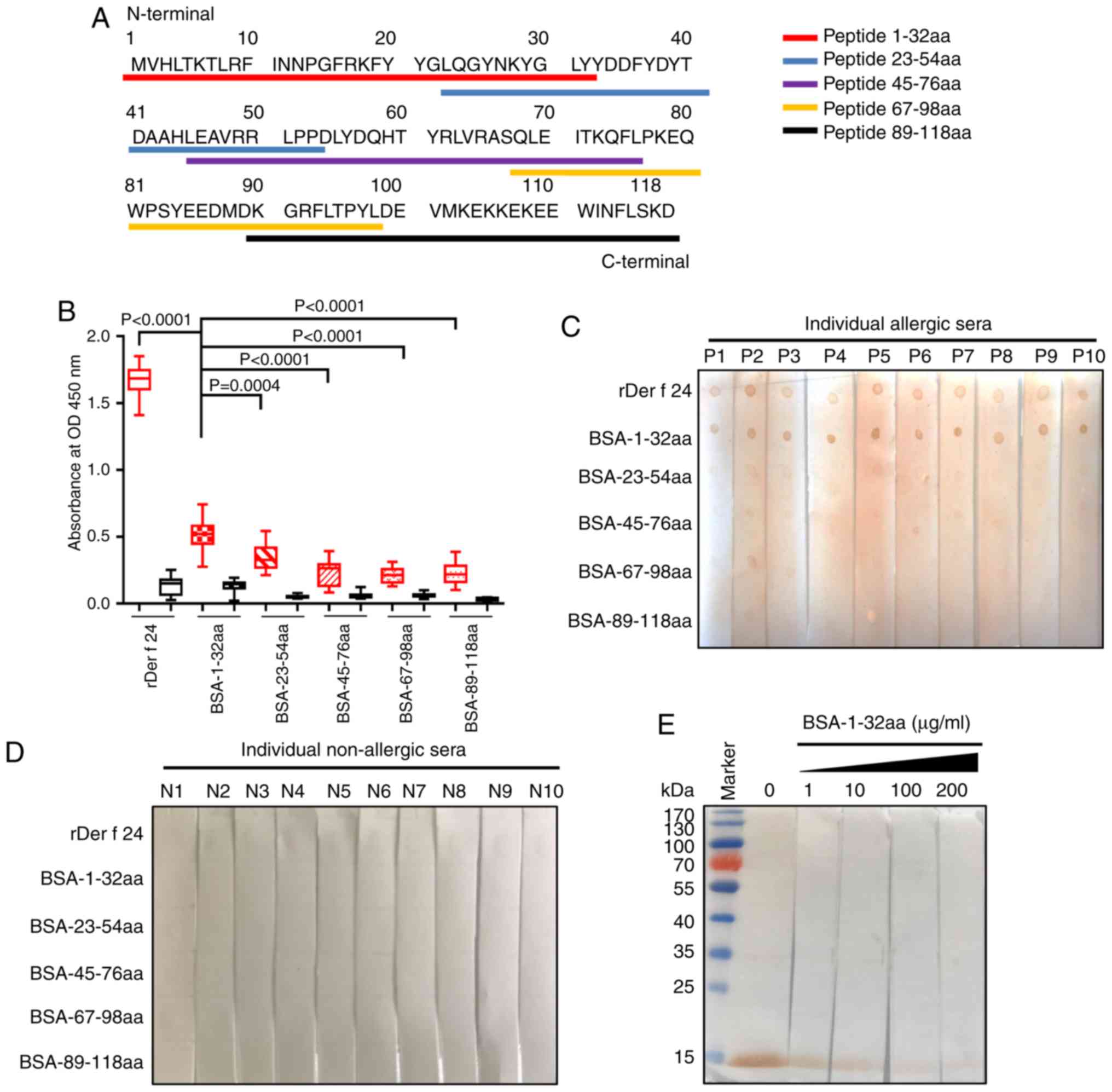
|
1
|
D'Amato G, Liccardi G, D'Amato M and
Holgate S: Environmental risk factors and allergic bronchial
asthma. Clin Exp Allergy. 35:1113–1124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciprandi G, Puccinelli P, Incorvaia C and
Passalacqua G: Italian Cometa Study Group: The relevance of house
dust mites allergy in clinical practice: The epidemiological impact
on allergen immunotherapy. Immunotherapy. 9:1219–1224. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bousquet PJ, Chinn S, Janson C, Kogevinas
M, Burney P and Jarvis D: European Community Respiratory Health
Survey I: Geographical variation in the prevalence of positive skin
tests to environmental aeroallergens in the European community
respiratory health survey I. Allergy. 62:301–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chew GL, Reardon AM, Correa JC, Young M,
Acosta L, Mellins R, Chew FT and Perzanowski MS: Mite sensitization
among Latina women in New York, where dust-mite allergen levels are
typically low. Indoor Air. 19:193–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang L and Zhu R: Immunotherapy of house
dust mite allergy. Hum Vaccin Immunother. 13:2390–2396. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pomés A, Davies JM, Gadermaier G, Hilger
C, Holzhaouser T, Lidholm J, Lopata AL, Mueller GA, Nandy A,
Radauer C, et al: WHO/IUIS allergen nomenclature: Providing a
common language. Mol Immunol. 100:3–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas WR, Smith WA, Hales BJ, Mills KL
and O'Brien RM: Characterization and immunobiology of house dust
mite allergens. Int Arch Allergy Immunol. 129:1–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weghofer M, Grote M, Resch Y, Casset A,
Kneidinger M, Kopec J, Thomas WR, Fernández-Caldas E, Kabesch M,
Ferrara R, et al: Identification of Der p 23, a peritrophin-like
protein, as a new major Dermatophagoides pteronyssinus allergen
associated with the peritrophic matrix of mite fecal pellets. J
Immunol. 190:3059–3067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan TF, Ji KM, Yim AK, Liu XY, Zhou JW,
Li RQ, Yang KY, Li J, Li M, Law PT, et al: The draft genome,
transcriptome, and microbiome of Dermatophagoides farinae reveal a
broad spectrum of dust mite allergens. J Allergy Clin Immunol.
135:539–548. 2015. View Article : Google Scholar
|
|
10
|
Gough L, Schulz O, Sewell HF and Shakib F:
The cysteine protease activity of the major dust mite allergen Der
p 1 selectively enhances the immunoglobulin E antibody response. J
Exp Med. 190:1897–902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gough L, Sewell HF and Shakib F: The
proteolytic activity of the major dust mite allergen Der p 1
enhances the IgE antibody response to a bystander antigen. Clin Exp
Allergy. 31:1594–1598. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trompette A, Divanovic S, Visintin A,
Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini
TL, Weiss JP and Karp CL: Allergenicity resulting from functional
mimicry of a Toll-like receptor complex protein. Nature.
457:585–588. 2009. View Article : Google Scholar
|
|
13
|
Park BS, Lee NR, Kim MJ, Kim SY and Kim
IS: Interaction of Der p 2 with Toll-like receptor 4 and its effect
on cytokine secretion. Biomed Sci Letters. 21:152–159. 2015.
View Article : Google Scholar
|
|
14
|
Hales BJ, Martin AC, Pearce LJ, Laing IA,
Hayden CM, Goldblatt J, Le Souef PN and Thomas WR: IgE and IgG
anti-house dust mite specificities in allergic disease. J Allergy
Clin Immunol. 118:361–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacquet A: Innate immune responses in
house dust mite allergy. ISRN Allergy. 2013.735031:2013.
|
|
16
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar
|
|
17
|
Pomes A: Relevant B cell epitopes in
allergic disease. Int Arch Allergy Immunol. 152:1–11. 2010.
View Article : Google Scholar :
|
|
18
|
Matsuo H, Yokooji T and Taogoshi T: Common
food allergens and their IgE-binding epitopes. Allergol Int.
64:332–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bronnert M, Mancini J, Birnbaum J,
Agabriel C, Liabeuf V, Porri F, Cleach I, Fabre A, Deneux I,
Grandné V, et al: Component-resolved diagnosis with commercially
available D. pteronyssinus Der p 1 Der p 2 and Der p 10: Relevant
markers for house dust mite allergy. Clin Exp Allergy.
42:1406–1415. 2012. View Article : Google Scholar
|
|
20
|
Chou H, Tam MF, Lee SS, Tang RB, Lin TH,
Tai HY, Chen YS and Shen HD: Asp159 is a critical core amino acid
of an IgE-binding and cross-reactive epitope of a dust mite
allergen Der f 7. Mol Immunol. 48:2130–2134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chruszcz M, Chapman MD, Vailes LD, Stura
EA, Saint-Remy JM, Minor W and Pomes A: Crystal structures of mite
allergens Der f 1 and Der p 1 reveal differences in surface-exposed
residues that may influence antibody binding. J Mol Biol.
386:520–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Halleux S, Stura E, VanderElst L,
Carlier V, Jacquemin M and Saint-Remy JM: Three-dimensional
structure and IgE-binding properties of mature fully active Der p
1, a clinically relevant major allergen. J Allergy Clin Immunol.
117:571–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takai T, Kato T, Yasueda H, Okumura K and
Ogawa H: Analysis of the structure and allergenicity of recombinant
pro- and mature Der p 1 and Der f 1: Major conformational IgE
epitopes blocked by prodomains. J Allergy Clin Immunol.
115:555–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho YS, Jung HJ, Seok SH, Payumo AY, Chen
JK and Kwon HJ: Functional inhibition of UQCRB suppresses
angiogenesis in zebrafish. Biochem Biophys Res Commun. 433:396–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai ZL, Chen JJ, Zhang Z, Hou YB, He YS,
Sun JL and Ji K: Identification of immunodominant IgE binding
epitopes of Der p 24, a major allergen of Dermatophagoides
pteronyssinus. Clin Transl Allergy. 9:282019. View Article : Google Scholar :
|
|
26
|
Chen ZG, Li YT, Wang WH, Tan KS, Zheng R,
Yang LF, Guan WJ, Hong HY and Yang QT: Distribution and
determinants of dermatophagoides mites sensitization of allergic
rhinitis and allergic asthma in China. Int Arch Allergy Immunol.
180:17–27. 2019. View Article : Google Scholar
|
|
27
|
Thomas WR: Geography of house dust mite
allergens. Asian Pac J Allergy Immunol. 28:211–224. 2010.
|
|
28
|
Liu XY, Ji KM, Gao B and Liu ZG:
Expression, purification and identification of the recombinant
allergen Der p2 from Dermatophagoides pteronyssinus and
investigation on its immunological activities. Chin J Zoonoses.
009:929–935. 2009.
|
|
29
|
Lauber B, Molitor V, Meury S, Doherr MG,
Favrot C, Tengvall K, Bergvall K, Leeb T, Roosje P and Marti E:
Total IgE and allergen-specific IgE and IgG antibody levels in sera
of atopic dermatitis affected and non-affected Labrador- and Golden
retrievers. Vet Immunol Immunopathol. 149:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taylor JA, Karas JL, Ram MK, Green OM and
Seidel-Dugan C: Activation of the high-affinity immunoglobulin E
receptor Fc epsilon RI in RBL-2H3 cells is inhibited by Syk SH2
domains. Mol Cell Biol. 15:4149–4157. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Wang P, Kim Y, Haste-Andersen P,
Beaver J, Bourne PE, Bui HH, Buus S, Frankild S, Greenbaum J, et
al: Immune epitope database analysis resource (IEDB-AR). Nucleic
Acids Res. 36:Web Server Issue. pp. W513–W518. 2008, View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Costa MA, Duro G, Izzo V, Colombo P,
Mirisola MG, Locorotondo G, Cocchiara R and Geraci D: The
IgE-binding epitopes of rPar j 2, a major allergen of Parietaria
judaica pollen, are heterogeneously recognized among allergic
subjects. Allergy. 55:246–250. 2000. View Article : Google Scholar
|
|
33
|
Banerjee B, Greenberger PA, Fink JN and
Kurup VP: Conformational and linear B-cell epitopes of Asp f 2, a
major allergen of Aspergillus fumigatus, bind differently to
immuno-globulin E antibody in the sera of allergic bronchopulmonary
aspergillosis patients. Infect Immun. 67:2284–2291. 1999.PubMed/NCBI
|
|
34
|
Szalai K, Fuhrmann J, Pavkov T, Scheidl M,
Wallmann J, Brämswig KH, Vrtala S, Scheiner O, Keller W, Saint-Remy
JM, et al: Mimotopes identify conformational B-cell epitopes on the
two major house dust mite allergens Der p 1 and Der p 2. Mol
Immunol. 45:1308–1317. 2008. View Article : Google Scholar
|
|
35
|
Stanley JS, King N, Burks AW, Huang SK,
Sampson H, Cockrell G, Helm RM, West CM and Bannon GA:
Identification and mutational analysis of the immunodominant IgE
binding epitopes of the major peanut allergen Ara h 2. Arch Biochem
Biophys. 34:244–253. 1997. View Article : Google Scholar
|
|
36
|
Laskowski RA, MacArthur MW, Moss DS and
Thornton JM: PROCHECK-a program to check the stereochemical quality
of protein structures. J App Cryst. 26:283–291. 1993. View Article : Google Scholar
|
|
37
|
Ramos JD, Cheong N, Lee BW and Chua KY:
Peptide mapping of immunoglobulin E and immunoglobulin G
immunodominant epitopes of an allergenic Blomia tropicalis
paramyosin, Blo t 11. Clin Exp Allergy. 33:511–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jung HJ, Kim KH, Kim ND, Han G and Kwon
HJ: Identification of a novel small molecule targeting UQCRB of
mitochondrial complex III and its anti-angiogenic activity. Bioorg
Med Chem Lett. 21:1052–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greene WK and Thomas WR: IgE binding
structures of the major house dust mite allergen Der p I. Mol
Immunol. 29:257–262. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van't Hof W, Driedijk PC, van den Berg M,
Beck-Sickinger AG, Jung G and Aalberse RC: Epitope mapping of the
Dermatophagoides pteronyssinus house dust mite major allergen Der p
II using overlapping synthetic peptides. Mol Immunol. 28:1225–1232.
1991. View Article : Google Scholar
|
|
41
|
Herrick CA, MacLeod H, Glusac E, Tigelaar
RE and Bottomly K: Th2 responses induced by epicutaneous or
inhalational protein exposure are differentially dependent on IL-4.
J Clin Invest. 105:765–775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moingeon P and Mascarell L: Novel routes
for allergen immuno-therapy: Safety, efficacy and mode of action.
Immunotherapy. 4:201–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen KW, Blatt K, Thomas WR, Swoboda I,
Valent P, Valenta R and Vrtala S: Hypoallergenic Der p 1/Der p 2
combination vaccines for immunotherapy of house dust mite allergy.
J Allergy Clin Immunol. 130:435–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Winther L, Arnved J, Malling HJ, Nolte H
and Mosbech H: Side-effects of allergen-specific immunotherapy: A
prospective multi-centre study. Clin Exp Allergy. 36:254–260. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nishiyama C, Fukada M, Usui Y, Iwamoto N,
Yuuki T, Okumura Y and Okudaira H: Analysis of the IgE-epitope of
Der f 2, a major mite allergen, by in vitro mutagenesis. Mol
Immunol. 32:1021–1029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ferreira F, Wallner M, Breiteneder H,
Hartl A, Thalhamer J and Ebner C: Genetic engineering of allergens:
Future therapeutic products. Int Arch Allergy Immunol. 128:171–178.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fanuel S, Tabesh S, Mokhtarian K,
Saroddiny E, Fazlollahi MR, Pourpak Z, Falak R and Kardar GA:
Construction of a recombinant B-cell epitope vaccine based on a Der
p1-derived hypoallergen: A bioinformatics approach. Immunotherapy.
10:537–553. 2018. View Article : Google Scholar : PubMed/NCBI
|