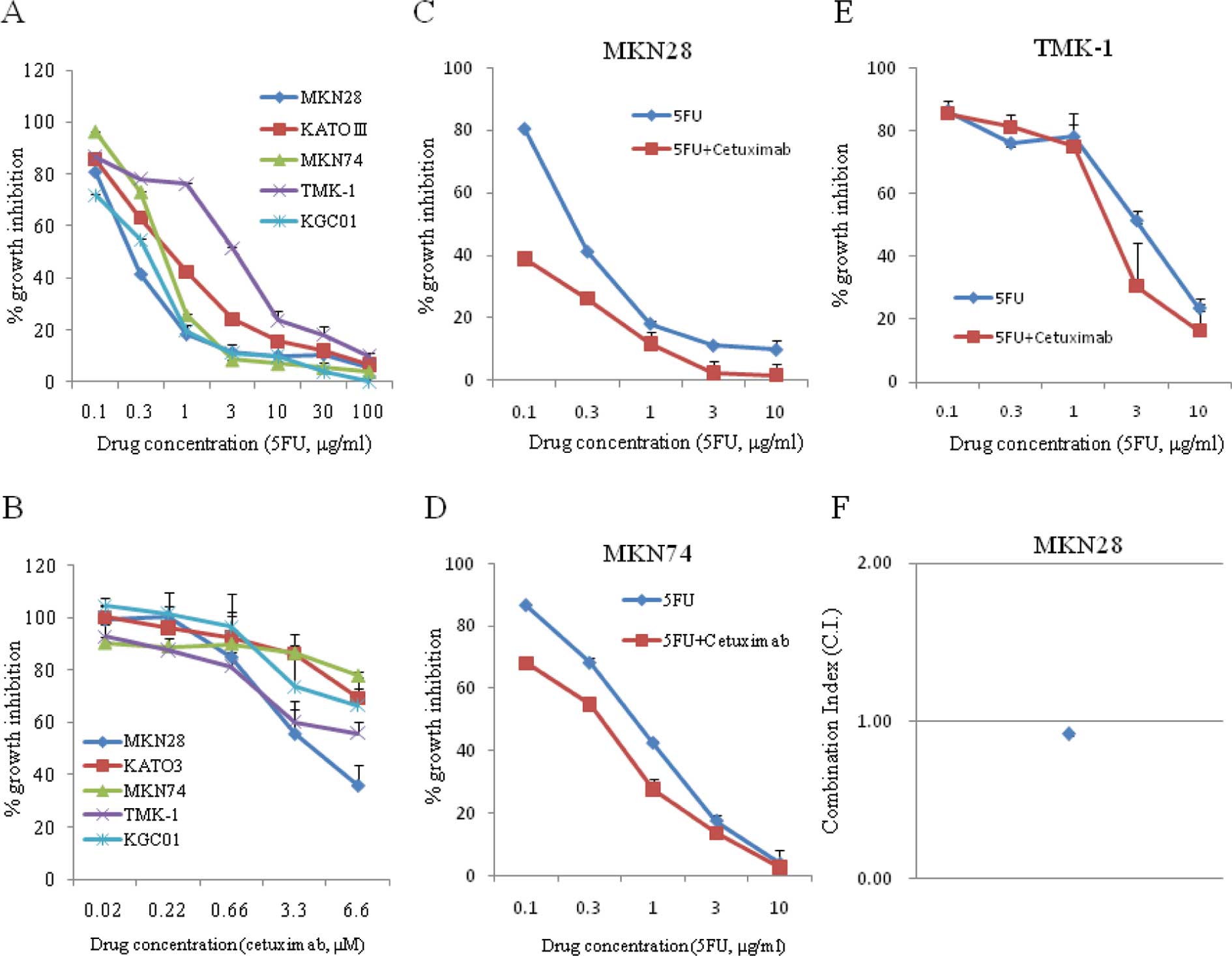
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Mari E, Floriani I, Tinassi A, et al:
Efficacy of adjuvant chemotherapy after curative resection for
gastric cancer: a meta-analysis of published randomized trials. Ann
Oncol. 11:837–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panzini I, Gianni L, Fattori PP, et al:
Adjuvant chemotherapy in gastric cancer: a meta-analysis of
randomized trials and a comparison with previous meta-analyses.
Tumori. 88:21–27. 2002.PubMed/NCBI
|
|
4
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1 an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boku N: Chemotherapy for metastatic
disease: review from JCOG trials. Int J Clin Oncol. 13:196–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
10
|
Wesolowski R, Lee C and Kim R: Is there a
role for second-line chemotherapy in advanced gastric cancer?
Lancet Oncol. 10:903–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iqbal S, Goldman B, Fenoglio-Preiser CM,
Lenz HJ, Zhang W, Danenberg KD, Shibata SI and Blanke CD: Southwest
Oncology Group study S0413: a phase II trial of lapatinib
(GW572016) as first-line therapy in patients with advanced or
metastatic gastric cancer. Ann Oncol. May 20–2011.(Epub ahead of
print).
|
|
13
|
Cappetta A, Lonardi S, Pastorelli D,
Bergamo F, Lombardi G and Zagonel V: Advanced gastric cancer (GC)
and cancer of the gastro-oesophageal junction (GEJ): focus on
targeted therapies. Crit Rev Oncol Hematol. Jan 19–2011.(Epub ahead
of print).
|
|
14
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saltz LB, Meropol NJ, Loehrer PJ Sr,
Needle MN, Kopit J and Mayer RJ: Phase II trial of cetuximabin
patients with refractory colorectal cancer that expresses the
epidermal growth factor receptor. J Clin Oncol. 22:1201–1208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1999.
|
|
19
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermorken JB, Mesia R, Rivera F, et al:
Platinum-based chemotherapy plus cetuximab in head and neck cancer.
N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanna N, Lilenbaum R, Ansari R, et al:
Phase II trial of cetuximab in patients with previously treated
non-small cell lung cancer. J Clin Oncol. 24:5253–5258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JC, Wang ST, Chow NH and Yang HB:
Investigation of the prognostic value of coexpressed erbB family
members for the survival of colorectal cancer patients after
curative surgery. Eur J Cancer. 38:1065–1071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porebska I, Harlozinska A and Bojarowski
T: Expression of the tyrosine kinase activity growth factor
receptors (EGFR, ERBB2, ERBB3) in colorectal adenocarcinomas and
adenomas. Tumor Biol. 21:105–115. 2002.PubMed/NCBI
|
|
24
|
Bancroft CC, Chen Z, Yeh J, et al: Effects
of pharmacologic antagonists of epidermal growth factor receptor,
PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8
and VEGF expression in human head and neck squamous cell carcinoma
lines. Int J Cancer. 99:538–548. 2002.
|
|
25
|
Raymond E, Fauvre S and Armand JP:
Epidermal growth factor receptor tyrosine kinase as a target for
anticancer therapy. Drugs. 60(Suppl 1): S15–S23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baselga J, Norton L, Masui H, Pandiella A,
Coplan K, Miller WH and Mendelsohn J: Antitumor effects of
doxorubicin in combination with anti-epidermal growth factor
receptor monoclonal antibodies. J Natl Cancer Inst. 85:1327–1333.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruns CJ, Harbison MT, Davis DW, et al:
Epidermal growth factor receptor blockade with cetuximab plus
gemcitabine results in regression of human pancreatic carcinoma
growing orthotopically in nude mice by antiangiogenic mechanisms.
Clin Cancer Res. 6:1936–1948. 2006.
|
|
28
|
Ciardiello F, Bianco R, Damiano V, et al:
Antitumor activity of sequential treatment with topotecan and
anti-epidermal growth factor receptor monoclonal antibody C225.
Clin Cancer Res. 4:909–916. 1999.PubMed/NCBI
|
|
29
|
Fan Z, Baselga J, Masui H and Mendelsohn
J: Antitumor effect of anti-EGF receptor monoclonal antibodies plus
cis-diamminedichloroplatinum (cis-ddp) on well established A431
cell xenografts. Cancer Res. 53:4637–4642. 1993.PubMed/NCBI
|
|
30
|
Huang SM, Bock JM and Harari PM: Epidermal
growth factor receptor blockade with C225 modulates proliferation,
apoptosis, and radiosensitivity in squamous cell carcinomas of the
head and neck. Cancer Res. 59:1935–1940. 1999.PubMed/NCBI
|
|
31
|
Huang SM and Harari PM: Modulation of
radiation response after epidermal growth factor receptor blockade
in squamous cell carcinomas: inhibition of damage repair, cell
cycle kinetics, and tumor angiogenesis. Clin Cancer Res.
6:2166–2174. 2000.
|
|
32
|
Inoue K, Slaton JW, Perrotte P, et al:
Paclitaxel enhances the effects of the anti-epidermal growth factor
receptor monoclonal antibody ImClone C225 in mice with metastatic
human bladder transitional cell carcinoma. Clin Cancer Res.
6:4874–4884. 2000.
|
|
33
|
Prewett MC, Hooper AT, Bassi R, Ellis LM,
Waksal HW and Hicklin DJ: Enhanced antitumor activity of
anti-epidermal growth factor receptor monoclonal antibody IMCC225
in combination with irinotecan (CPT-11) against human colorectal
tumor xenografts. Clin Cancer Res. 8:994–1003. 2002.
|
|
34
|
Shin DM, Donato NJ, Perez-Soler R, et al:
Epidermal growth factor receptor-targeted therapy with C225 and
cisplatin in patients with head and neck cancer. Clin Cancer Res.
7:1204–1213. 2001.PubMed/NCBI
|
|
35
|
Han SW, Oh DY, Im SA, Park SR, Lee KW,
Bang YJ and Kim TY: Phase II study and biomarker analysis of
cetuximab combined with modified FOLFOX6 in advanced gastric
cancer. Br J Cancer. 100:298–304. 2009. View Article : Google Scholar : PubMed/NCBI
|