polyclonal anti-β actin antibody
(cat. no. ab8227; 1:1,000). After washing, the membranes were
incubated for 1 h with the respective horseradish
peroxidase-conjugated secondary antibodies at room temperature.
Finally, the membranes were developed by enhanced chemiluminescence
using a ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Densitometric analysis of the bands was
performed using Gel Pro Analyzer version 6.0 (Media Cybernetics,
Inc., Rockville, MD, United States).
Statistical analysis
Results are expressed as the mean ± standard
deviation of three independent experiments. Comparisons between
different groups were performed using a Student's t-test or one-way
analysis of variance followed by post hoc analysis with Tukey's
multiple comparison test using Prism 5.00 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
MRF prevents ISO-induced cardiac injury
in rats
The levels of serum cardiac enzymes (CK, AST and
LDH) and the activities of anti-oxidant enzymes (MDA, SOD, and CAT)
were determined in the different animal groups to analyze the role
of MRF on ISO-induced myocardial injury in rats. Fig. 2A and B demonstrated that MRF
treatment significantly decreased the levels of LDH, CK, AST and
MDA (P<0.05), as well as enhanced the activities of SOD and CAT,
relative to those of the ISO group (P<0.05). Morphological
alterations in the myocardial cells were determined through H&E
staining under a light microscope. No abnormal changes were
detected in the control and positive control groups, whereas
considerable myocardial necrosis, fibrosis and neutrophil
granulocyte infiltration of the heart were observed in the ISO
group. MRF pretreatment groups markedly reduced the pathological
alterations by ISO (Fig. 2C).
 | Figure 2Effects of MRF on ISO-induced
myocardial injury in vivo. (A) The effects of MRF on the
levels of serum cardiac enzymes (CK, AST and LDH). (B) The effects
of MRF on the activities of MDA, SOD and CAT in myocardial
homogenates in rats. (C) The effects of MRF on the pathological
alterations in rat hearts as indicated by hematoxylin and eosin
staining (magnification, x200). Data (n=10 per group) are expressed
as the mean ± standard deviation. #P<0.05,
##P<0.01 and ###P<0.001 vs. the control group;
*P<0.05, **P<0.01 and
***P<0.001 vs. the ISO group. MRF, Myrica
rubra flavonoids; Di-ao, Di-ao-xin-xue-kang capsule; ISO,
isoproterenol; CK, creatine kinase; AST, aspartate
aminotransferase; LDH: Lactate dehydrogenase; MDA, malondialdehyde
SOD, superoxide dismutase; CAT, catalase. |
MRF ameliorates the H/R-induced
cytotoxicity in H9c2 cardiomyocytes
The protective effect of MRF against H/R-induced
cell death was detected using an MTT assay. The cells were exposed
to hypoxia for 6 h to mimic injury and then subjected to different
MRF concentrations (1.5625, 3.125 and 6.25 µg/ml) for different
periods (4, 12 and 24 h). Fig. 3
demonstrates that MRF treatment significantly alleviated the
H/R-induced reduction in cell viability and 6.25 µg/ml MRF for 12 h
exhibited the most significant protective effect (P<0.05).
Therefore, 6.25 µg/ml MRF for 12 h was chosen for further
experiments. As an indicator of cell injury, LDH levels were
measured. As presented in Fig.
3B, MRF treatment significantly dose-dependently decreased the
LDH levels in the culture medium (P<0.05).
MRF reduces oxidative stress by H/R in
H9c2 cardiomyocytes
The membrane lipid oxidation level in oxidative
damage was detected by MDA formation (23). In Fig. 4, the H/R group exhibited a
significant increase in intracellular MDA levels (P<0.01),
whereas the MRF treatment groups prevented MDA formation compared
with the H/R group. In addition, MRF treatment effectively enhanced
the activities of the endogenous antioxidative enzymes SOD, CAT and
GSH-Px relative to that of the H/R group.
 | Figure 4Effects of MRF on lipid oxidation and
antioxidative activities in H/R-induced H9c2 cell injury. Cells
incubated with different concentrations (1.5625, 3.125 and 6.25
µg/ml) of MRF for 12 h following hypoxia for 6 h. The data are
presented as the mean ± standard deviation, n=3.
#P<0.05 vs. the control, ##P<0.01 vs.
the control; *P<0.05 vs. H/R-treated cells,
**P<0.01 vs. the H/R-treated cells. MRF, Myrica
rubra flavonoids; H/R, hypoxia/reoxygenation; SOD, superoxide
dismutase; CAT, catalase; GSH-Px, glutathione-peroxidase; MDA,
malondiadehyde. |
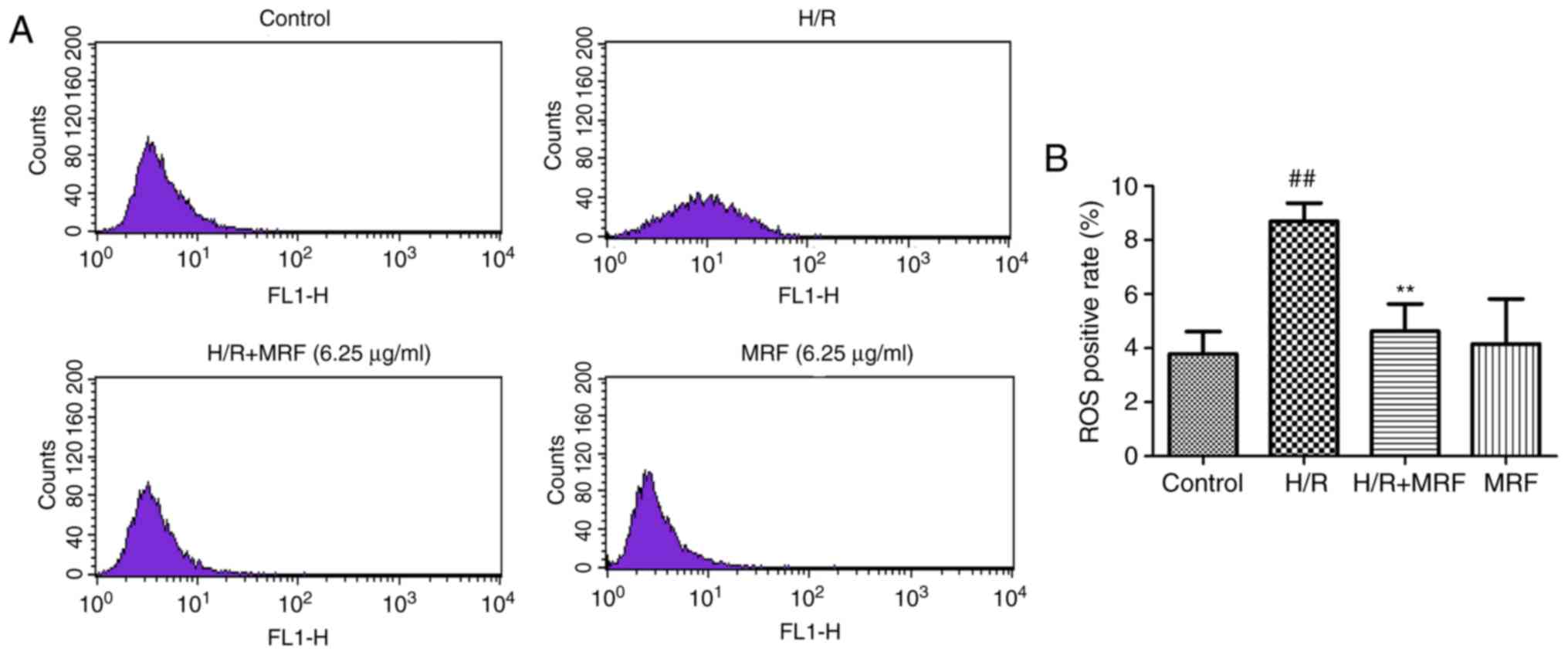
ROS generation is a common response to cell injury
(3). ROS production was monitored
by flow cytometry (Fig. 5A). As
presented in Fig. 5B, the H/R
group significantly increased the intracellular ROS levels compared
with the control (P<0.01). However, MRF post-conditioning
significantly attenuated the intracellular ROS levels induced by
H/R in H9c2 cells (P<0.01). These results revealed that MRF
protects H/R-induced cell injury by inhibiting ROS production.
MRF inhibits H/R-induced apoptosis in
H9c2 cells
The disruption of ∆Ψm is an early marker of
apoptosis (22). Therefore, the
possible effect of MRF on ΔΨm was assessed by JC-1 staining, which
exhibited a potential-dependent accumulation in the mitochondria
(Fig. 6A). The anti-apoptotic
effect of MRF was further corroborated through FITC-Annexin V/PI
double staining (Fig. 6B). Cells
incubated with MRF demonstrated its strong protective effect
against the H/R-induced mitochondrial membrane potential
depolarization (Fig. 6C). The
apoptosis rate significantly increased in the H/R group compared
with the control group (P<0.01), while MRF treatment effectively
alleviated the ratio of apoptotic cells compared with the H/R group
(Fig. 6D).
Caspase-3 serves a key role in regulating the
apoptotic cascade. As presented in Fig. 6E, the caspase-3 activity
signifi-cantly increased in the H/R group but relatively decreased
in the H/R combined with MRF group (P<0.05). Therefore, MRF
exhibited an inhibitory effect on the caspase-3 activity of H9c2
cells.
MRF-induced cell protection depends on the
PI3K/Akt/GSK-3β signal pathway. The PI3K/Akt/GSK-3β pathway
serves an important protective role in myocardial H/R injury
(24). To investigate the
potential signaling pathways contributing to the anti-apoptotic
function of MRF, western blot analysis was used to investigate the
effects of MRF on the expression of proteins associated with the
PI3K/Akt/GSK-3β signaling pathways. The H/R group significantly
decreased the levels of Akt and GSK-3β phosphorylation compared
with the control group (P<0.05; Fig. 7). In response to MRF treatment,
the levels of phospho-Akt and phospho-GSK-3β were well
preserved.
To further assess whether the PI3K/Akt/GSK-3β
signaling pathway is essential for the antiapoptotic effect of MRF,
the pharmacological inhibitor PI3K (LY294002) was adopted in the
experiment. LY294002 reversed the cytoprotection of MRF against H/R
injury by decreasing the cell viability (Fig. 8A), increasing ROS levels (Fig. 8B), downregulating phosphorylation
levels of the pro-survival proteins Akt and GSK-3β, and
antiapoptotic protein Bcl-2 expression as well as upregulating the
pro-apoptotic protein Bax expression (Fig. 8C and D). These results indicated
that the activation of the PI3K/Akt/GSK-3β signaling pathway is
involved in the protective effect of MRF against H/R injury in
cardiomyocytes.
 | Figure 8Effects of LY294002 (PI3K/Akt
inhibitor) on the protection of MRF against H/R-stimulated cell
death and apoptosis. (A) Effects of MRF and LY on cell viability in
H/R-treated cardiomyocytes. (B) Effects of MRF and LY on ROS levels
in H/R-treated cardiomyocytes. The intracellular ROS levels were
measured with a fluorometric assay. (C) Representative western blot
analysis of the protein expression levels of p-Akt, Akt, p-GSK-3β,
GSK-3β, Bcl-2 and Bax in cardiomyocytes. (D) The ratios of
p-Akt/Akt, p-GSK3β/GSK-3β and Bcl-2/Bax were represented in bar
graph. β-actin expression was examined as the protein loading
control. The data are expressed as the mean ± standard deviation
from three independent experiments. #P<0.05 vs. the
control, ##P<0.01 vs. the control;
*P<0.05 vs. the H/R-treated cells,
**P<0.01 vs. the H/R-treated cells.
*P<0.05 vs. H/R+MRF-treated cells and
**P<0.01 vs. the H/R+MRF-treated cells. MRF,
Myrica rubra flavonoids; H/R, hypoxia/reoxygenation; Akt,
protein kinase B; p-GSK-3β, phosphorylated glycogen synthase kinase
3β; LY, LY294002; ROS, reactive oxygen species; PI3K,
phosphoinositide 3 kinase. |
Discussion
Natural flavonoids, which constitute a major part of
effective components in traditional Chinese herbal medicines, are
used as antioxidants for preventing and treating cardiovascular
diseases for a number of years (25). For example, the leaf extracts of
Ginkgo biloba that contain 24-27% flavonoids as the major
effective components have been widely known for their antioxidant
capacities and are utilized to treat cardiovascular diseases
worldwide (26). The bark of
M. rubra is used as an astringent, antidote and
antidiarrheal agent in oriental traditional medicine (12). A number of these flavonoid
compounds possess outstanding antioxidant, anti-inflammatory and
anti-hyperlipidemic effects, which are highly associated with the
prevention of cardiovascular diseases (14,15,27). Particularly, myricitrin and
quercetin 3-rhamnoside, the major constituents of MRF, have been
reported to prevent atherosclerosis and other associated
cardiovascular diseases (28-31). In accordance with previous studies
(25-31) supporting that flavonoids possess
antioxidant actions and the present in vivo study, it was
demonstrated that along with the increased SOD, GSH-Px and CAT
activities and decreased MDA levels, MRF treatment fully prevented
the overgeneration of ROS and neutralized the reduction in cell
viability during H/R. These results directly revealed that the
cardioprotective effects of MRF contributed to their role in
reducing oxidative damage.
Oxidative stress is an essential mechanism causing
cardiac myocyte apoptosis in pathological conditions, including I/R
(32). ROS overproduction alters
the mitochondrial structure and induces mitochondrial
depolarization and the loss of ΔΨm (33), leading to the release of
proapoptotic molecules from the mitochondria and resulting in
apoptosis (33). The results of
the present study strongly demonstrated that MRF conditioning
significantly improved the mitochondrial membrane potential,
decreased the number of apoptotic cells and reduced caspase-3
activation against H/R. Several studies have demonstrated that the
Bcl-2/Bax ratio may decide the cellular threshold for apoptosis
(34,35). It was demonstrated that MRF
enhanced the ratio of Bcl-2/Bax compared with the H/R group. Given
these results, it was hypothesized that MRF exerts cardioprotective
effects by modulating intracellular ROS levels and regulating the
apoptotic cascade.
Extensive studies have demonstrated that activating
PI3K/Akt-dependent signaling prevents cardiac myocyte apoptosis and
attenuates the myocardium from I/R injury (19,29,36). GSK-3β is a critical, active enzyme
that functions downstream of Akt (37). GSK-3β phosphorylation by Akt
results in enzyme inactivation (37). GSK-3β inhibition protects against
organ ischemic injury, oxidative stress and apoptosis (38). Therefore, it was hypothesized that
the cardioprotective effect of MRF against H/R-induced apoptosis in
H9c2 cardiomyocytes is associated with the PI3K/Akt/GSK3β signaling
pathways. As expected, the results of the present study
demonstrated that MRF treatment increased the levels of
phosphorylated Akt and GSK3β compared with the H/R group. Notably,
using the PI3K inhibitor LY294002 demonstrated that the
pharmacological inhibition of PI3K blocked the MRF-induced
cardioprotection against H/R injury as demonstrated by the
decreased cell viability, Bcl-2/Bax ratio and phosphorylation
levels of GSK-3β. Therefore, it was concluded that elevated
myocardial PI3K/Akt signaling and the subsequent increased
phosphorylation of GSK3-β may serve important roles in the
cardioprotective effects of MRF.
In conclusion, this study revealed that MRF protects
H/R-induced apoptosis in H9c2 cardiomyocytes. The pharmacological
actions of MRF promote cardioprotection by attenuating oxidative
stress and inhibiting apoptosis. Furthermore, the results of the
present study indicate that the PI3K/Akt/GSK-3β pathway serves a
critical role in the protective effects associated with MRF
treatment. The present study provides rational evidence on MRF for
further preclinical development of a formulation to improve
cardiovascular disease. However, the overall mechanisms underlying
the antiapoptotic effect of MRF require further investigations by
TUNEL assay. In addition, further studies on the active flavo-noids
and their synergistically mechanisms of MRF against ischemic heart
disease will be necessary.
Funding
The present study was supported by the Major
Scientific and Technological Special Project for 'Significant New
Drugs Formulation' (grant nos. 2012ZX09103201-004 and
2012ZX09501001-004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GBS and XBS made substantial contributions to the
design of the work and interpretation of data for the work. JXY, MW
and RYW took part in the animal experiments in this study. MW, YL,
RLP, RYW, SLD and WRD performed the experiments and collected the
data. MW and YL analyzed the data and wrote the paper. All authors
read and approved the manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Laboratory
Animal Ethics Committee of the Institute of Medicinal Plant
Development, Peking Union Medical College (Beijing, China) and
complied with the Guide for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Moran AE, Roth GA, Narula J and Mensah GA:
1990-2010 Global cardiovascular disease atlas. Glob Heart. 9:3–16.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma V, Bell RM and Yellon DM: Targeting
reperfusion injury in acute myocardial infarction: A review of
reperfusion injury pharmacotherapy. Expert Opin Pharmacother.
13:1153–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerczuk PZ and Kloner RA: An update on
cardioprotection: A review of the latest adjunctive therapies to
limit myocardial infarction size in clinical trials. J Am Coll
Cardiol. 59:969–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marczin N, El-Habashi N, Hoare GS, Bundy
RE and Yacoub M: Antioxidants in myocardial ischemia-reperfusion
injury: Therapeutic potential and basic mechanisms. Arch Biochem
Biophys. 420:222–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu Y, Qiao L, Cao Y, Zhou X, Liu Y and Ye
X: Structural elucidation and antioxidant activities of
proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et
Zucc.) leaves. PLoS One. 9:e961622014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu H, Qi X, Cao S and Li P: Protective
effect of flavonoid extract from Chinese bayberry (Myrica rubra
Sieb. et Zucc.) fruit on alcoholic liver oxidative injury in mice.
J Nat Med. 68:521–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng C, Chen M, Xu CJ, Bai L, Yin XR, Li
X, Allan AC, Ferguson IB and Chen KS: Transcriptomic analysis of
Chinese bayberry (Myrica rubra) fruit development and ripening
using RNA-Seq. BMC Genomics. 13:192012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai GH, Meng GM, Tong YL, Chen X, Ren ZM,
Wang K and Yang F: Growth-inhibiting and apoptosis-inducing
activities of Myricanol from the bark of Myrica rubra in human lung
adeno-carcinoma A549 cells. Phytomedicine. 21:1490–1496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun C, Huang H, Xu C, Li X and Chen K:
Biological activities of extracts from Chinese bayberry (Myrica
rubra Sieb. et Zucc.): A review. Plant Foods Hum Nutr. 68:97–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao J, Morikawa T, Toguchida I, Ando S,
Matsuda H and Yoshikawa M: Inhibitors of nitric oxide production
from the bark of Myrica rubra: Structures of new biphenyl type
diaryl-heptanoid glycosides and taraxerane type triterpene. Bioorg
Med Chem. 10:4005–4012. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda H, Morikawa T, Tao J, Ueda K and
Yoshikawa M: Bioactive constituents of Chinese natural medicines.
VII. Inhibitors of degranulation in RBL-2H3 cells and absolute
stereo-structures of three new diarylheptanoid glycosides from the
bark of Myrica rubra. Chem Pharm Bull (Tokyo). 50:208–215. 2002.
View Article : Google Scholar
|
|
13
|
Hobbs CA, Swartz C, Maronpot R, Davis J,
Recio L, Koyanagi M and Hayashi SM: Genotoxicity evaluation of the
flavonoid, myricitrin, and its aglycone, myricetin. Food Chem
Toxicol. 83:283–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He K, Li X, Xiao Y, Yong Y, Zhang Z, Li S,
Zhou T, Yang D, Gao P and Xin X: Hypolipidemic effects of Myrica
rubra extracts and main compounds in C57BL/6j mice. Food Funct.
7:3505–3515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Chen S, Wei C, Gong H, Li L and
Ye X: Chemical and cellular assays combined with in vitro digestion
to determine the antioxidant activity of flavonoids from Chinese
Bayberry (Myrica rubra Sieb. et Zucc.) leaves. PLoS One.
11:e01674842016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin W, Sun G, Pan R, Shen S and Wang T:
Protective effects of myricetin against injury induced by
H2O2 in vascular endothelial cells. Modern
Chinese Med. 17:443–447. 2015.
|
|
17
|
Wang M, Meng XB, Yu YL, Sun GB, Xu XD,
Zhang XP, Dong X, Ye JX, Xu HB, Sun YF and Sun XB: Elatoside C
protects against hypoxia/reoxygenation-induced apoptosis in H9c2
cardiomyocytes through the reduction of endoplasmic reticulum
stress partially depending on STAT3 activation. Apoptosis.
19:1727–1735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Sun G, Meng X, Wang H, Wang M, Qin
M, Ma B, Luo Y, Yu Y, Chen R, et al: Ginsenoside RK3 prevents
hypoxia-reoxygenation induced apoptosis in H9c2 cardiomyocytes via
AKT and MAPK pathway. Evid Based Complement Alternat Med.
2013:6901902013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Sun GB, Sun X, Wang HW, Meng XB,
Qin M, Sun J, Luo Y and Sun XB: Cardioprotective effect of
salvianolic acid B against arsenic trioxide-induced injury in
cardiac H9c2 cells via the PI3K/Akt signal pathway. Toxicol Lett.
216:100–107. 2013. View Article : Google Scholar
|
|
20
|
Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu
HB, Meng XB, Qin M, Sun J, Wang HW and Sun XB: Oxidative stress
suppression by luteolin-induced heme oxygenase-1 expression.
Toxicol Appl Pharmacol. 265:229–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Wu Y, Liu X, Yan C, Liu D, Pan Y,
Yang G, Yin F, Weng Z, Zhao D, et al: Antioxidant properties of
cis-Z, Z'-3a.7a',7a.3a'-dihydroxyligustilide on human umbilical
vein endothelial cells in vitro. Molecules. 18:520–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun X, Sun GB, Wang M, Xiao J and Sun XB:
Protective effects of cynaroside against
H2O2-induced apoptosis in H9c2
cardio-myoblasts. J Cell Biochem. 112:2019–2029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodrigo R, Libuy M, Feliu F and Hasson D:
Oxidative stress-related biomarkers in essential hypertension and
ischemia-reperfusion myocardial damage. Dis Markers. 35:773–790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu Y, Li L, Yin W, Shen L, You B and Gao
H: Protective effect of proanthocyanidins on anoxia-reoxygenation
injury of myocardial cells mediated by the PI3K/Akt/GSK-3beta
pathway and mitochondrial ATP-sensitive potassium channel. Mol Med
Rep. 10:2051–2058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar
|
|
26
|
Mahady GB: Ginkgo biloba for the
prevention and treatment of cardiovascular disease: A review of the
literature. J Cardiovasc Nurs. 16:21–32. 2002. View Article : Google Scholar
|
|
27
|
Kim HH, Kim DH, Kim MH, Oh MH, Kim SR,
Park KJ and Lee MW: Flavonoid constituents in the leaves of Myrica
rubra sieb. et zucc. with anti-inflammatory activity. Arch Pharm
Res. 36:1533–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE−/− mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin M, Luo Y, Meng XB, Wang M, Wang HW,
Song SY, Ye JX, Pan RL, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathways. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Sun GB, Du YY, Tian Y, Liao P, Liu
XS, Ye JX and Sun XB: Myricitrin protects cardiomyocytes from
hypoxia/reoxygenation injury: Involvement of heat shock protein 90.
Front Pharmacol. 8:3532017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi JS, Bae JY, Kim DS, Li J, Kim JL, Lee
YJ and Kang YH: Dietary compound quercitrin dampens VEGF induction
and PPARgamma activation in oxidized LDL-exposed murine
macrophages: Association with scavenger receptor CD36. J Agric Food
Chem. 58:1333–1341. 2010. View Article : Google Scholar
|
|
32
|
van Empel VP, Bertrand AT, Hofstra L,
Crijns HJ, Doevendans PA and De Windt LJ: Myocyte apoptosis in
heart failure. Cardiovasc Res. 67:21–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaushal GP, Liu L, Kaushal V, Hong X,
Melnyk O, Seth R, Safirstein R and Shah SV: Regulation of caspase-3
and -9 activation in oxidant stress to RTE by forkhead
transcription factors, Bcl-2 proteins, and MAP kinases. Am J
Physiol Renal Physiol. 287:F1258–F1268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu D, Bai XC, Gui L, Su YC, Deng F, Liu B,
Li XM, Zeng WS, Cheng BL and Luo SQ: Hydrogen peroxide in the
Burkitt's lymphoma cell line Raji provides protection against
arsenic trioxide-induced apoptosis via the phosphoinositide-3
kinase signalling pathway. Br J Haematol. 125:512–520. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu CW, Yang F, Cheng SZ, Liu Y, Wan LH
and Cong HL: Rosuvastatin postconditioning protects isolated hearts
against ischemia-reperfusion injury: The role of radical oxygen
species, PI3K-Akt-GSK-3beta pathway, and mitochondrial permeability
transition pore. Cardiovasc Ther. 35:3–9. 2017. View Article : Google Scholar
|
|
37
|
Martin M, Rehani K, Jope RS and Michalek
SM: Toll-like receptor-mediated cytokine production is
differentially regulated by glycogen synthase kinase 3. Nat
Immunol. 6:777–784. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu T, Fang Y, Liu S, Yu X, Zhang H, Liang
M and Ding X: Limb ischemic preconditioning protects against
contrast-induced acute kidney injury in rats via phosphorylation of
GSK-3beta. Free Radic Biol Med. 81:170–182. 2015. View Article : Google Scholar
|