1. Introduction
The severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) is the etiological agent of the coronavirus disease
2019 (COVID-19) that was declared as a global pandemic on March 11,
2020. At the time of writing (June 16, 2020) it has infected almost
6 million people with some 450,000 deaths, with numbers rising
daily.
Coronaviruses are single-stranded RNA viruses that
can infect several host species (1) and can be subdivided into α, β, γ,
and δ genera, and with SARS-CoV, Middle East respiratory syndrome
coronavirus (MERS-CoV) and SARS-CoV-2 belonging to β coronaviruses
(1).
Similar to SARS-CoV, SARS-CoV-2 mainly transmits
through respiratory droplets and direct contact (2,3).
However, SARS-CoV-2 seems more infective but less virulent than
SARS-CoV (4,5) as it is also consistent with its
lower rate of lethality, that seems to range between 0.3 and 0.9%
of infected patients (1-4). Like SARS-CoV and other
coronaviruses, SARS-CoV-2 enters into the host cells through
binding with the receptor via the S-spike on the surface of the
virus (4). In addition,
SARS-CoV-2 is uniquely endowed with a furin cleavage site ('RPPA'
sequence) at the S1/S2 site that is likely responsible of its
strong pathogenicity (1). In a
manner similar to SARS-CoV, SARS-CoV-2 employs the
angiotensin-converting enzyme 2 (ACE2) as its receptor but with ten
times higher affinity than the former (6-8).
ACE2 is abundantly expressed in different cells of the lung, heart,
ileum, kidney, bladder and brain and this may explain the variable
clinical symptom-atology induced by SARS-CoV-2 infection (6).
2. The variable clinical courses of COVID-19
infection
Clinical features
COVID-19 manifests with a wide clinical spectrum
ranging from asymptomatic patients to septic shock and multi-organ
dysfunction. Interestingly, it has been reported that smell and
taste dysfunction are associated with COVID-19 (7).
COVID-19 infection is classified into mild,
moderate, severe, and critical depending on the symptoms that are
shown in Table I. The
asymptomatic or mild course is seen in some 80% of the patients,
another 15% experience serious course requiring hospitalization and
5% have a critical illness. The symptoms appear after an incubation
period of approximately 1 week (8). The period from the onset of COVID-19
symptoms to death varies from 1 to 6 weeks with a median of 14 days
(9). This time-frame depends on
the age of the patient and the presence of other comorbidities
being shorter among patients >70-years old (9). It has been reported that for
critical Chinese patients the case fatality rate is 49% (10), that is increased by preexisting
comorbidities such as diabetes (7.3%), respiratory disease (6.5%),
cardiovascular disease (10.5%), hypertension (6%), and oncological
complications (5.6%) (11). The
lack of comorbidities markedly lowers the case fatality rate to
0.9% (11). The clinical
manifestations of COVID-19 infection in the different clinical
course of the disease are synoptically summarized in Table I.
 | Table IClinical manifestation of SARS-CoV-2
infection. |
Table I
Clinical manifestation of SARS-CoV-2
infection.
| Mild | Moderate | Severe | Critical |
|---|
| Fever | Persistent
fever | Dyspnea and
respiratory | Respiratory
failure |
| Dry cough | Shortness of
breath | frequency
≥30/min | Septic shock |
| Fatigue | Early signs of
pneumonia in imaging (multiple ground glass opacities with
consolidation in the peripheral zone of the lung, and/or with
vascular thickening air bronchogram sign, or halo sign) | Blood oxygen
saturation ≤93%, PaO2/FiO2 ratio
<300
Lung infiltrates >50% of the lung field within 24-48 h | Multiple organ
dysfunction/ failure acute kidney injury
Thromboembolic events (stroke, myocardial infarction)
Disseminated intravascular coagulation |
| Sputum
production |
| Sore throat |
| Headache |
| Myalgia or
arthralgia |
| Chills |
| Nausea or
vomiting |
| Nasal
congestion |
| Diarrhea and
hemoptysis |
| Anosmia |
| Ageusia |
3. Aim of the review
This review will focus on the occurrence of mild to
severe derangement of the coagulation system that may range from
inapparent thrombosis to arterial and venous thrombosis in multiple
sites and organs to potentially lethal disseminated intravascular
coagulation (DIC).
On the basis of empirical observations and emerging
laboratoristic findings, we will elaborate the hypothesis that
several cases of thrombotic events during COVID-19 infection
represent the clinical epiphenomenon of a viral-induced secondary
anti-phospholipid antibody syndrome (APS) that, in the most severe
cases, may develop as catastrophic anti-phospholipid antibody
syndrome (CAPS). Diagnostic and therapeutic consequences of this
are discussed.
4. Coagulopathy, thromboembolic events and
DIC during COVID-19 infection
Clinical evidence and emerging data from
pathological examinations indicate that a thrombotic diathesis,
potentially leading to venous thromboembolism (VTE), and to DIC in
some of the most severe cases, may occur in a substantial
proportion of patients with COVID-19 infection, also in a manner
independent of long-term bed rest and eventual hormonal treatment.
We will discuss, in this chapter, laboratoristic analyses, clinical
evidences and interventional studies with anticoagulant therapies
that lend support to the concept that the coagulation system is
severely deranged during COVID-19 infection and may play a key role
in determining the severity of the disease and its rate of
lethality.
Laboratoristic analyses
In an initial and important study, Tang et al
(12) retrospectively analyzed
conventional coagulation results and outcomes of 183 consecutive
patients with confirmed COVID-19 infection. The study demonstrated
that, when evaluated at baseline levels on hospital admission, the
patients that died during the course of the infection by COVID-19
had higher levels of D-dimer and fibrin degradation products (FDP),
along with longer prothrombin and activated partial thromboplastin
times than survivors. In addition, 71.4% of non-survivors and 0.6%
survivors met the criteria of DIC.
This study attracted much attention on the
occurrence and pathogenically significant role of abnormal
coagulation results during severe COVID-19 infection (12).
Lending support to the pathogenic implication of the
abnormal coagulation pathways during COVID-19 infection was a
meta-analysis carried out by Li et al (13) on 10 studies entailing a total of
1,995 cases that reported a significant increase of D-dimer in a
substantial number of patients. Along this line of research, Zou
et al (14) evaluated
retrospectively the abnormalities of the coagulation system and
correlated them with the disease status. The patients were divided
into two groups with mild and severe disease. More males (76.9 vs.
49.8%) and older patients (median age 65 vs. 50) and higher
frequency of other comorbidities were observed in patients with
severe disease. Altogether, 209 abnormalities (69.0%) of
coagulation indexes were observed in the cohort of 303 patients and
were more frequent in patients affected by severe disease (100 vs.
66.1%). The international normalized ratio, the prothrombin time,
the activated partial thromboplastin time, the fibrinogen, the FDP,
and the D-dimer were all significantly augmented in the patients
with severe diseases as compared to those with mild disease. This
study further and clearly supports the concept that coagulation
dysfunction, in particular fibrinogen and D-dimer elevation, is
common in patients with COVID-19, and the degree of elevation is
related to the severity of the disease. The reduction of both
fibrinogen and activated partial thromboplastin time are associated
with recovery (14).
Clinical evidence
Propelled from these laboratoristic observations,
several clinical studies investigated the role of the abnormalities
of coagulation system during COVID-19 infection. An Italian study
evaluated symptomatic patients with laboratory-proven COVID-19
(15). A total of 388 patients
were recruited. In spite of the thromboprophylaxis administered to
all patients, thromboembolic events occurred in 28 (21%) of them.
Forty-four patients underwent VTE imaging tests, that were
confirmed in 16 (36%). Pulmonary embolism was confirmed in 10 out
of 30 patients (33 and 7.7% of total). The rate of ischemic stroke
and acute coronary syndrome /myocardial infarction was 2.5 and
1.1%, respectively. Overt DIC was present in 8 (2.2%) patients.
This study demonstrates that venous and arterial thromboembolic
events is frequent during COVID-19 infection and independent of
thromboprophylaxis and that 50% of events are diagnosed within 24 h
of hospital admission. In addition out of the 11% of total patients
undergoing VTE imaging tests, 16 were positive (36% of tests),
suggesting an underestimation of thromboembolic complications
(15).
Therapeutic intervention with
anticoagulant therapies
That thromboembolism is involved in the clinical
course of COVID-19 infection concurs with the reduction of
mortality rate observed in one study that treated COVID-19 infected
patients with anticoagulant treatment (16). Another retrospective study was
conducted on 449 patients with severe COVID-19 and 99 of them were
on heparin for 7 days or longer (17). The 28-day mortality rate was
positively associated to D-dimer, prothrombin time, and age and
negatively with platelet count. Interestingly, the 28-day mortality
of heparin users was lower than non-users in patients stratified by
the sepsis-induced coagulopathy (SIC) score or D-dimer result with
SIC score ≥4, or D-dimer >6-fold of the upper limit of normal.
These data represent a valuable proof of concept for biomarker
driven approach to heparin use in patients infected with COVID-19
(17).
Evidence is also emerging that ethnicity has major
effects on thrombotic risk, with a 3-4-fold lower risk in Chinese
compared to Caucasians and a significantly higher risk in
African-Americans. When studying coagulopathy in Caucasian patients
infected with COVID-19, Fogarty et al (18) demonstrated that, when treated with
low molecular weight heparin, these patients rarely develop overt
DIC, which is eventually limited to the late stage of the disease.
The authors also propose that the diffuse bilateral pulmonary
inflammation observed in COVID-19 is associated with a novel
pulmonary-specific vasculopathy, named intravascular coagulopathy
(PIC). In agreement with the well-established thrombotic diathesis
of COVID-19 patients, it seems possible that PIC may contribute to
the unexplained emerging differences that highlight racial
susceptibility to COVID-19 mortality.
Taken as a whole, these laboratoristic and clinical
observations and both prospective and retrospective outcomes from
interventional studies employing anticoagulant therapies indicate
an important and probably underestimated role of thromboembolic
complications during COVID-19 and warns on the urgent necessity of
proper diagnostic and therapeutic monitoring of the coagulation
system during the infection. Particularly so in those COVID-19
infected patients with preexisting thrombotic disease, or those who
need prevention or care for their thrombotic disease during the
COVID-19 pandemic (16).
Nonetheless, a recent study demonstrated occurrence
of heparin resistance in some patients with COVID-19 infection as
defined by the need of dose unfractionated heparin of more than
35,000 IU/day to achieve the target aPTT ratio or the impossibility
of doing so (19). Heparin
resistance in these patients was associated to highly increased
levels of Factor VIII level, fibrinogen and d-dimer while almost
all of the antithrombin levels were in the normal range (19).
The emerging role of cytokines complement
as effector mechanisms of inflammation and thrombosis during
COVID-19
Increasing evidence indicates that upregulated
release of proinflammatory cytokines of the innate immune system
secreted either in the vicinity of organ targeted from the virus
(e.g., the alveolar cells of the lung) or in the peripheral
circulation in response to infection by SARS-CoV-2 may represent
the culprit of the immunoinflammatory process (20). Evidence for the occurrence of a
cytokine storm during the occurrence of SARS-CoV-2 infection and
its pathogenic role in determining immunoinflammatory pneumonia and
thrombosis have been repeatedly described with independent
confirmation of augmented circulating levels of these
proinflammatory cytokines (20).
These observations led to the adoption of the anti-IL-6 receptor
monoclonal antibody tocilizumab for the treatment of pneumonia
associated to cytokine storm. It has also been demonstrated that
the local immunoinflammatory response triggered from these
cytokines may lead to complement activation that may amplify the
circuit of immunoinflammation and thrombosis (21). In fact, skin and lung tissues from
5 patients with severe COVID-19 associated with respiratory failure
(n=5) and purpuric skin rash (n=3) had marked deposition of
different terminal complement components in the microvasculature,
and co-localization of COVID-19 spike glycoproteins with C4d and
C5b-9 in the interalveolar septa and the cutaneous microvasculature
of 2 cases examined (22). In a
similar manner, the purpuric skin lesions exhibited a
pauciinflammatory thrombogenic vasculopathy, with deposition of
C5b-9 and C4d in both grossly involved and normally-appearing skin.
These observations suggest that at least certain cases of severe
COVID-19 may be secondary to activation of the complement that
leads to catastrophic microvascular injury and an associated
procoagulant state. That complement may represent an important
therapeutic target for the treatment of severe cases of COVID-19
has been suggested (22-24). A case of a patient with severe
ARDS due to COVID-19 pneumonia who was successfully treated with
the complement C3 inhibitor AMY-101 has been reported (25).
5. Can the thromboembolic diathesis during
some cases of COVID-19 represents secondary form of
anti-phospholipid antibody syndrome? Combining Cartesio deductivism
and Baconian inductivism to prove the hypothesis
Background
The APS is characterized by the occurrence of
multiple episodes of venous and arterial thromboses and recurrent
fetal losses, frequently accompanied by a moderate
thrombocytopenia, in the presence of antiphospholipid (auto)
antibodies (aPL Abs) that are directed against cardiolipin (aCL) or
β2 glycoprotein1 (β2-GP1) (26).
Activation of the complement is also required for the full clinical
manifestation of the APS.
APS can occur idiopathically or it can be associated
with other autoimmune diseases such as systemic lupus erythematosus
(26). Catastrophic
antiphospholipid syndrome (CAPS) is a severe manifestation of APS
(26). Although affecting only 1%
of patients with APS, the condition is frequently fatal if not
recognized and treated early.
Secondary cases of APS due to viral
infections have been reported
Secondary cases of APS due to infectious agents
potentially evolving into CAPS have been reported and include
infections from hepatitis C virus, herpes zoster, as well as
bacteria, fungi and parasites and acute Q fever (27).
The induction of molecular mimicry that leads to
production of anti-β2-GPI autoantibodies has been proposed as
putative cause of secondary APS and CAPS (28,29).
The immunopathogenetic mechanisms that are
subsequently activated entail a network of multiple proinflammatory
factors including the Toll-like receptor 4 (TLR-4), which triggers
a cytokine storm, followed by endothelial alterations that induce a
procoagulant state (30).
Diagnosis and classification criteria of
APS
According to the original criteria formulated in
Sapporo in 1988 and revised in Sidney in 2004, APS can be diagnosed
in the presence of least one clinical (vascular thrombosis or
pregnancy morbidity) and one laboratory including aCL, or
anti-β2-GPI autoantibodies with lupus anticoagulant (LA) (31). LA is a somehow enigmatic
laboratoristic phenomenon observed in patients with APS and
represents a paradox that is still unsolved. LA causes a
phospholipid-dependent prolongation of the clotting time but is
associated with an increased risk of thrombosis and pregnancy
morbidity (32). Recently, Pengo
et al (32) have
demonstrated that LA positivity may identify two different groups
of patients with or without anti-β2-GPI
(LA+/anti-β2-GPI+ and
LA+/anti-β2-GPI-). The
LA+/anti-β2-GPI- group of patients had
anti-phosphatidylserine/prothrombin autoantibodies and consisted of
significantly older patients, with a lower rate of previous
thromboembolic events and a weaker LA activity.
Epidemiology
The incidence of the APS is reported as
approximately 5 new cases per 100,000 persons per year and the
prevalence approximately 40-50 cases per 100,000 persons. The aPL
Abs are positive in approximately 13% of patients with stroke, 11%
with myocardial infarction, 9.5% of patients with deep vein
thrombosis and 6% of patients with pregnancy morbidity (33,34). As it occurs for other autoimmune
diseases, the presence of autoantibodies directed against CL and/or
β2-GPI has been observed in a percentage of healthy individuals
without clinical symptoms of APS ranging from 4.5 to 5.5% (35). In addition, the prevalence of
anti-CL autoantibodies varies with age, having been reported of 2%
in young healthy individuals as compared to 12% of the elderly
(mean age 70) healthy individuals (36). In a recent study conducted in 956
elderly individuals (mean age of 81.1 years; 72% women) positivity
for a PL Abs including aCL, anti-β2-GPI and antiphosphatidyl-serine
autoantibodies was found in 197 (20.6%) of them (37).
What converts aPL Ab(s) positive healthy
individual into APS patients?
In agreement to the second hit hypothesis, it is
thought that inflammatory events, the use of tobacco, alcohol or
obesity and the associated metabolic syndrome may trigger the full
clinical development of APS. These risk factors may be observed in
up to 50% of patients with APS. Findings from an epidemiological
study showed that the risk of myocardial infarction or stroke in
young women with LA is increased in those who smoke or take oral
oestrogenic therapy.
As discussed above for development of secondary
CAPS, it is proposed that after activation of endothelial cells,
monocytes, and platelets by aPL Abs, a procoagulant state is
induced, which is mainly mediated by the increased synthesis of
tissue factor and thromboxane A2. Activation of the complement
cascade might close the loop and provoke thrombosis, often in the
presence of a second hit (38).
Clinical manifestations of APS
APS is characterized by arterial, venous, or small
vessel thrombosis and/or recurrent early pregnancy loss, fetal
loss, or pregnancy morbidity in the presence of aPL Abs that
include the lupus anticoagulant, or moderate-high titer of aCL or
anti-β2-GPI autoanti-bodies (26). CAPS is characterized by thrombosis
in multiple organs and a cytokine storm developing over a short
period, with histopathologic evidence of multiple microthromboses,
and laboratory confirmation of high aPL Abs titers and it is
characterized as widespread acute thrombotic microangiopathy
(26,39).
Standard of care (SOC) treatment for
APS
The only approved SOC treatment for APS relies on
the use of indefinite anticoagulation with a vitamin K antagonist
as the standard care (26).
In addition, several anecdotic reports have proposed
beneficial effects of immunomodulatory agents including
corticosteroids, rituximab, IvIg, D3 vitamin, plasmapheresis and
chloroquine, anti-complement antibody eculizumab and the mTOR
inhibitor rapamycin (26,40,41).
Can thrombosis during COVID-19 and APS be
the same face of two different coins? Exercizing with Cartesio and
the restricted but initiating power of the deductivism
From what we have stated above there are several
similar clinical characteristics that associate COVID-19 and APS
and that culminate with thrombosis and which may represent the
ultimate clinical outcome of eventually overlapping
immunopathogenic pathways.
The 3 main culprits responsible for activating
immunoinflammatory responses and thrombosis appear to be the same
in both diseases, namely upregulated cytokine secretion from cells
of the innate immune system and activated macrophages, thrombus
formation and complement activation. In a similar manner, the
causes of comorbidities that are emerging as capable of worsening
the course of COVID-19 infection are remarkably similar to those
postulated for the second hit hypothesis of APS and entail
immunoinflammatory disorders, such as metabolic syndrome and
obesity and hypertension, as well as the use of tobacco.
Interestingly, elderly patients have more severe course of COVID-19
infection (9) and exhibit per se
higher frequency of aPL-Abs (37).
One would reason that a significant proof
substantiating the deductive reasoning would be that defective
function of B lymphocytes would ameliorate the course of SARS-CoV-2
infection if the hypothesis that these aPL Abs are involved in the
thrombotic diathesis, and this seems actually to be the case, as
patients with agammaglobulinemia, who are unable to produce
anti-aPL Abs, have a moderate course of the disease (42).
Last, but not least, and lending further support to
a deductive exercise of reasoning, both some cases of COVID-19 and
APS seem to respond well to anticoagulant treatment. The beneficial
effects observed with hydroxychloroquine in APS patients (26,43) has also been claimed in COVID-19
patients but it needs, however, formal demonstration (43,44).
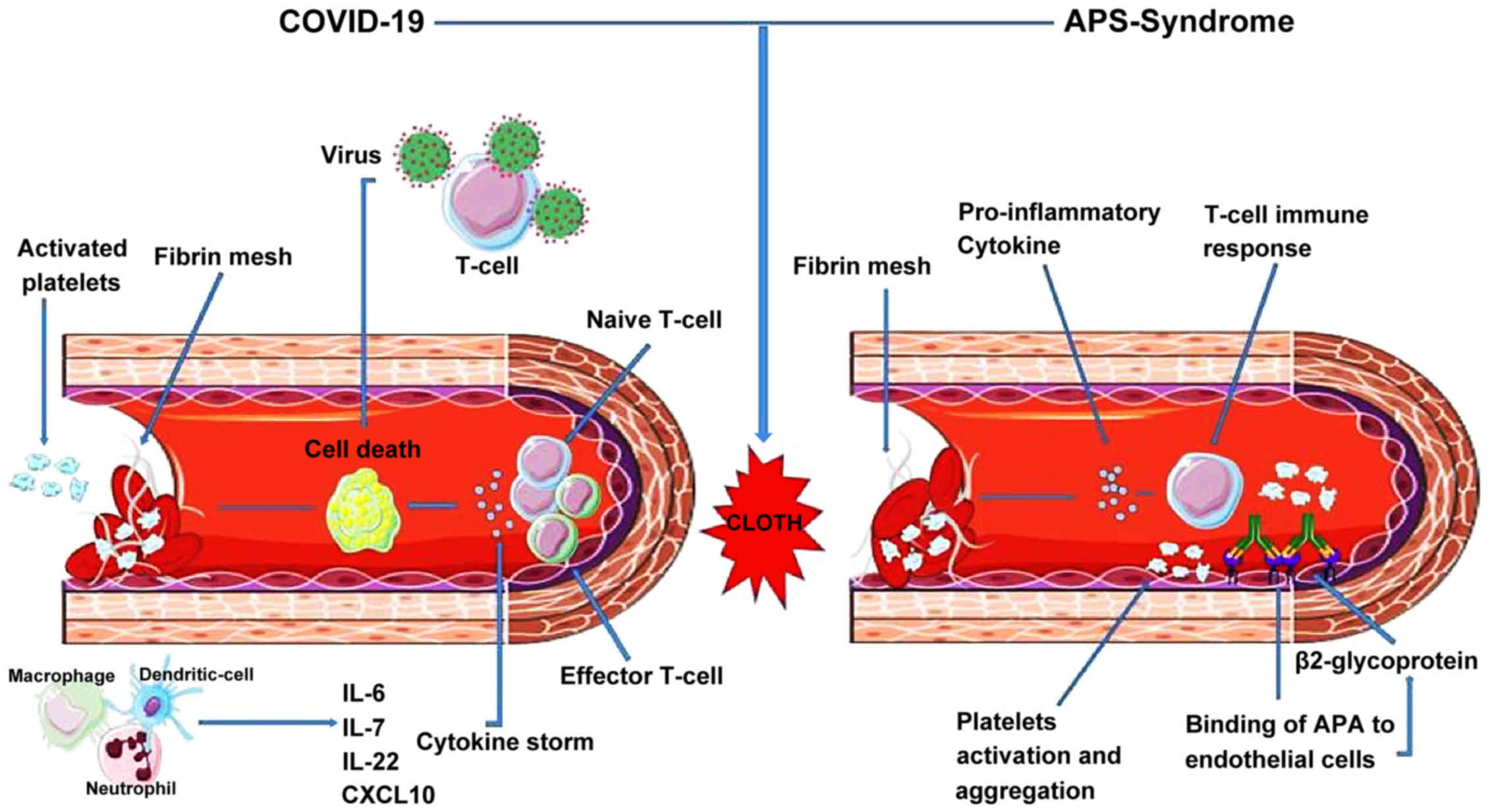
A summary of the similarities between COVID-19 and
Thrombosis/APS is presented in Table
II and in Fig. 1.
 | Table IISimilarities between COVID-19 and
thrombosis/APS. |
Table II
Similarities between COVID-19 and
thrombosis/APS.
| Antiphospholipid
syndrome (revised Sydney classification criteria) | COVID-19 |
|---|
| Altered APTT,
D-dimer elevated | Significantly
higher D-dimer and FDP levels, longer prothrombin time and
activated partial thromboplastin time in non-survivor as compared
to survivors on admission. 71.4% of non-survivors and 0.6%
survivors met the criteria of disseminated intravascular
coagulation (12) |
| Vascular thrombosis
(≥1 clinical episode of arterial, venous, or small vessel
thrombosis) | Abnormal
coagulation parameters in 69.0% (out of 303) cases: FIB, D-dimer,
prolonged PT, altered APTT, elevated FDP. Median INR, PT, APTT,
FIB, FDP, and D-dimer significantly higher in the COVID-19 severe
group compared to the mild group (14) Thromboembolic events among COVID-19
patients occurred at a cumulative rate of 21% (15) |
| Pulmonary
involvement | Large-vessel stroke
in five patients younger than 50 years of age (57) |
| Complement
activation | Pulmonary
intravascular coagulopathy (18)
Deposits of complement components C5b-9, C4d, and MASP2, in the
microvasculature of lung and skin (22) |
| Pregnancy
morbidity | |
| Disregulated
production of cytokine in APS cytokine storm in CAPS | Cytokine storm |
| Anticardiolipin IgG
and/or IgM | Anticardiolipin IgA
antibodies and anti-β2-GP1 |
| Anti-β2-GP1 IgG
and/or IgM | IgA and IgG
antibodies in three COVID-19 patients (45) |
| Lupus
anticoagulant | Lupus anticoagulant
(50,51) |
Satisfying Baconian inductivism to
substantiate the case
Lending important proof of concept support to the
empiric observation that thrombotic phenomena observed during
SARS-CoV-2 infection and APS may recognize common pathogenetic
pathways, and represent eventually the same nosological entity, is
the observation that 3 patients with COVID-19 and ischemic stroke
had anti-CL IgA, as well as anti-β2-GPI IgA and IgG autoantibodies
(45). In another article,
Beyrouti et al (46)
reported 5 cases of ischemic stroke with presence of LA in COVID-19
patients without history of APS. Five of six patients had a
positive LA, one with medium-titre IgM aCL and low-titre IgG and
IgM anti-β2-GPI autoantibodies. Another case report of cerebral
stroke with multiple infarctions during COVID-19 infection and
associated with aCL Abs has also been reported (47).
These emerging lines of evidence have put forward
the hypothesis of performing a routine screening for LA and aPL Abs
in patients with COVID-19 infection (48). Along this line of research,
another group demonstrated that 45% of 56 patients infected with
COVID-19 were positive for LA, while anti-CL or anti-β2-GPI
autoantibodies were detected in only 5 out of 50 tested patients
(10%, 3 associated to LA) using IgG and IgM detection (49). However, thrombotic complications
were not reported in these patients. It is noteworthy that Zhang
et al (45) found IgA
autoantibodies directed against CL or β2-GPI, however, it is not
clear from the paper whether or not the authors have also searched
for the IgA subclass of anti-CL and anti-β2-GPI. Finally, other two
independent studies have demonstrated that 31 out of 34 (50) and 50 out of 57 patients with
COVID-19 infection were positive for LA (51).
Additional important evidence in support of the
contribution of secondary APS as potential mechanism of thrombosis
during COVID-19 infection was provided by Pineton de Chambrun et
al (52) who retrospectively
analyzed LA positivity, aCL (IgM, IgG, IgA), anti-β2-GPI (IgM,
IgG/IgA) and anti-phospholipid (IgM/IgG) autoantibodies in 25
patients with confirmed SARS-CoV-2 infection that were hospitalized
at a tertiary ICU in Paris from March 14 to April 8, 2020. The mean
age of patients at admission was 47.7 (range, 35-64) and
male-to-female ratio was 2.1. All patients had refractory
COVID-19-related ARDS requiring extracorporeal membrane oxygenation
and receiving non-fractioned heparin with an aimed aPPT ratio of
1.5-2. LA, aCL, anti-β2-GP1 and anti-phospholipid Abs were positive
in 23 (92%), 13 (52%), 3 (12%), and 18 (72%) patients,
respectively. The most frequent autoantibody isotype was IgG for
aCL (n=10), IgA for anti-β2-GPI (n=3) and IgG for anti-phospholipid
(n=11) autoasntibodies. When considering LA positivity with any aCL
Abs and any anti-β2-GPI single, double and triple positivity was
found in 8 (32%), 13 (52%) and 3 (12%) of the patients. Triple
negativity was observed only in 1 patient (4%). In addition, serum
fibrinogen was elevated in most patients (72%) at the time of LA
measurement and D-dimer was increased in all the patients. Massive
pulmonary embolism was observed in 6 patients that were all aPL Abs
positive (52). Considering LA,
any anti-cardiolipin and any anti-β2-GP1 antibodies, 8 (32%)
patients had single APLa positivity, 13 (52%) had double
positivity, 3 (12%) had triple positivity and only one (4%) was
triple negative. Other case reports have also demonstrated that
antiCL Abs are associated with thrombosis during COVID-19 infection
(53).
However, not all studies have confirmed these
initial data. In a study conducted in a Hospital in Madrid in 24
patients with COVID-19 infection and VTE, the Authors found that
only two patients (8.3%) were weakly positive for aCL IgM and
anti-β2-glycoprotein I IgM Abs. Anti-CL IgG and
anti-β2-glycoprotein I IgG were negative in all patients (54). The reason for these discrepant
results are unknown and may be due to different ethnicity and/or
different ELISA kits used (54).
Also in light of this latter report, it is clear
that several points remain to be studied to fully dismantle the
potential contribution of SARS-CoV-2-induced secondary APS to at
least some cases of thrombotic events that occur during the
infection with this virus. Testing of aPL Abs and LA in a much
larger number of COVID-19 patients, as well as the eventual
correlation of their titres, with the course of the disease and
thrombotic events is mandatory. Potential fluctuation of aPL Abs in
response to therapy is of clinical relevance as it may represent an
important biomarker. Longitudinal follow-up studies in individuals
that have recovered from COVID-19 infection and that are positive
for aPL Abs and LA will be important to ascertain whether they
revert at recovery of the infection or whether they persist
independently. This is particularly so for the elderly patients
that are per se at higher risk of thrombosis (55).
Understanding if and to what extent COVID-19
infection induces different production of aPL Abs in the elderly
population than in younger individuals is of great relevance as the
temporary or persistent presence of these autoantibodies may
increase further the thrombotic risk and would require particular
therapeutic attention for thromboprophylaxis. Along the same line,
routine testing for LA and aPL Abs should be warranted in elderly
patients during COVID-19 infection, as these patients are at higher
risk of severe infection.
In a similar manner, future studies are required for
those individuals with single, dual or triple positivity for LA and
aPL Abs that are clinically asymptomatic to prove whether they are
at increased risks of thromboembolism in case of COVID-19
infection. Although thromboprophylaxis is currently not approved
for these individuals (26) this
therapeutic approach may need to be revised during COVID-19
pandemic. The testing of large cohort of COVID-19 patients for aPL
Abs is also of outmost relevance to understand the real prevalence
of these autoantibodies in patients with SARS-CoV-2. The available
data seem to indicate that while the percentage of LA positivity
range is approximately 50-80% of the patients, the positivity for
aPL Abs is instead significantly lower and higher for aCL Abs than
anti-β2-GPI Abs according to the study of Pineton de Chambrun et
al (52).
It has also been suggested that the measurement of
aPL Abs in COVID-19 patients may be hindered by the large formation
of microparticles (MP) that are released in the circulation. The
production of these MPs that are endowed with procoagulant activity
is thought to be secondary to the activation induced by cytokines
of several cells including platelets, leukocytes, and also of
endothelial cells that provokes cell blebbing with the shedding of
MPs into the circulation (56).
We strongly believe that the possible definite
demonstration that some or most cases of thrombosis triggered by
COVID-19 are secondary forms of APS is not only important for
semantic classification or diagnostic criteria but may prove of
outmost theranostic relevance for the patients. In particular, on
the basis of their positivity for aPL Abs, it would be possible to
identify the patients that are at greatest risk for developing
thrombotic complications during SARS-CoV-2 infection, including
those healthy individuals that are positive for aPL Abs or LA. In
addition, understanding whether some fatal and unresponsive cases
of DIC observed in COVID-19 may represent cases of CAPS is also of
great relevance as considerable experience has been gained during
these years in the understanding of immunopathogenesis of CAPS. In
particular, though the outcome of CAPS remain unsatisfactory, the
generally accepted therapeutic regime consisting of a triple
combination entailing anticoagulation, corticosteroids and plasma
exchange or intravenous seem to considerably improve the clinical
course of patients who received this treatment (40).
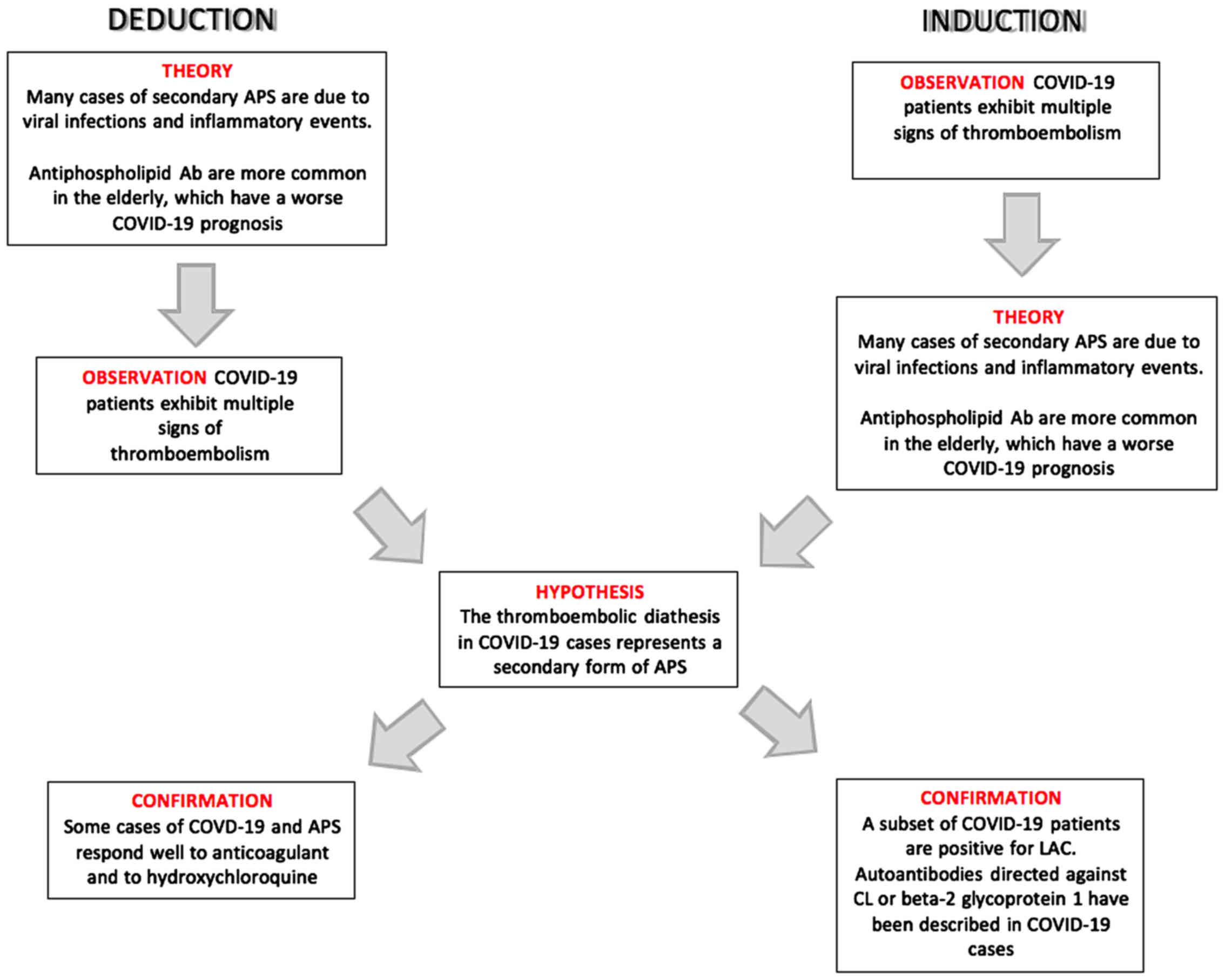
A summary of the Deduction and Induction processes
that lead to the hypothesis of the occurrence of a secondary form
of APS in COVID-19 patients is presented in Fig. 2.
6. Future therapeutic directions
Although, the diagnosis of secondary APS would not
immediately change the therapeutic approach to prevention and
treatment of thrombosis in COVID-19 patients, this demonstration
could propel tailored pathogenic approaches for the treatment of a
B cell dependent disorders under an unprecedently urgent pandemic
situation. Repurposing of specific anti-B cell therapies such as
rituximab and ocrelizumab can be anticipated. The anti-C5a mAb
eculizumab would also receive attention though its costs would
hinder its repurposing in a very large arena such as is that of
COVID-19 currently. The use of plasmapheresis should warrant
further consideration as well as that of IvIg that seem effective
in some cases of APS and CAPS (26,40). Small molecule inhibitors of
Toll-like receptor 4 signaling such as resatorvid or GLS-1027 R
that may also inhibit neutrophil extracellular traps associated
with thrombosis, might be considered in the therapeutic management
of COVID-19-related thrombosis (58,59).
Also inhibitors of mTOR pathway such as rapamycin
that has been reported to have some beneficial effects in APS and
we have identified in silico as a drug candidate for
COVID-19 seems of particular interest (30,60,61). Especially so, for the combined
ability of rapamycin to exert immunomodulatory and certain
antiviral efficacy that remains, however, to be eventually
demonstrated on SARS-CoV-2 (60,62,63).
Last, but not least, if some or most cases of
COVID-19 associated thrombosis represent a secondary APS, this
could give strong impetus to preclinical compounds that represent
first class inhibitors of the thrombogenic properties of
autoanti-bodies to β2-GP1. Two of these compounds have demonstrated
in vivo efficacy in multiple forms of APS.
The A1-A1 peptide
β2-GP1 is known to interact with A1, the first
ligand-binding domain of ApoER2. The A1-A1 peptide is a soluble
analogue of ApoE receptor 2 with a high affinity for
β2-GP1/antibody complexes. A1-A1 inhibits at least two
prothrombotic interactions of β2-GP1/antibody complexes: the
binding to ApoER2 and anionic phospholipids on the cellular
surfaces. A distinctive feature of A1-A1 compared to A1 is that
A1-A1 preferentially interacts with β2-GP1 bound to anti-β2-GP1
antibodies (64,65). Because β2-GP1 is present in the
blood at high concentration, 4 µM (66), it is important that a potential
drug binds predominantly to pathological β2-GP1/antibody complexes.
The A1-A1 molecule has been shown to be effective in several rodent
models of APS (64,67,68).
AUR-1001
Aur-1001 is a minibody lacking the CH2 domain and
thus incapable of activating the complement that is being developed
from Aura Biopharm (Oslo, Norway). The minibody has been shown to
have a higher affinity than endogenous anti-β2-GPI autoAbs that are
displaced in vitro. AUR-1001 has been shown to be capable of
preventing development of both vascular and obstetric APS in
preclinical model that are challenged with purified IgG from
patients with APS (69).
Acknowledgments
Not applicable.
Funding
This study was supported by the current research
funds 2020 of IRCCS 'Centro Neurolesi Bonino-Pulejo' (Messina,
Italy).
Availability of data and materials
Not applicable.
Authors' contributions
EC, PB, YS and FN were involved in the conception of
the study. AB, RC, AT, AG, CB and PF were involved in the
literature search and critical reviewing of the manuscript. EC and
FN were involved in the preparation of the draft of the manuscript.
AG, PF, CB, YS and FN were involved in the revising and editing of
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
FN is cofounder and shareholders of Aura Biopharm.
The others authors declare that they have no competing
interests.
References
|
1
|
Yuki K, Fujiogi M and Koutsogiannaki S:
COVID-19 pathophysiology: A review. Clin Immunol. 215:1084272020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al China Medical Treatment
Expert Group for Covid-19: Clinical characteristics of coronavirus
disease 2019 in China. N Engl J Med. 382:1708–1720. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rothan HA and Byrareddy SN: The
epidemiology and pathogenesis of coronavirus disease (COVID-19)
outbreak. J Autoimmun. 109:1024332020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Zhou W, Yang L and You R:
Physiological and pathological regulation of ACE2, the SARS-CoV-2
receptor. Pharmacol Res. 157:1048332020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doobay MF, Talman LS, Obr TD, Tian X,
Davisson RL and Lazartigues E: Differential expression of neuronal
ACE2 in transgenic mice with overexpression of the brain
renin-angio-tensin system. Am J Physiol Regul Integr Comp Physiol.
292:R373–R381. 2007. View Article : Google Scholar
|
|
7
|
Yan CH, Faraji F, Prajapati DP, Boone CE
and DeConde AS: Association of chemosensory dysfunction and
COVID-19 in patients presenting with influenza-like symptoms. Int
Forum Allergy Rhinol. April 12–2020.Epub ahead of print. View Article : Google Scholar
|
|
8
|
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong
Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al: Early transmission
dynamics in Wuhan, China, of novel coronavirus-infected pneumonia.
N Engl J Med. 382:1199–1207. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Tang J and Wei F: Updated
understanding of the outbreak of 2019 novel coronavirus (2019-nCoV)
in Wuhan, China. J Med Virol. 92:441–447. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cascella M, Rajnik M, Cuomo A, Dulebohn SC
and Di Napoli R: Features, evaluation and treatment coronavirus
(COVID-19). StatPearls Publishing; Treasure Island, FL: 2020
|
|
11
|
Nishiura H, Jung SM, Linton NM, Kinoshita
R, Yang Y, Hayashi K, Kobayashi T, Yuan B and Akhmetzhanov AR: The
extent of transmission of novel coronavirus in Wuhan, China, 2020.
J Clin Med. 9:3302020. View Article : Google Scholar :
|
|
12
|
Tang N, Li D, Wang X and Sun Z: Abnormal
coagulation parameters are associated with poor prognosis in
patients with novel coronavirus pneumonia. J Thromb Haemost.
18:844–847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y,
Huang TB, Zhang HY, Sun W and Wang Y: COVID-19 patients' clinical
characteristics, discharge rate, and fatality rate of
meta-analysis. J Med Virol. 92:577–583. 2010. View Article : Google Scholar
|
|
14
|
Zou Y, Guo H, Zhang Y, Zhang Z, Liu Y,
Wang J, Lu H and Qian Z: Analysis of coagulation parameters in
patients with COVID-19 in Shanghai, China. Biosci Trends. April
30–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lodigiani C, Iapichino G, Carenzo L,
Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C,
Alexia B, et al: Venous and arterial thromboembolic complications
in COVID-19 patients admitted to an academic hospital in Milan,
Italy. Thromb Res. 191:9–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bikdeli B, Madhavan MV, Jimenez D, Chuich
T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y,
et al Global COVID-19 Thrombosis Collaborative Group, Endorsed by
the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working
Group on Pulmonary Circulation and Right Ventricular Function:
COVID-19 and thrombotic or thromboembolic disease: implications for
prevention, antithrombotic therapy, and follow-up: JACC
State-of-the-Art Review. J Am Coll Cardiol. 75:2950–2973. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang N, Bai H, Chen X, Gong J, Li D and
Sun Z: Anticoagulant treatment is associated with decreased
mortality in severe coronavirus disease 2019 patients with
coagulopathy. J Thromb Haemost. 18:182020.
|
|
18
|
Fogarty H, Townsend L, Ni Cheallaigh C,
Bergin C, Martin-Loeches I, Browne P, Bacon CL, Gaule R, Gillett A,
Byrne M, et al: More on COVID-19 coagulopathy in Caucasian
patients. Br J Haematol. 189:1060–1061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beun R, Kusadasi N, Sikma M, Westerink J
and Huisman A: Thromboembolic events and apparent heparin
resistance in patients infected with SARS-CoV-2. Int J Lab Hematol.
42(Suppl 1): 19–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye Q, Wang B and Mao J: The pathogenesis
and treatment of the 'Cytokine Storm' in COVID-19. J Infect.
80:607–613. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaninov N: In the eye of the COVID-19
cytokine storm. Nat Rev Immunol. 20:2772020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magro C, Mulvey JJ, Berlin D, Nuovo G,
Salvatore S, Harp J, Baxter-Stoltzfus A and Laurence J: Complement
associated microvascular injury and thrombosis in the pathogenesis
of severe COVID-19 infection: A report of five cases. Transl Res.
220:1–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Risitano AM, Mastellos DC, Huber-Lang M,
Yancopoulou D, Garlanda C, Ciceri F and Lambris JD: Complement as a
target in COVID-19? Nat Rev Immunol. 20:343–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campbell CM and Kahwash R: Will complement
inhibition be the new target in treating COVID-19-related systemic
thrombosis? Circulation. 141:1739–1741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mastaglio S, Ruggeri A, Risitano AM,
Angelillo P, Yancopoulou D, Mastellos DC, Huber-Lang M, Piemontese
S, Assanelli A, Garlanda C, et al: The first case of COVID-19
treated with the complement C3 inhibitor AMY-101. Clin Immunol.
215:1084502020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodziewicz M and D'Cruz DP: An update on
the management of antiphospholipid syndrome. Ther Adv Musculoskelet
Dis. 12:1759720X209108552020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Million M, Bardin N, Bessis S, Nouiakh N,
Douliery C, Edouard S, Angelakis E, Bosseray A, Epaulard O, Branger
S, et al: Thrombosis and antiphospholipid antibody syndrome during
acute Q fever: A cross-sectional study. Medicine (Baltimore).
96:e75782017. View Article : Google Scholar
|
|
28
|
Mendoza-Pinto C, García-Carrasco M and
Cervera R: Role of infectious diseases in the antiphospholipid
syndrome (including its catastrophic variant). Curr Rheumatol Rep.
20:622018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Catoggio C, Alvarez-Uría A, Fernandez PL,
Cervera R and Espinosa G: Catastrophic antiphospholipid syndrome
triggered by fulminant disseminated herpes simplex infection in a
patient with systemic lupus erythematosus. Lupus. 21:1359–1361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garcia-Carrasco M, Mendoza-Pinto C,
Macias-Diaz S, Vazquez de Lara F, Etchegaray-Morales I,
Galvez-Romero JL, Mendez-Martinez S and Cervera R: The role of
infectious diseases in the catastrophic antiphospholipid syndrome.
Autoimmun Rev. 14:1066–1071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gómez-Puerta JA and Cervera R: Diagnosis
and classification of the antiphospholipid syndrome. J Autoimmun.
48-49:20–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pengo V, Del Ross T, Ruffatti A, Bison E,
Cattini MG, Pontara E, Testa S, Legnani C, Pozzi N, Peterle D, et
al: Lupus anticoagulant identifies two distinct groups of patients
with different antibody patterns. Thromb Res. 172:172–178. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cervera R: Antiphospholipid syndrome.
Thromb Res. 151(Suppl 1): S43–S47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erkan D, Espinosa G and Cervera R:
Catastrophic antiphospholipid syndrome: Updated diagnostic
algorithms. Autoimmun Rev. 10:74–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi W, Krilis SA, Chong BH, Gordon S and
Chesterman CN: Prevalence of lupus anticoagulant and
anticardiolipin antibodies in a healthy population. Aust N Z J Med.
20:231–236. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fields RA, Toubbeh H, Searles RP and
Bankhurst AD: The prevalence of anticardiolipin antibodies in a
healthy elderly population and its association with antinuclear
antibodies. J Rheumatol. 16:623–625. 1989.PubMed/NCBI
|
|
37
|
Arvanitakis Z, Capuano AW, Brey R,
Fleischman DA, Arfanakis K, Buchman AS, Schneider JA, Levine SR and
Bennett DA: Antiphospholipid Antibodies: Cognitive and Motor
Decline, Neuroimaging and Neuropathology. Neuroepidemiology.
53:100–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruiz-Irastorza G, Crowther M, Branch W and
Khamashta MA: Antiphospholipid syndrome. Lancet. 376:1498–1509.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carmi O, Berla M, Shoenfeld Y and Levy Y:
Diagnosis and management of catastrophic antiphospholipid syndrome.
Expert Rev Hematol. 10:365–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rodríguez-Pintó I, Lozano M, Cid J,
Espinosa G and Cervera R: Plasma exchange in catastrophic
antiphospholipid syndrome. Presse Med. 48:347–353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Islam MA, Alam F, Wong KK, Kamal MA and
Gan SH: Thrombotic management of antiphospholipid syndrome: towards
novel targeted therapies. Curr Vasc Pharmacol. 15:313–326. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quinti I, Lougaris V, Milito C, Cinetto F,
Pecoraro A, Mezzaroma I, Mastroianni CM, Turriziani O, Bondioni MP,
Filippini M, et al: A possible role for B cells in COVID-19? Lesson
from patients with agammaglobulinemia. J Allergy Clin Immunol.
April 22–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McKee DL, Sternberg A, Stange U, Laufer S
and Naujokat C: Candidate drugs against SARS-CoV-2 and COVID-19.
Pharmacol Res. 157:1048592020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chighizola CB, Andreoli L, Gerosa M,
Tincani A, Ruffatti A and Meroni PL: The treatment of
anti-phospholipid syndrome: a comprehensive clinical approach. J
Autoimmun. 90:1–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Xiao M, Zhang S, Xia P, Cao W,
Jiang W, Chen H, Ding X, Zhao H, Zhang H, et al: Coagulopathy and
antiphos-pholipid antibodies in patients with Covid-19. N Engl J
Med. 382:e382020. View Article : Google Scholar
|
|
46
|
Beyrouti R, Adams ME, Benjamin L, Cohen H,
Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, et
al: Characteristics of ischaemic stroke associated with COVID-19.
Neurol Neurosurg Psychiatry. April 30–2020.Epub ahead of print.
View Article : Google Scholar
|
|
47
|
Zayet S, Klopfenstein T, Kovacs R,
Stancescu S and Hagenkötter B: Acute cerebral stroke with multiple
infarctions and COVID-19, France, 2020. Emerg Infect Dis.
26:262020. View Article : Google Scholar
|
|
48
|
Aubignat M and Godefroy O: COVID-19 and
ischemic stroke: Should we systematically look for lupus
anticoagulant and antiphospholipid antibodies? Rev Neurol (Paris).
176:505–506. 2020. View Article : Google Scholar
|
|
49
|
Harzallah I, Debliquis A and Drénou B:
Lupus anticoagulant is frequent in patients with Covid-19. J Thromb
Haemost. 2020.
|
|
50
|
Bowles L, Platton S, Yartey N, Dave M, Lee
K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, et al:
Lupus anticoagulant and abnormal coagulation tests in patients with
Covid-19. N Engl J Med. May 5–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Helms J, Tacquard C, Severac F,
Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R,
Schenck M, Fagot Gandet F, et al CRICS TRIGGERSEP Group (Clinical
Research in Intensive Care and Sepsis Trial Group for Global
Evaluation and Research in Sepsis): High risk of thrombosis in
patients with severe SARS-CoV-2 infection: A multicenter
prospective cohort study. Intensive Care Med. 46:1089–1098. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pineton de Chambrun M, Frere C, Miyara M,
Amoura Z, Martin-Toutain I, Mathian A, Hekimian G and Combes A:
High frequency of antiphospholipid antibodies in critically-ill
COVID-19 patients: a link with hypercoagulability? J Intern Med.
June 12–2020.Epub ahead of print. View Article : Google Scholar
|
|
53
|
Hossri S, Shadi M, Hamarsha Z, Schneider R
and El-Sayegh D: Clinically significant anticardiolipin antibodies
associated with COVID-19. J Crit Care. 59:32–34. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galeano-Valle F, Oblitas CM,
Ferreiro-Mazón MM, Alonso-Muñoz J, Del Toro-Cervera J, di Natale M
and Demelo-Rodríguez P: Antiphospholipid antibodies are not
elevated in patients with severe COVID-19 pneumonia and venous
thromboembolism. Thromb Res. 192:113–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chaudhary R, Pagali S, Garg J, Murad MH,
Wysokinski WE and McBane RD 2nd: DOACs versus VKAs in older adults
treated for acute venous thromboembolism: systematic review and
meta-analysis. J Am Geriatr Soc. May 22–2020.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Marchandot B, Sattler L, Jesel L,
Matsushita K, Schini-Kerth V, Grunebaum L and Morel O: COVID-19
Related coagulopathy: A distinct entity? J Clin Med. 9:92020.
View Article : Google Scholar
|
|
57
|
Oxley TJ, Mocco J, Majidi S, Kellner CP,
Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger
KA, et al: Large-vessel stroke as a presenting feature of Covid-19
in the young. N Engl J Med. 382:e602020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Plunk MA, Alaniz A, Olademehin OP,
Ellington TL, Shuford KL and Kane RR: Design and Catalyzed
Activation of Tak-242 Prodrugs for Localized Inhibition of
TLR4-Induced Inflammation. ACS Med Chem Lett. 11:141–146. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stojanovic I, Cuzzocrea S, Mangano K,
Mazzon E, Miljkovic D, Wang M, Donia M, Al Abed Y, Kim J, Nicoletti
F, et al: In vitro, ex vivo and in vivo immunopharmacological
activities of the isoxazoline compound VGX-1027: Modulation of
cytokine synthesis and prevention of both organ-specific and
systemic autoimmune diseases in murine models. Clin Immunol.
123:311–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fagone P, Ciurleo R, Lombardo SD,
Iacobello C, Palermo CI, Shoenfeld Y, Bendtzen K, Bramanti P and
Nicoletti F: Transcriptional landscape of SARS-CoV-2 infection
dismantles pathogenic pathways activated by the virus, proposes
unique sex-specific differences and predicts tailored therapeutic
strategies. Autoimmun Rev. 19:1025712020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nicoletti F, Lapenta C, Donati S, Spada M,
Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C and Aquaro
S: Inhibition of human immunodeficiency virus (HIV-1) infection in
human peripheral blood leucocytes-SCID reconstituted mice by
rapamycin. Clin Exp Immunol. 155:28–34. 2009. View Article : Google Scholar :
|
|
63
|
Maiese K: The mechanistic target of
rapamycin (mTOR): Novel considerations as an antiviral treatment
and possibilities for COVID-19. Curr Neurovasc Res. April
25–2020.Epub ahead of print. View Article : Google Scholar
|
|
64
|
Kolyada A, Lee CJ, De Biasio A and Beglova
N: A novel dimeric inhibitor targeting Beta2GPI in
Beta2GPI/antibody complexes implicated in antiphospholipid
syndrome. PLoS One. 5:e153452010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee CJ, De Biasio A and Beglova N: Mode of
interaction between β2GPI and lipoprotein receptors suggests
mutually exclusive binding of β2GPI to the receptors and anionic
phospholipids. Structure. 18:366–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin F, Murphy R, White B, Kelly J,
Feighery C, Doyle R, Pittock S, Moroney J, Smith O, Livingstone W,
et al: Circulating levels of β2-glycoprotein I in thrombotic
disorders and in inflammation. Lupus. 15:87–93. 2006. View Article : Google Scholar
|
|
67
|
Kolyada A, Karageorgos I, Mahlawat P and
Beglova N: An A1-A1 mutant with improved binding and inhibition of
β2GPI/antibody complexes in antiphospholipid syndrome. FEBS J.
282:864–873. 2015. View Article : Google Scholar :
|
|
68
|
Kolyada A, Porter A and Beglova N:
Inhibition of thrombotic properties of persistent autoimmune
anti-β2GPI antibodies in the mouse model of antiphospholipid
syndrome. Blood. 123:1090–1097. 2014. View Article : Google Scholar :
|
|
69
|
Agostinis C, Durigutto P, Sblattero D,
Borghi MO, Grossi C, Guida F, Bulla R, Macor P, Pregnolato F,
Meroni PL, et al: A non-complement-fixing antibody to β2
glycoprotein I as a novel therapy for antiphospholipid syndrome.
Blood. 123:3478–3487. 2014. View Article : Google Scholar : PubMed/NCBI
|